Abstract
Background
Iron supplementation improves the erythropoiesis-stimulating agents’ (ESAs) response in chemotherapy-related anemia. The primary aim of our study is to assess the efficacy of sucrosomial iron, a new oral iron formulation, in cancer patients with chemotherapy-induced anemia treated with ESAs. The secondary objectives included the efficacy into two subgroups of patients (iron replete and functional iron deficiency) between the two study arms, safety and the effect on transfusion need.
Methods
In this randomized, multicentre, open-label, phase III clinical trial, 60 cancer patients were enrolled. Each patient was randomly assigned (1:1) to receive 12 weeks of oral sucrosomial iron at the dose of 30 mg daily in combination with ESAs or no supplementation to ESA treatment. The endpoint considered for efficacy was the proportion of patients achieving complete hematological response at 12 weeks (increase in Hb > 2 g/dL from baseline, without RBC transfusions in the previous 28 days or achieving Hb ≥ 12 g/dL).
Results
There was a statistically significant association between oral sucrosomial iron supplementation in combination with ESAs and the achievement of a complete hematological response. This response was achieved within 12 weeks by 31% of patients in the control group and by 52% of patients supplemented with oral sucrosomial iron. A trend of greater response in sucrosomial iron arm was found in both subgroups. No difference was observed about safety and transfusion need.
Conclusions
Sucrosomial iron is well tolerated and its combination with ESAs improves the hematological response in cancer patients with chemotherapy-related anemia.
Trial registration number and date of registration
This study has been reviewed by the Institutional Ethics Committee of the IRCCS Policlinico San Matteo Foundation, Pavia, Italy (28/04/2015; prot. N. 20,150,002,059), and by the Institutional Ethics Committee of the other Italian oncological centers involved in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is one of the most frequent complications in cancer patients, significantly affecting their quality of life and contributing to cancer’s morbidity. It is usually characterized by hemoglobin values (Hb) ≤ 10 g/dL, reduced reticulocyte count, reduced serum iron level and iron-binding capacity, normal or reduced transferrin saturation, and increased serum ferritin with normal levels of vitamin B12 and B9.
Cancer-related anemia is a multifactorial disease: it may be caused by a blood loss (hemolysis, bleeding) or a reduced erythrocyte production (involvement of the bone marrow by neoplastic cells, nutritional deficiencies), but more frequently, it is a consequence of the antiblastic treatments (chemotherapy-associated anemia, both for direct effect on the bone marrow and for indirect effect on the production of erythropoietin) and the chronic inflammatory process, a condition that defines the chronic-disease anemia [1].
Erythropoiesis-stimulating agents (ESAs) are often used in the treatment of cancer-related anemia and their administrations can lead to a significant improvement in quality of life and a decrease in transfusion need [2, 3].
According to the recent recommendations for anemia treatment in cancer patients, ESA administration is indicated in patients with solid tumors and symptomatic anemia under chemotherapy with Hb level < 10 g/dL or asymptomatic anemia with Hb < 8 g/dL. Red blood cell (RBC) transfusions are recommended in patients having severe symptoms of anemia with Hb < 7–8 g/dL or Hb < 9 g/dL in case of cardiovascular risk factors [4].
Blood transfusions determine a rapid increase in Hb values (and a rapid subjective benefit) but this practice can lead to complications such as transfusion reactions and transmission of infectious diseases (significantly reduced in recent years).
Although ESAs represent an important therapeutic resource in the treatment of cancer-related anemia, a relatively high percentage of patients (40–50%) do not respond adequately to these drugs. This could be related in part to the dysregulation of iron metabolism leading to functional iron deficiency [5]. This condition characterizes inflammatory disorders, including cancer, and it is linked to the increase of hepcidin in response to inflammatory cytokines. High levels of hepcidin inhibit ferroportin with consequent iron sequestration in enterocytes and macrophages. This leads to a decrease of serum iron and a reduction in erythropoiesis as iron is retained inside cells, despite normal iron status [6, 7].
Furthermore, in cancer-related anemia, a functional iron deficiency may be present or may appear during the therapy with ESAs, due to the intense erythropoietic stimulus.
In recent years, several clinical trials have been conducted to evaluate the role of martial therapy in improving the effectiveness of ESAs in the treatment of cancer-associated anemia [8,9,10,11,12,13,14,15]. Although the basal body iron status was different depending on the study, a benefit was observed from iron addition in improving the hematopoietic response.
A meta-analysis of 8 studies published in 2011 [8] confirmed the goodness of the association of intravenous (IV) iron in obtaining greater hematopoietic responses to erythropoietin (RR 1.29), a reduced risk of transfusion (RR 0.77), and also a greater speed in response (1 month benefit).
The data were confirmed by another meta-analysis published in recent years by Pedrazzoli P. et al., which aimed to clarify the role of intravenous martial therapy in association with ESAs [5]. The target population included 1784 patients (776 had received parenteral iron, 1008 oral iron, or no martial therapy). The results confirmed that the use of intravenous iron improves the percentage of patients responsive to ESA therapy.
A review published in 2019 [6] summarizes seven clinical trials on chemotherapy-induced anemia using IV iron with ESAs. These trials, one of them after a reanalysis, reported positive results such as reduction in transfusion need, improving in hematological response, and lowering in ESA dose. This is a positive goal for patient care considering that ESAs are associated with potential adverse events and their use has several limitations.
Intravenous martial therapy associated with ESAs may have a role not only in subjects with functional iron deficiency, but also in patients with normal iron status [5, 9,10,11,12,13, 16].
The efficacy of intravenous iron in the treatment of functional iron deficiency and correction of anemia has been documented in kidney failure during dialysis [17] and in patients with inflammatory rheumatic diseases such as rheumatoid arthritis, spondyloarthritis, or other autoimmune connective tissue diseases [18].
Previous experiences have shown that the use of intravenous iron in cancer patients undergoing chemotherapy and/or in patients undergoing dialysis induces an increase in responses to ESA therapy and reduces the number of blood transfusions [16]. In fact, in anemic patients on dialysis, the use of parenteral iron is a routine practice and allows significant savings in terms of ESA dose and costs [14].
The guidelines of the main scientific societies are not entirely in agreement in suggesting the use of iron in cancer patients receiving ESAs. The European Society for Medical Oncology recommends a “basal and periodic check of the martial state”, reiterating “the effectiveness of intravenous iron compared to iron administered orally, in obtaining a greater increase in hemoglobin response” [19]. The American Society of Clinical Oncology guidelines conclude that “although the martial supplement is generally recommended to increase responses to ESA therapy, there is insufficient evidence for parenteral iron to be a standard of care” [20]. Finally, the Italian guidelines of the Associazione Italiana di Oncologia Medica suggest “parenteral iron therapy in association with ESA, not only in subjects with functional martial deficiency, but also in those with normal iron status (ferritin > 100 ng / ml and TSAT > 20%)” [21].
However, it is known that intravenous iron formulations are associated with high management costs and a significant incidence of adverse reactions compared to oral iron, traditionally in form of ferrous salts, which in turn is associated with frequent gastrointestinal intolerance [15, 22, 23].
On the other hand, oral iron may be ineffective in functional iron deficiency due to the intestinal hepcidin-ferroportin pathway that seize iron in enterocytes and macrophages. Consequently, intravenous iron may represent a useful option, bypassing the intestinal iron block and leading to a faster Hb increase [6, 24].
In the recent times, a new oral iron formulation (Sideral® Forte, PharmaNutra SpA, Pisa, Italy) has become available in clinical practice.
SiderAL Forte is a food supplement containing 30 mg Sucrosomial Iron (SIDERAL r.m.) and 70 mg of vitamin C. In this formulation, ferric pyrophosphate is covered by phospholipids and sucrose ester of fatty acid matrix in a structure called sucrosomes, ensuring high iron bioavailability and considerable reduction of gastrointestinal side effects [25]. In recent years, some clinical trials have been carried out in patients suffering from chronic refractory anemia, confirming these data [26, 27]. In 2012, an Italian trial conducted in 72 cancer patients receiving chemotherapy and Epoetin Alfa for anemia plus sucrosomial iron showed a significant increase in the Hb level compared to baseline (> 2 g/dL), an improvement in the quality of life and high tolerability. Furthermore, none of the enrolled patients required red blood cell transfusions during the treatment period [8]. More recent studies compared sucrosomial iron with intravenous iron in the treatment for anemia in various diseases confirming that sucrosomial iron is a safe and effective alternative to intravenous iron [28,29,30]. Furthermore, intravenous iron administrations required higher health care costs versus sucrosomial iron as showed by a recent Italian study in non-dialysis chronic kidney disease (ND-CKD) patients [31].
Our knowledge about sucrosomial iron effectiveness in chemotherapy-related anemia in cancer patients is based on limited data. The aim of our study was therefore to assess the efficacy of sucrosomial iron supplementation versus no additional treatment in combination with ESAs in chemotherapy-induced anemia in cancer patients. Between 2015 and 2020, several Italian oncological centers were active recruiting patients.
Patients and methods
Study design and treatment
This is a multicentre, randomized, open-label phase III trial.
All patients received a treatment with an ESA chosen by the referring physician from those available on the market. All the types of ESA chosen were equally distributed among the various patients. The chosen drug was administered by subcutaneous injection at the dosage indicated in the specific data sheet, once a week for 12 weeks. At any time during the study, ESA administration was discontinued if the Hb value was found to be > 12 g/dL. Oral sucrosomial iron (Sideral® Forte, PharmaNutra SpA, Pisa, Italy) was given at a dosage of one capsule (30 mg)/day for 12 weeks.
RBC transfusions were recommended if the Hb value was found to be ≤ 8 g/dL. Transfusions with Hb values > 8 g/dL were however accepted if there were clinical signs/symptoms related to anemia such as drowsiness, congestive heart failure, angina, and dyspnea.
Each patient was required to sign and date the informed consent form before starting any study-related procedure.
Patient population
Inclusion criteria were age ≥ 18 years, life expectancy ≥ 6 months, Eastern Cooperative Oncology Group Performance Score (ECOG PS) 0–2, solid tumor or non-myeloid hematological neoplasms, chemotherapy-associated anemia, functional iron deficiency (transferrin saturation < 20% and ferritin ≥ 30 ng/mL) or normal iron status (transferrin saturation > 20% and ferritin ≥ 30 ng/mL), at least 12 weeks of residual chemotherapy, and adequate kidney and hepatic function.
Exclusion criteria included bone marrow diseases, absolute iron deficiency (TSAT < 20% or ferritin < 30 ng/mL) or iron overload (TSAT > 50% and ferritin > 1000 ng/mL), severe comorbid cardiovascular disease, previous or concomitant pelvic radiation therapy, bone marrow metastasis, more than 2 RBC transfusions, or ESA treatment respectively within 4 and 8 weeks prior to enrollment.
Study objectives and endpoints
The primary aim of the study was to assess the efficacy of sucrosomial iron supplementation versus no additional treatment, in combination with ESAs, for the treatment of chemotherapy-related anemia in cancer patients with functional iron deficiency or normal iron status undergoing chemotherapy. The endpoint considered for efficacy was the proportion of patients achieving complete hematological response at 12 weeks (increase in Hb > 2 g/dL from baseline, without RBC transfusions in the previous 28 days or achieving Hb ≥ 12 g/dL).
The secondary objectives included the efficacy into two subgroups of patients, those with normal martial reserve identified as iron replete (transferrin saturation percentage, TS%, ≥ 20), and those with functional iron deficiency (TS% < 20) between the two study arms. Other secondary objectives were safety (evaluating the occurrence of adverse events, NCI-CTC v. 4.0 grading) and the effect on number of RBC transfusions.
Statistical analysis
The planned sample size for this two-arms study was 282 patients (141 per group). On the basis of previous clinical trials [9], the complete hematological response rate in the control group appears to be 62%; we assumed that the treatment achieved a response rate of 77%. With the planned sample size and a2-sided alpha error of 5%, a log rank test for equality of complete response free survival curves has 80% power to detect a difference between a response-free survival at 12 weeks of 38% in the control group and 23% in the treated group (a constant hazard ratio, (HR) of 0.66) (taking into account a dropout rate of 5%).
Categorical variables were described as counts and percentages. Quantitative variables were summarized as median and interquartile range (IQR).
The cumulative incidence of complete response was defined as the time between the basal visit and the achieving of complete hematological response (event) or last follow-up (censored) and was estimated with the Kaplan–Meier failure function. The disease-free survival was defined as the time between the achieving of complete response and the loss of complete response (event) or last follow-up (censored) and was estimated by the Kaplan–Meier product limit method.
The complete hematological response incidence rate over time (and 95% confidence interval, 95% CI) was calculated in each group. Two arms were compared both for cumulative incidence of complete response and for disease-free survival. Hazard ratio (HR) and 95% CI has been derived by Cox model. The cumulative incidence of complete response was also compared between two arms within the 2 sub-groups of functional iron deficiency. In addition, the association between arm and complete response was also estimated with a repeated measure logistic regression model, with standard error estimated with clustered sandwich method to take into account the intra-patient correlation.
The number of adverse events and the number of transfusions during the trial were compared using the negative binomial model. A 2-sided p-value < 0.05 was considered statistically significant.
All statistical analyses were performed by Stata 16software (release 16, StataCorp, College Station, TX, USA).
Results
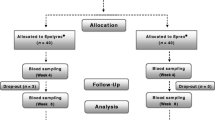
Due to lower than expected accrual, a total of only 60 cancer patients with chemotherapy-related anemia were enrolled in the period between July 2015 and March 2020. This was mainly due to the poor performance status of many patients and to comorbidities contraindicating ESA treatment (e.g., uncontrolled hypertension, previous episodes of venous thrombosis and/or embolism). Each patient was randomly assigned to receive 12 weeks of oral sucrosomial iron 30 mg daily (Sideral® Forte) in combination with ESAs or no supplementation to ESA treatment.
A randomization list has been used and managed by the coordinating center IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
There were drop-outs and two deaths due to worsening of clinical conditions and sepsis. Some patients stopped or changed cancer treatment for clinical decision. Characteristics of the analyzed patients (n = 56) are reported in Table 1. Proportion of ECOG performance status of 1–2 was lower in the sucrosomial iron arm (26.9 vs 34.5%). Not all patients were classified into the two subgroups (iron replete and functional iron deficiency) for missing laboratory data.
Primary endpoint
Efficacy
Complete hematological response was achieved within 12 weeks by 31% of patients in the control group and by 52% of patients supplemented with oral sucrosomial iron. The incidence rate of complete response was higher in the sucrosomial iron arm (94 × 1000 person-weeks; 95% CI 56–159) compared to the control arm (37 × 1000 person-weeks; 95% CI 19–72). Figure 1 shows the cumulative incidence of complete response in two arms (sucrosomial iron vs control arm: HR = 2.7, 95% CI 1.2–6.3, p = 0.021). Cumulative incidence of complete response was higher in the sucrosomial iron arm compared to the control arm also after adjusting for ECOG performance status (HR = 3.8, 95% CI 1.5–9.7, p = 0.005).
The repeated measure logistic regression analysis confirmed a statistically significant association between the treatment arm and the achievement of complete hematological response (OR 3.2, 95% CI 1.3–8.1, p = 0.014).
Considering only the patients who have achieved a complete hematological response, disease-free survival was not different between the two arms (HR = 0.6, 95% CI 0.2–2.5, p = 0.499). Again, these results do not change after adjusting for ECOG performance status (data not shown).
Secondary endpoints
Efficacy within subgroups
When comparing sucrosomial iron vs control within the two iron subgroups, a trend towards a higher incidence of complete response in sucrosomial iron arm was found both in iron replete patients (HR = 4.7, 95% CI 0.9–24.2, p = 0.068, Fig. 2) and in functional iron deficiency patients (HR = 2.1, 95% CI 0.7–6.8, p = 0.199, Fig. 3). The data do not reach statistical significance probably due to the small number of patients analyzed.
Safety
Seventy-three percent of patients has experienced at least one adverse event (74% in sucrosomial iron arm and 72% in the control arm). The collected adverse events are reported in Table 2. The number of adverse events was not different between two arms (IRR = 1.2, 95% CI 0.6–2.4, p = 0.594). In the patients supplemented with oral sucrosomial iron, no adverse event was found to be related to this supplementation.
RBC transfusions
One patient in the control arm and two patients in the treatment arm required one transfusion while four patients in the control arm required two transfusions. The number of transfusions needed was not significantly different between the two study arms (IRR = 0.2; 95% CI 0.0–1.6; p = 0.144).
Discussion
Functional iron deficiency has an important role in determining an inadequate ESA response in a considerable percentage of cancer patients with chemotherapy-induced anemia [5]. Iron supplementation improves this response and recent studies confirm the safety and the efficacy of a new oral iron formulation (sucrosomial iron, Sideral® Forte) in the treatment of anemia in various diseases [8, 28,29,30]. This formulation ensures high iron bioavailability and considerable gastrointestinal tolerance [25].
Considering the limited literature about sucrosomial iron effectiveness in chemotherapy-related anemia, the primary aim of our study was to assess the efficacy of sucrosomial iron supplementation in the optimization of ESA treatment for chemotherapy-related anemia in cancer patients versus no iron supplementation. We have also considered the efficacy within subgroups (iron replete and functional iron deficiency), safety and transfusion need.
Our results showed a statistically significant association between oral sucrosomial iron supplementation in combination with ESAs and the achievement of a complete hematological response. This response was achieved within 12 weeks by 31% of patients in the control group and by 52% of patients supplemented with oral sucrosomial iron.
Furthermore, an indicative trend of greater response was observed comparing the two arms for the complete hematological response rate within iron replete and functional iron deficiency subgroups, which however did not reach statistical significance. This is probably related to the failure to achieve the planned sample size, one of the limitations of our study, which lead consequently to a small patients number in each subgroup. Other limitations of this study are represented by the different type of the primary tumor into the sample, the stage of disease, the chemotherapy treatment regimen, and its dosing schedule. These limits may have influenced the iron status and the anemia treatment response on a case by case basis. Furthermore, ECOG performance status 1–2 is less present in the treatment arm compared to the control arm. Despite the lack of balance of these characteristics, our results do not change after adjusting the efficacy analysis and the logistic regression analysis for ECOG PS. These data suggest that the statistically significant difference between the two study arms in terms of efficacy and the retention time of complete response was not influenced by the different baseline clinical condition of the two patient groups. This finding reinforces the positive information about the efficacy of oral sucrosomial iron in chemotherapy-related anemia provided by our study.
Considering the number of adverse events and the need for transfusions, no significant difference was found between the two study arms. Between patients supplemented with oral sucrosomial iron, no adverse event was found to be related to this supplementation. This data highlights the known high tolerance of this supplement [25].
In the end, the most significant result that emerged from our trial is that sucrosomial iron supplementation improves the hematological response in association with ESAs in patients with cancer-related anemia undergoing chemotherapy. Another food for thought that this study provides is the possibility of using martial supplementation with Sideral Forte in patients with functional iron deficiency anemia, a very frequent condition during chemotherapy, which is often difficult to treat and which can limit compliance to treatment and consequently its effectiveness.
Given that our findings are based on a limited number of patients and are promising to optimize the ESA treatment in chemotherapy-related anemia, further studies need to be performed to extend the analysis on a larger sample size.
Conclusions
Iron supplementation is demonstrated to promote the achieving of a complete hematological response in combination with ESAs in the treatment of chemotherapy-related anemia. Traditionally, iron supplementation is accomplished through IV iron or oral iron salts, which are related to significant incidence of adverse events. Furthermore, iron supplementation promotes the administration of lower doses of ESAs reducing the incidence of the potential adverse events linked to them. As its high gastrointestinal tolerance, high bioavailability, and low adverse events incidence, oral sucrosomial iron could represent a valid method of iron supplementation improving the treatment of chemotherapy-related anemia and the quality of life in cancer patients.
Data availability
Data are available on request from the authors.
References
Rodgers GM III et al (2012) Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw 10(5):628–653. https://doi.org/10.6004/jnccn.2012.0064
Melosky BL (2008) Erythropoiesis-stimulating agents: benefits and risks in supportive care of cancer. Curr Oncol 15(Suppl 1):S10–S15. https://doi.org/10.3747/co.2008.172
Bohlius J, et al (2006) Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev (3):CD003407. https://doi.org/10.1002/14651858.CD003407.pub4
Escobar Álvarez Y et al (2021) SEOM clinical guidelines for anaemia treatment in cancer patients (2020). Clin Transl Oncol 23(5):931–939. https://doi.org/10.1007/s12094-021-02580-2
Pedrazzoli P et al (2009) Iron supplementation and erythropoiesis-stimulatory agents in the treatment of cancer anemia. Cancer 115(6):1169–1173. https://doi.org/10.1002/cncr.24115
Rodgers GM et al (2019) The role of intravenous iron in the treatment of anemia associated with cancer and chemotherapy. Acta Haematol 142(1):13–20. https://doi.org/10.1159/000496967
Natalucci V et al (2021) Cancer related anemia: an integrated multitarget approach and lifestyle interventions. Nutrients 13(2):482. https://doi.org/10.3390/nu13020482
Mafodda A et al (2017) Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: a pilot study. Support Care Cancer 25(9):2779–2786. https://doi.org/10.1007/s00520-017-3690-z
Pedrazzoli P et al (2008) Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alfa. J Clin Oncol 26(10):1619–1625. https://doi.org/10.1200/JCO.2007.12.2051
Petrelli F et al (2012) Addition of iron to erythropoiesis-stimulating agents in cancer patients: a meta-analysis of randomized trials. J Cancer Res Clin Oncol 138(2):179–187. https://doi.org/10.1007/s00432-011-1072-3
Hedenus M et al (2007) Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 21(4):627–632. https://doi.org/10.1038/sj.leu.2404562
Henry DH et al (2007) Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 12(2):231–242. https://doi.org/10.1634/theoncologist.12-2-231
Glaspy J et al (2009) Erythropoietin in cancer patients. Annu Rev Med 60:181–192. https://doi.org/10.1146/annurev.med.60.050307.110718
Mikhail A et al (2017) Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol 18:345. https://doi.org/10.1186/s12882-017-0688-1
Steensma DP et al (2011) Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J Clin Oncol 29(1):97–105. https://doi.org/10.1200/JCO.2010.30.3644
Auerbach M et al (2004) Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 22(7):1301–1307. https://doi.org/10.1200/JCO.2004.08.119
Macdougall IC, et al (2016) Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 89(1) pp 28-39, ISSN 0085-2538https://doi.org/10.1016/j.kint.2015.10.002
Salvadori U et al (2020) (2020) Intravenous ferric carboxymaltose is effective and safe in patients with inflammatory rheumatic diseases. Blood Transfus 18(3):176–181. https://doi.org/10.2450/2019.0207-19
Aapro M et al (2018) Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann Oncol 29(Suppl 4):iv96–iv110. https://doi.org/10.1093/annonc/mdx758
Bohlius J et al (2019) Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. Blood Adv 3(8):1197–1210. https://doi.org/10.1182/bloodadvances.2018030387
AIOM (2019) Linee guida AIOM 2019 - Gestione della tossicità ematopoietica in Oncologia. pp 1–47, 2019
Michael B et al (2002) Sodium ferric gluconate complex in hemodialysis patients: adverse reactions compared to placebo and iron dextran. Kidney Int 61(5):1830–1839. https://doi.org/10.1046/j.1523-1755.2002.00314.x
Chertow GM et al (2006) Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 21(2):378–382. https://doi.org/10.1093/ndt/gfi253
Abdel-Razeq H et al (2020) Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol 145:102837. https://doi.org/10.1016/j.critrevonc.2019.102837
Gómez-Ramírez S et al (2018) Sucrosomial® iron: a new generation iron for improving oral supplementation. Pharmaceuticals (Basel) 11(4):97. https://doi.org/10.3390/ph11040097
Yuan L et al (2013) Effect of iron liposomes on anemia of inflammation. Int J Pharm 454(1):82–89. https://doi.org/10.1016/j.ijpharm.2013.06.078
Capra AP et al (2017) A child with severe iron-deficiency anemia and a complex TMPRSS6 genotype. Hematology 22(9):559–564. https://doi.org/10.1080/10245332.2017.1317990
Pisani A et al (2015) Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: a randomized trial. Nephrol Dial Transplant 30(4):645–652. https://doi.org/10.1093/ndt/gfu357
Giordano G et al (2020) Oral high-dose sucrosomial iron vs intravenous iron in sideropenic anemia patients intolerant/refractory to iron sulfate: a multicentric randomized study. Ann Hematol. https://doi.org/10.1007/s00277-020-04361-3
Bertani L et al (2021) Oral sucrosomial iron is as effective as intravenous ferric carboxy-maltose in treating anemia in patients with ulcerative colitis. Nutrients 13(2):608. https://doi.org/10.3390/nu13020608
Riccio E et al (2020) Oral Sucrosomial® iron versus intravenous iron for recovering iron deficiency anaemia in ND-CKD patients: a cost- minimization analysis. BMC Nephrol 21(1):57. https://doi.org/10.1186/s12882-020-01716-w
Funding
The authors disclosed receipt of the following financial support for the research and authorship: The study was funded by PharmaNutra SpA, Pisa, Italy.
Author information
Authors and Affiliations
Contributions
The authors of this manuscript have directly participated in the planning, execution, and analyses of the study. All authors have provided comments and critical revisions to the manuscript. The final version was approved by all authors prior to submission.
Corresponding author
Ethics declarations
Ethics approval
The protocol and all amendments were approved by the appropriate ethics committee at each center. The trial was done in accordance with the protocol, its amendments, the standards of Good Clinical Practice, the Declaration of Helsinki (Edinburgh 2000, with clarification note from Washington, 2002), and the regulations in force in clinical trials (Legislative Decree no. 211 of 06/24/03).
Consent to participate
All participants have provided informed consent.
Consent for publication
All authors agreed to publish the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zuccarini, A., Cicognini, D., Tancredi, R. et al. Randomized trial of sucrosomial iron supplementation in patients with chemotherapy-related anemia treated with ESA. Support Care Cancer 30, 7645–7653 (2022). https://doi.org/10.1007/s00520-022-07184-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07184-2