Abstract
Purpose
Guidelines recommend primary prophylactic (PP) granulocyte colony stimulating factor (G-CSF) for prevention of febrile neutropenia (FN) in patients receiving myelosuppressive chemotherapy with high risk (HR: > 20%), or intermediate risk (IR:10–20%) of FN and ≥ 1 patient risk factor (e.g., age ≥ 65y). The current retrospective cohort study describes patterns of PP-G-CSF in older Medicare patients undergoing myelosuppressive chemotherapy with HR/IR of FN.
Methods
Patients aged ≥ 66y initiating chemotherapy regimens with HR/IR of FN to treat breast, colorectal, lung, or ovarian cancer, or Non-Hodgkin’s Lymphoma were selected using Medicare 20% sample (2013–2015) and 100% cancer patient (2014–2017) data. PP-G-CSF use was identified in the first cycle. Timing of pegfilgrastim pre-filled syringe (PFS) administration, proportion of patients completing all cycles (adherence) with pegfilgrastim PFS or on-body injector (OBI), and duration of short-acting G-CSF (sG-CSF) was described across all cycles.
Results
Of 64,893 patients receiving HR/IR for FN, 71% received HR and 29% IR regimens. Overall, PP-G-CSF use in the first cycle was 53% (HR: 74%; IR: 44%) and varied across cancers. Adherence with pegfilgrastim was slightly higher among OBI initiators (78%) than PFS (74%). Number of PP-sG-CSF administrations (mean [SD]) per cycle was 5.1 (SD: 2.7) overall, 5.4 (2.6) for HR, and 4.9 (2.7) for IR.
Conclusion
Despite cancer treatment guidelines recommending PP-G-CSF use to reduce risk of FN associated with HR and IR (with ≥ 1 patient risk-factor) regimens, PP-G-CSF remains underutilized in older patients, across cancer types and regimens. Opportunities exist for improvement in use of PP-G-CSF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients undergoing chemotherapy for the treatment of cancer are at risk of chemotherapy-induced complications including fatigue, cognitive dysfunction, gastrointestinal or cardiovascular toxicity, and febrile neutropenia (FN) [1]. FN following myelosuppressive chemotherapy is a serious complication associated with morbidity and mortality, often leading to reduced treatment efficacy due to dose delays and reductions [2]. Approximately 25–40% of treatment-naïve patients receiving common myelosuppressive chemotherapy experience FN [3]. The risk of developing FN varies by chemotherapy regimen and patient risk factors such as age, bone marrow involvement, performance status, persistent neutropenia, and prior radiation [4,5,6]. Since, granulocyte colony-stimulating factors (G-CSF) have been proven to be effective in reducing FN risk [7,8,9,10], the National Comprehensive Care Network (NCCN) and other clinical guidelines recommend G-CSF prophylaxis for patients undergoing myelosuppressive chemotherapy with high (≥ 20%), or intermediate (10%–20%) FN risk and at least one patient-level risk factor [11,12,13,14]. Neutropenic complications are more frequent and severe in older patients with cancer resulting in longer hospitalizations and a higher mortality rate [16, 17]. Consequently, older patients often experience chemotherapy dose delays and reductions leading to reduced chemotherapy effectiveness and a decrease in overall survival as compared to younger patients [16, 18]. G-CSF therapy in older patients have been shown to be effective in reducing the incidence of FN thereby improving chemotherapy persistence [18, 19]. Age ≥ 65 years is a patient-level risk factor for FN and thus should be taken into account when deciding on G-CSF treatment [2, 12, 15].
Observational studies have reported increased FN risk with filgrastim treatment durations shorter than ten to eleven days [20,21,22]. However, in most clinical practices primary prophylactic (PP)-filgrastim is administered on average for four to seven days [20,21,22,23] or even less [24]. Pegfilgrastim has been routinely administered as a pre-filled syringe (PFS) 1 day after the last day of chemotherapy completion (day zero). NCCN also considers it preferable to administer pegfilgrastim from day one to days three/four after the last day of chemotherapy [11]. Yet, some patients receive pegfilgrastim on the same day as the last day of chemotherapy [25], despite available evidence indicating that same day administration is associated with higher risk of FN incidence [25, 26]. In 2015, a new on-body injector (OBI) treatment modality was introduced as a delivery option for pegfilgrastim to eliminate the requirement of a clinic visit one day after chemotherapy [27].
As the US population ages, more patients are entering the Medicare program, thus it is important to evaluate achievement of recommended guideline care to ensure quality outcomes. Treatment with myelosuppressive chemotherapy and supportive care medicines remains a cornerstone for the care of patients diagnosed with various types of cancer [28,29,30,31,32]. In this study, we examined the use, adherence, and timing of administration of PP-G-CSF among Medicare patients diagnosed with different cancer types and receiving myelosuppressive chemotherapy with high risk or intermediate risk for FN.
Materials and methods
Study design and data source
We conducted a retrospective cohort study of older Medicare patients who received chemotherapy regimens with intermediate-risk or high-risk of FN for breast, colorectal, lung, or ovarian cancer, or Non-Hodgkin’s Lymphoma (NHL), from 2013 to 2017 (see Appendix A for study schema). The study design has been described in detail earlier [33]. Briefly, 20% Medicare sample data from 2012–2015 and 100% Medicare cancer patient file (2014–2017) from the Centers for Medicare & Medicaid Services were used and included the annual Master Beneficiary Summary File file (including demographic information and Medicare enrollment status), and the annual claims-based standard analytical files (including Part A institutional and Part B carrier files) [33, 34]. This study was approved by the Office for Human Subjects Research of Hennepin Healthcare System.
Study cohort
For all eligible patients, the date of initiation of the first chemotherapy course was defined as the cohort entry date. We created cohorts of patients diagnosed with breast, colorectal, lung, or ovarian cancer, or NHL, initiating myelosuppressive chemotherapy from January 1, 2013 through June 30, 2017, with regimens classified as intermediate risk (10%–20%) or high risk (> 20%) for FN as defined by the NCCN guidelines (Appendix B). All eligible patients were continuously enrolled in Medicare Part A and Part B without enrollment in a Medicare Advantage (MA) program for at least 365 days before and 6 days after chemotherapy initiation, aged ≥ 66 years at chemotherapy initiation and survived the subsequent 6 days to effectively identify regimen and day of chemotherapy completion in the first cycle. Initiation of the first cycle of chemotherapy required at least 365 days with no prior myelosuppressive chemotherapy (Appendix C). Patients who had undergone radiotherapy or stem cell transplant (Appendices D1–D2) in the 365 days before or six days after chemotherapy initiation were excluded.
Chemotherapy course and cycle in Medicare Part A outpatient and Part B carrier claims were identified using the algorithm by Weycker et al. (Appendix E) [35]. Chemotherapy regimens were defined based on the Healthcare Common Procedure Coding System (HCPCS) Level II codes for parenterally administered antineoplastic agents (myelosuppressive and non-myelosuppressive; Appendix B) with service dates from day one to day six of the first cycle [33]. The same claims contained the diagnosis codes for breast, colorectal, lung, or ovarian cancer, or NHL based on the International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification (ICD-9-CM/ICD-10-CM) (Appendix F). To ensure that the identified regimen was used to treat the cancer of interest, patients with diagnosis codes for more than one cancer reported in the chemotherapy claims during the first 6 days of the cycle were excluded. Regimens were classified as “intermediate” or “high” with regard to risk of FN based on the NCCN® guideline for use of myeloid growth factors [11, 33]. The last date of administration of myelosuppressive chemotherapy agent during the initial 6 days of the cycle was defined as the chemotherapy completion date in the cycle.
Study period
The baseline period started 12 months prior to chemotherapy initiation (Appendix A). The study follow-up period started on the chemotherapy initiation date and continued until the earliest date of the chemotherapy course end date of the last cycle (up to eight cycles), first occurrence of neutropenia-related hospitalization, first occurrence of Sargramostim use, bone marrow/stem cell transplant/radiation therapy (Appendix D1–D2), death, disenrollment from Medicare Part A/B, enrollment in an MA program, or November 30, 2017.
Covariate assessment
As described previously, age in years was assessed at the index date [1]. At least one inpatient claim or two outpatient claims, separated by at least 30 days, were required for defining comorbidities during the baseline period (Appendix G). Neutropenia hospitalization was defined through an inpatient claim with a diagnosis of neutropenia (ICD-9 288.0x; ICD-10 D70.x) in any position [36]. The modified Charlson comorbidity index (CCI) adapted for use with administrative claims data was calculated [1, 36, 37].
Outcome assessment
Primary prophylactic G-CSF (PP-G-CSF) was defined as at least one administration of a long-acting G-CSF (pegfilgrastim) or an sG-CSF (filgrastim, tbo-filgrastim, filgrastim-sndz) from Part A outpatient or Part B claims via corresponding HCPCS Level II codes (Appendix H1) from the initiation of chemotherapy to five days after chemotherapy completion in the first cycle [27, 38,39,40,41,42,43]. Pegfilgrastim administration mode (PFS and OBI) were distinguished via National Drug Codes (NDC) and Current Procedural Terminology (CPT) codes (Appendix H2). Claims with HCPCS codes for pegfilgrastim alone without NDC or CPT codes indicating administration mode were categorized as “pegfilgrastim, route unknown.” Time of administration of the PP-pegfilgrastim PFS was identified by a claim for pegfilgrastim PFS relative to day zero (day of chemotherapy completion) through day five after chemotherapy completion. Prophylactic sG-CSF administrations were defined by the number of days with sG-CSF claims during the entire cycle; multiple claims on the same day were considered as a single administration. Use of PP-G-CSF, timing for administration of PFS, and number of sG-CSF administrations in the second through subsequent cycles were defined using the same methods.
Up to eight completed chemotherapy cycles were identified for each eligible patient in the study cohort. After March 1, 2015 (post-OBI approval period), adherence of PP-pegfilgrastim was defined (for patients who received PP-pegfilgrastim PFS or OBI in the first cycle) as the proportion of patients completing 100% of their cycles (up to eight cycles) with the same mode of pegfilgrastim administration they used in the first cycle.
Analysis
Descriptive statistics were reported. Continuous variables were expressed as mean (standard deviation [SD]) or median and interquartile range (IQR). Categorical variables were expressed as count and percentage with 95% confidence intervals (CIs). The mean (SD) number of prophylactic sG-CSF administrations per cycle was calculated using all eligible cycles that received at least one administration of prophylactic sG-CSF and results stratified by cancer type, and FN risk category of the chemotherapy regimen.
Results
A total of 64,893 patients initiated myelosuppressive chemotherapy for breast, colorectal, lung, or ovarian cancer, or NHL, between January 1, 2013, and June 30, 2017 (Appendix I). The most common cancer type was breast (29.6%), followed by lung cancer (25.7%), NHL (21.9%), colorectal cancer (21.8%), and ovarian cancer (1%). Among all patients, 71.2% received chemotherapy regimens with intermediate FN risk and 28.8% received regimens with high FN risk. Most patients with breast cancer (92%) received chemotherapy regimens with high FN risk while chemotherapy regimens with intermediate risk were primarily given to patients with colorectal cancer (100%) followed by lung (99.8%), NHL (94.9%), and ovarian cancer (62.3%).
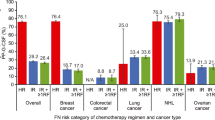
PP-G-CSF use in the first cycle varied across cancer-type and FN risk categories of chemotherapy regimen (Fig. 1). The combined proportion of patients with cancer receiving PP-G-CSF in the first cycle was 43.9% (95% CI: 43.4%, 44.3%) for chemotherapy with intermediate risk for FN and 74.2% (95% CI, 73.6%, 74.8%) for chemotherapy with high risk for FN. Among patients receiving chemotherapy regimens with intermediate FN risk, PP-G-CSF use was most common in NHL (79.2%), followed by ovarian cancer (60.6%), lung cancer (45.2%), breast cancer (19.3%), and colorectal cancer (10.9%). Among patients receiving chemotherapy regimens with high FN risk, prophylactic G-CSF use was most common in patients with NHL (79.4%) and breast cancer (75.0%), followed by lung cancer (31.4%) and ovarian cancer (11.3%).
Proportion of patients receiving prophylactic G-CSF in the first chemotherapy cycle stratified by the FN risk category of the chemotherapy regimen, overall and by cancer type. Error bars represent 95% CI. CI, confidence interval, FN, febrile neutropenia; NHL, non-Hodgkin lymphoma; PP-G-CSF, primary prophylactic G-CSF
Among patients receiving chemotherapy regimens with high FN risk, mean age at the initiation of their first chemotherapy course, presence of baseline comorbid conditions, and mean CCI were similar between those who received compared with those who did not receive PP-G-CSF overall, and by cancer types (Table 1). Among patients receiving chemotherapy regimens with intermediate FN risk, those who received PP-G-CSF were less likely to have metastasis (18.6% vs 37.5%) and had lower CCI (mean CCI [SD]: 3.7 [3.2] vs 5 [3.6]) at baseline compared with those who did not receive PP-G-CSF, results driven mostly by patients with breast cancer (Table 1). While the overall mean number of completed chemotherapy cycles was similar for patients receiving PP-G-CSF (3.8) or not receiving PP-G-CSF (3.7), those with breast cancer receiving PP-G-CSF had a higher mean number of completed chemotherapy cycles (4.3) than those who didn’t receive PP-G-CSF (3.2) at baseline and across FN risk categories of chemotherapy regimen.
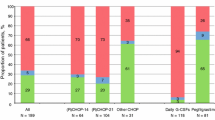
Among patients receiving PP-G-CSF in the first cycle of chemotherapy in each year of the study period, 98% received pegfilgrastim which remained unchanged over the study period (Fig. 2A). Use of the OBI formulation increased from 1.8% in 2015 to 21.7% in 2017 while use of PFS decreased from 30.2% in 2015 to 11.6% in 2017, although 67 to 78% of pegfilgrastim use could not be classified as either PFS or OBI (Fig. 2B). In patients receiving prophylactic sG-CSFs, biosimilar filgrastim use increased to ≥ 70% by the end of 2017. Filgrastim use decreased from 100% in 2013 to 25% in 2017 while filgrastim-sndz and tbo-filgrastim use increased to 38.3% and 36.7%, respectively, by 2017 (Fig. 2C). Among all patient cycles receiving prophylactic sG-CSFs, mean (SD) number of administrations per cycle was 5.1 (2.7) overall, 5.4 (2.6) and 4.9 (2.7) for patients receiving high or intermediate FN risk regimens, respectively. By cancer type, patients with NHL had the highest mean number of prophylactic sG-CSF administrations per cycle (mean [SD]: 6.1 [2.9]), followed by breast cancer (5.1 [2.4]), lung cancer (4.4 [2.5]), ovarian cancer (3.9 [2.4]), and colorectal cancer (3.7 [2.0]).
Proportion of prophylactic G-CSF use by type among patients receiving chemotherapy with intermediate or high FN risk and prophylactic G-CSF in the first chemotherapy cycle by calendar year 2013–2017, and by (A) Type of G-CSF (B) Type of Pegfilgrastim, and (C) Type of sG-CSF. aRoute unknown: pegfilgrastim users who could not be classified as pegfilgrastim pre-filled syringe or OBI. FN, febrile neutropenia; G-CSF, granulocyte colony-stimulating factor; OBI, on-body injector; PP-G-CSF, primary prophylaxis G-CSF; sG-CSF, short-acting G-CSF
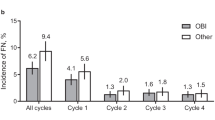
Among all patients receiving chemotherapy regimens with intermediate FN risk and PP-pegfilgrastim in the first cycle of chemotherapy (Fig. 3), the proportion of patients completing all cycles with the same modes of administration (i.e. adherence) was slightly higher among those who initiated PP-pegfilgrastim with the OBI (78.2% [95% CI, 75.7%, 80.7%]) versus those that were treated with the PFS (74% [95% CI, 72.2%, 75.75%]). For patients with NHL, the adherence was higher among OBI users (89% [95% CI, 81.2%, 96.9%]) than PFS users (72% [95% CI, 69.2%, 74.5%]). Similar patterns of PP-pegfilgrastim were found among patients receiving chemotherapy regimens with high FN risk (Fig. 3).
Proportion of patients completing all chemotherapy cycles with prophylactic pegfilgrastim OBI versus pre-filled syringe stratified by FN risk category of the chemotherapy regimen, overall and by cancer types (March 1, 2015 to November 30, 2017). * Results are suppressed due to less than 11 patients completing all cycles with prophylactic pegfilgrastim pre-filled syringe or OBI. ^ No chemotherapy regimens with high FN risk for colorectal cancer. FN, febrile neutropenia; HR, chemotherapy regimen with high FN risk; IR, chemotherapy regimen with intermediate FN risk; NHL, non-Hodgkin lymphoma; OBI, pegfilgrastim on-body injector
Among all patients receiving chemotherapy regimens with intermediate-risk or high-risk for FN and PP-pegfilgrastim PFS in the first cycle, the proportion of patients receiving PFS on the same day as completion of chemotherapy was 4.3% overall and ranged from 4.1% for breast cancer to 18.8% for ovarian cancer (Fig. 4). The proportion of patients receiving PFS on day four-five after completion of chemotherapy was 3.5% overall and ranged from 1.7% for breast cancer to 6.6% for colorectal cancer. Similar patterns were seen for all cycles in patients receiving chemotherapy with intermediate or high FN risk and PP-pegfilgrastim PFS (results not shown).
Discussion
The current retrospective cohort study evaluated the pattern of PP-G-CSF in older Medicare patients with cancer receiving myelosuppressive chemotherapy regimens associated with high or intermediate FN risk. Clinical guidelines state that older patients (aged ≥ 65 years) with cancer receiving certain types of myelosuppressive chemotherapy have a markedly higher risk for developing FN than younger patients [33, 44]. Since age ≥ 65 years is a known patient-level risk factor for FN, older Medicare patients receiving regimen with intermediate-risk for FN, are inherently at an increased risk of FN and thus are eligible to receive G-CSF. In this study, we observed that overall 71.2% of patients received chemotherapy regimens with intermediate FN risk, of whom 43.9% received prophylactic G-CSF during the first cycle indicating less than optimal G-CSF utilization in intermediate FN risk regimens. Among patients with IR regimens, patients receiving PP-G-CSF were less likely to have metastasis at baseline and had lower CCI compared with those who did receive PP-G-CSF, which was most strongly reflected in patients with breast cancer. Of 28.8% of patients that received high-risk for FN regimen, results primarily driven by breast cancer patients (92%), only 74.2% received PP-G-CSF during the first cycle of chemotherapy. Among all patients receiving PP-G-CSF in the first cycle, 98% received pegfilgrastim and 2% received sG-CSF. Adherence with pegfilgrastim was slightly higher among those who were treated with the OBI (78.2%) compared with those treated with PFS (74%). Among patients receiving prophylactic sG-CSFs, overall mean number of administrations per cycle was 5.1 (5.4 for high FN risk and 4.9 for intermediate FN risk regimens) which was considerably lower than the recommended ten to eleven administrations per cycle for best efficacy [24, 25, 40, 45].
Earlier studies have reported suboptimal use of PP-G-CSF, particularly for chemotherapy regimens with intermediate risk for FN, consistent with our results [1, 29, 46]. In the TULIP study, Laribi and colleagues observed PP-G-CSF use of 33% among patients receiving chemotherapy regimens with intermediate FN risk, and 66% among patients receiving chemotherapy regimens with high FN risk [16, 29]. Aligned with our findings, Sosa and colleagues observed that 74% of breast cancer patients and 62% of NHL patients undergoing chemotherapy regimens with high FN risk, received PP-G-CSF in the first cycle [46]. The inconsistent use of PP-G-CSF in our study and others may be due to multiple factors including differences among physicians’ experience and training, practice setting, reimbursement, the geographical location of care, along with factors inherent to the patient or disease [32]. Given that all patients had at least one risk factor, higher PP-G-CSF utilization was anticipated and highlights opportunities for improvement.
We observed that during the post-OBI approval period (after March 1, 2015), use of OBI increased in 2015 to 2017 while use of PFS decreased, indicating higher patient/provider/facility preference for OBI which may also be reflected in the higher adherence in patients receiving OBI (78.2%) versus those receiving PFS (74%). Our results are consistent with other observational studies which have shown a steady increase in the use of the OBI delivery device since its approval [1]. We analyzed adherence by modality and only for patients who had received PP-G-CSF in the first cycle. This did not allow for secondary prophylaxis of G-CSF or a change in modality of G-CSF in subsequent cycles, since patients were excluded from the analysis upon termination of their specific PP-G-CSF after the first cycle. However, a few earlier studies including the TULIP and NEXT studies in France and the MONITOR-GCSF European study have shown PP-G-CSF to be more effective in older patients with cancer for preventing chemotherapy induced dose delays and reductions [16, 47, 48]. Conversely, a couple of studies on younger patients reported significantly lower proportion of patients receiving PP-G-CSF [49, 50].
Utilization of PP-sG-CSF remained < 2% across the study period even though biosimilar sG-CSF use had increased steadily with filgrastim-sndz and tbo-filgrastim accounting for ≥ 70% biosimilar filgrastim use by 2017. This corroborated our finding that more convenient treatment modalities such as OBI and PFS were preferred given reduced need for repeated visits following chemotherapy. For patients with NHL, the adherence with PP-pegfilgrastim was higher among OBI users (89%) than the PFS users (72%), while, for patients with other cancers, no large differences were observed in the adherence between the two modes of administration. Although guidelines recommend next day administration for best efficacy, overall, we observed 4.3% patients received pegfilgrastim PFS on the last day of chemotherapy (4.1% for breast cancer; 18.8% for ovarian cancer). The trend was similar across all chemotherapy cycles and FN risk regimens. The issue might be resolved by using pegfilgrastim-OBI which would help eliminate the suboptimal administration of PFS by delivering the required dose on the optimal day thereby increasing efficacy.
Medicare claims information has been shown to be generally valid and we anticipated a high level of accuracy in the reporting of these claims. However, there was potential for missing or inaccurate codes that might have led to underestimation or failure to identify certain regimens, comorbid conditions, or PP-G-CSF administrations and/or differentiation of G-CSFs in the database. We did not control for the administered dose and dose delays of chemotherapy regimens, thus making it impossible to determine whether the intermediate or high-risk regimens were modified for a lower dose, thereby possibly decreasing the risk for FN. Additionally, “diagnosed” comorbid conditions were possibly reported/documented more for sicker patients (higher risk for FN and more likely to receive G-CSF) than for healthier patients (less likely to receive G-CSF). However, since the study population included older patients with cancer with multiple co-morbidities and regular physician visits, the possibilities of missed diagnoses were low. While we attempted to restrict the study population to patients warranted to receive PP-G-CSF by including patients aged ≥ 66 years initiating chemotherapy regimens with HR/IR of FN, the study findings may not be applicable to younger patients with other possible indications for use of PP-G-CSF including bone marrow involvement, persistent neutropenia, and recent surgery. Lastly, we could only discern 22–33% of pegfilgrastim administrations as either OBI or PFS, given the J-Code discernment applied in this study. Grater adoption of such modifiers would help ensure more appropriate measurement of the guidelines of care.
Conclusion
The results from this retrospective cohort study demonstrate that older patients with cancer at risk for FN, receiving myelosuppressive chemotherapy regimens with intermediate or high FN risk, may be vulnerable because of mistimed or underuse of PP-G-CSF. There is still room for improvement with a focus on targeted use of supportive care when patients are administered myelosuppressive chemotherapy with an intermediate-risk or high-risk of FN. Further studies are required to better understand G-CSF utilization, and the impact of different treatment patterns on the risk of FN.
Data availability
Not applicable.
Code availability
Not applicable.
References
Gawade PL, Li S, Henry D et al (2020) Patterns of granulocyte colony-stimulating factor prophylaxis in patients with cancer receiving myelosuppressive chemotherapy. Support Care Cancer 28(9):4413–4424
Aapro MS, Cameron DA, Pettengell R et al (2006) EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 42(15):2433–2453
Dale DC (2002) Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs 62(Suppl 1):1–15
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol/Hematol 90(3):190–199
Lyman GH, Kuderer NM, Crawford J et al (2011) Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117(9):1917–1927
Lyman GH, Kuderer NM, Crawford J et al (2006) Prospective validation of a risk model for first cycle neutropenic complications in patients receiving cancer chemotherapy. J Clin Oncol 24(18_suppl):8561–8561
Mhaskar R, Clark OA, Lyman G, Engel Ayer Botrel T, Morganti Paladini L, Djulbegovic B (2014) Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev 10:CD003039
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25(21):3158–3167
Vogel CL, Wojtukiewicz MZ, Carroll RR et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23(6):1178–1184
Crawford J, Ozer H, Stoller R et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325(3):164–170
Crawford J, Becker PS, Armitage JO et al (2017) Myeloid Growth Factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15(12):1520–1541
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32
Freifeld AG, Bow EJ, Sepkowitz KA et al (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 52(4):427–431
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33(28):3199–3212
Smith RE Jr, Aapro MS, Ludwig H et al (2008) Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 26(7):1040–1050
Laribi K, Badinand D, Janoray P et al (2019) Filgrastim prophylaxis in elderly cancer patients in the real-life setting: a French multicenter observational study, the TULIP study. Supportive Care Cancer : Off J Multinatl Assoc Support Care Cancer 27(11):4283–4292
Morrison VA, Picozzi V, Scott S et al (2001) The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin’s lymphoma receiving initial CHOP chemotherapy: a risk factor analysis. Clin Lymphoma 2(1):47–56
Balducci L, Lyman GH (2001) Patients aged > or = 70 are at high risk for neutropenic infection and should receive hemopoietic growth factors when treated with moderately toxic chemotherapy. J Clin Oncol 19(5):1583–1585
Repetto L, Biganzoli L, Koehne CH et al (2003) EORTC Cancer in the Elderly Task Force guidelines for the use of colony-stimulating factors in elderly patients with cancer. Eur J Cancer 39(16):2264–2272
Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG (2006) Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother 40(3):402–407
Scott SD, Chrischilles EA, Link BK, Delgado DJ, Fridman M, Stolshek BS (2003) Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin’s lymphoma treated with chemotherapy. J Manag Care Pharm 9(2 Suppl):15–21
Rajan SS, Lyman GH, Stearns SC, Carpenter WR (2011) Effect of primary prophylactic granulocyte-colony stimulating factor use on incidence of neutropenia hospitalizations for elderly early-stage breast cancer patients receiving chemotherapy. Med Care 49(7):649–657
Mitchell S, Li X, Woods M et al (2016) Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: A systematic review. J Oncol Pharm Pract 22(5):702–716
Schwartzberg LS, Lal LS, Balu S et al (2018) Clinical outcomes of treatment with filgrastim versus a filgrastim biosimilar and febrile neutropenia-associated costs among patients with nonmyeloid cancer undergoing chemotherapy. J Manag Care Spec Pharm 24(10):976–984
Weycker D, Li X, Figueredo J, Barron R, Tzivelekis S, Hagiwara M (2016) Risk of chemotherapy-induced febrile neutropenia in cancer patients receiving pegfilgrastim prophylaxis: does timing of administration matter? Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 24(5):2309–2316
Burris HA, Belani CP, Kaufman PA et al (2010) Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non-small-cell lung cancer, ovarian cancer, and Non-Hodgkin’s Lymphoma: Results of four multicenter, double-blind, randomized phase ii studies. J Oncol Pract 6(3):133–140
Food and Drug Administration. Pegfilgrastim [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125031s180lbl.pdf. Accessed 3 Dec 2019
Potosky AL, Malin JL, Kim B et al (2011) Use of colony-stimulating factors with chemotherapy: opportunities for cost savings and improved outcomes. J Natl Cancer Inst 103(12):979–982
Ramsey SD, McCune JS, Blough DK et al (2010) Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care 16(9):678–686
Goyal RK, Tzivelekis S, Rothman KJ, Candrilli SD, Kaye JA (2018) Time trends in utilization of G-CSF prophylaxis and risk of febrile neutropenia in a Medicare population receiving adjuvant chemotherapy for early-stage breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 26(2):539–548
Langeberg WJ, Siozon CC, Page JH, Morrow PK, Chia VM (2014) Use of pegfilgrastim primary prophylaxis and risk of infection, by chemotherapy cycle and regimen, among patients with breast cancer or non-Hodgkin’s lymphoma. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 22(8):2167–2175
Barnes G, Pathak A, Schwartzberg L (2014) G-CSF utilization rate and prescribing patterns in United States: associations between physician and patient factors and GCSF use. Cancer Med 3(6):1477–1484
Li S, Liu J, Gong T et al (2020) Duration of short-acting granulocyte colony-stimulating factor for primary prophylaxis and risk of neutropenia-related hospitalization in older patients with cancer. J Geriar Oncol 11(8):1309–1315
Mues KE, Liede A, Liu J et al (2017) Use of the Medicare database in epidemiologic and health services research: a valuable source of realworld evidence on the older and disabled populations in the US. Clin Epidemiol 9:267–277
Weycker D, Li X, Tzivelekis S et al (2017) Burden of chemotherapy-induced febrile neutropenia hospitalizations in US clinical practice, by use and patterns of prophylaxis with Colony-stimulating factor. Support Care Cancer 25(2):439–447
Quan H, Sundararajan V, Halfon P et al (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139
Quan H, Li B, Couris CM et al (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173:676–682
Food and Drug Administration. Pegfilgrastim-jmdb [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761075s000lbl.pdf. Accessed 9 Feb 2021
Food and Drug Administration. Pegfilgrastim-cbqv [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761039s000lbl.pdf. Accessed 9 Feb 2021
Food and Drug Administration. Filgrastim [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5183lbl.pdf. Accessed 9 Feb 2021
Food and Drug Administration. Tbo-filgrastim [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125294s0000lbl.pdf. Accessed 9 Feb 2021
Food and Drug Admnistration. Filgrastim-sndz [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125553lbl.pdf. Accessed 9 Feb 2021
Food and Drug Administration. Filgrastim-aafi [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761080s000lbl.pdf. Accessed 9 Feb 2021
Roché H, Eymard JC, Radji A et al (2018) Biosimilar filgrastim treatment patterns and prevention of febrile neutropenia: a prospective multicentre study in France in patients with solid tumours (the ZOHé study). BMC Cancer 18(1):1127
Cornes P, Gascon P, Chan S et al (2018) Systematic Review and Meta-analysis of Short- versus Long-Acting Granulocyte Colony-Stimulating Factors for Reduction of Chemotherapy-Induced Febrile Neutropenia. Adv Ther 35(11):1816–1829
Sosa R, Li S, Molony JT, Liu J, Stryker S, Collins AJ (2017) Use of prophylactic growth factors and antimicrobials in elderly patients with cancer: a review of the Medicare database. Support Care Cancer 25(10):3123–3132
Leprêtre S, Maloisel F, Kamioner D, Berthou C, Albrand H (2015) Safety of biosimilar filgrastim in patients with breast cancer undergoing neutropenia-inducing chemotherapy: A subanalysis of the NEXT study. J Clin Oncol 33(15_suppl):e20711–e20711
Gascón P, Aapro M, Ludwig H et al (2016) Treatment patterns and outcomes in the prophylaxis of chemotherapy-induced (febrile) neutropenia with biosimilar filgrastim (the MONITOR-GCSF study). Support Care Cancer 24(2):911–925
Tesch H, Ulshöfer T, Vehling-Kaiser U, Ottillinger B, Bulenda D, Turner M (2015) Prevention and treatment of chemotherapy-induced neutropenia with the biosimilar filgrastim: a non-interventional observational study of clinical practice patterns. Oncol Res Treat 38(4):146–152
Almenar D, Mayans J, Juan O et al (2009) Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain–results of the LEARN Study. Eur J Cancer Care 18(3):280–286
Acknowledgements
Medical writing support was provided by Debasri Mukherjee of Cactus Lifesciences (part of Cactus Communications Pvt Ltd.) and Nancy Iskander of Amgen Inc.
Funding
This study was supported by Amgen, Inc.
Author information
Authors and Affiliations
Contributions
The study strategy was discussed with all authors. P Gawade and S Li were involved in developing the study concepts. Data were acquired by M Kelsh, S Li, TT Gong, and Y Peng. Statistical analyses were performed by S Li, TT Gong, and Y Peng. All authors were involved in quality control and interpretation of acquired data and algorithms. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Office for Human Subjects Research of Hennepin Healthcare System.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
JS, MK, and BB are employees of and hold stock in Amgen, INC; TG, YP, and SL are employees of Chronic Disease Research Group; DH, no conflicts; PG is currently an employee of and holds stock in Vertex Pharmaceuticals and is a former employee of Amgen and currently holds stock in Amgen.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Appendix A. Study Schema; Appendix B. FN risk of chemotherapy regimens classified per the NCCN® clinical guidelines for myeloid growth factors; Appendix C. Chemotherapeutic agents; Appendix D1. Codes used to identify radiation therapy in Medicare claims; Appendix D2. Codes used to identify stem cell transplant in Medicare claims; Appendix E. Details of chemotherapy course and cycle in Medicare Part A outpatient and Part B carrier claims; Appendix F. ICD-9-CM and ICD-10-CM Diagnosis Codes Used to Identify Cancers; Appendix G. ICD-9-CM and ICD-10-CM Diagnosis Codes Used to Identify Comorbid Conditions; Appendix H1. HCPCS codes used to identify colony-stimulating factor agents in Medicare claims; Appendix H2. Identification of Pegfilgrastim by route of administration in Medicare claims; Appendix I. Cohort selection
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schenfeld, J., Gong, T., Henry, D. et al. Patterns of primary prophylactic granulocyte colony-stimulating factor use in older Medicare patients with cancer receiving myelosuppressive chemotherapy. Support Care Cancer 30, 6327–6338 (2022). https://doi.org/10.1007/s00520-022-06967-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06967-x