Abstract
Purpose
To investigate the effects of exercise training on cancer-related fatigue (CRF) in colorectal cancer survivors.
Methods
Randomized controlled trials published between 1 January 2010 and 19 October 2020, selected through online search conducted in PubMed, Scopus, Web of Science, SPORTDiscus and PEDro databases, were included. Eligible trials compared the effect of exercise training interventions, versus non-exercise controls on CRF, in colorectal cancer survivors, during or after treatment. The methodological quality of individual studies was analysed using the Physiotherapy Evidence Database (PEDro) scale. Standardized mean differences (SMD) that were pooled using random-effects models were included as the effect size. In addition, 95% prediction intervals (PI) were calculated.
Results
Six trials involving 330 colorectal cancer patients met the inclusion criteria and presented reasonable to good methodological quality. An overall small-to-moderate effect of exercise training on CRF was found (SMD = − 0.29: 95% CI: [− 0.53; − 0.06]; p = 0.01; PI: [− 0.63; 0.04]; low-quality evidence). Subgroup analysis revealed moderate effects of exercise interventions performed during chemotherapy (SMD = − 0.63; 95% CI: [− 1.06; − 0.21]; p = 0.003) and small, non-significant effects, when exercise training was performed after cancer treatment (SMD = − 0.14; 95% CI: [− 0.43; 0.14]; p = 0.32). Steady improvements were achieved when a combination of aerobic plus resistance exercise was used, in interventions lasting 12 to 24 weeks.
Conclusion
Exercise training could be regarded as a supportive therapy for the clinical management of CRF in colorectal cancer patients undergoing chemotherapy, but further studies are necessary to clarify the effects of exercise interventions on CRF after cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The epidemiologic relevance of cancer is growing worldwide. Over 19 million new cases were estimated in 2020 and previsions are showing that these numbers will continue to increase, up to 28.4 million of new cancer cases in 2040 [1].
Colorectal cancer (CRC) is the second leading cause of cancer death and the third most commonly diagnosed form of cancer globally, comprising 10% of all cancer diagnoses [1]. With improvements in survival being consistently demonstrated [2,3,4], the burden of CRC is expected to increase in the future, which has led oncology research and health systems increasingly concerned about symptoms that interfere with the quality of life of these patients [5,6,7,8,9].
One of the most prevalent and distressing symptoms affecting the quality of life of CRC patients is cancer-related fatigue (CRF) [8, 10]. CRF has been described as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with functioning” [6]. Patients with cancer frequently report CRF as the symptom that mostly disturbs their daily life, even more than pain or nausea, which can generally be managed by medication [11]. CRF, on the other hand, is often undiagnosed, left untreated or poorly managed [6].
In CRC survivors, fatigue is present in approximately half of patients with localized tumours, and in 2/3 of those with metastatic/recurrent disease [10]. This debilitating symptom usually peaks immediately after adjuvant chemotherapy, being experienced by 70% of patients, but remains a significant problem until 10-year post-diagnosis, persisting in 39% of long-term CRC survivors [10, 12]. In this view, efforts to better manage this symptom throughout the trajectory of the disease are now considered a high priority in clinical practice [6, 8].
Current clinical guidelines for supportive care in oncology [6, 13] recommend exercise training, as an effective intervention in preventing or improving CRF, during active and post-treatment phases. The claims of beneficial effects of exercise training on this symptom, however, have been mainly demonstrated in patients with breast cancer, advising caution in extrapolating this recommendation to other types of cancer [14,15,16,17]. In fact, the value of Clinical Practice Guidelines [6] is disputed in this regard because a systematic review published in 2014 found no valuable short- or long-term effects of exercise training in CRC [18]. However, the results from that review have been pooled from a limited and small number of eligible trials [18], and, more recently, two systematic reviews integrating new studies about this topic found that exercise training leads to improvements in fatigue symptoms among CRC survivors [19, 20].
The strength of these findings was however limited by the inclusion of clinical trials combining exercise training with health education and dietary interventions [19, 20], which could have influenced the effect estimates given that these interventions may also improve CRF [21,22,23]. In addition, none of these reviews performed a subgroup analysis exploring whether exercise training effect differs between patients during and following CRC treatment. This could be clinically relevant because CRF is more severe during chemotherapy [10], and the effect of exercise training is predictively larger in cancer patients with higher fatigue levels [24]. Lastly, despite the recommendations to routinely report prediction interval in meta-analysis, representing the most sensible way to summarize the results of heterogeneous studies and allowing more robust conclusions [25,26,27], previous systematic reviews only reported summary effect size combined with a confidence interval [19, 20], which is considered insufficient for clinical decision making since it only summarizes the average treatment effect [27].
Taken together, these limitations justify an updated systematic review and meta-analysis, reporting the prediction interval in addition to the summary estimate that will illustrate which range of true effects can be expected in future clinical trials and including a subgroup analysis aiming to investigate if the exercise training effect on CRF varies between patients during and after CRC treatment.
Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis. (PRISMA) [28].
Protocol and registration
The protocol for this systematic review and meta-analysis was pre-registered on the International Prospective Register of Systematic Reviews (PROSPERO) (record no. CRD42020206435).
Eligibility criteria
The eligibility criteria were developed using the participants, intervention, comparator, outcome and type of study (PICOS) approach [29].
Participants
Adult (≥ 18 years of age) colorectal cancer survivors were defined by the Nacional Cancer Institute: an individual is considered a cancer survivor from the time of diagnosis, through the balance of his or her life [30].
Intervention
Exercise training was defined as a type of physical activity that consists of a well-defined and structured plan that aims to increase or maintain the person’s physical conditioning [15]. Studies in which the experimental group combined exercise training with another type of intervention (e.g. cognitive-behavioural therapy or nutrition) were excluded.
Comparison
Participants receiving usual care, with no exercise training (e.g. chemotherapy/radiotherapy or instructions for the continuation of usual activities).
Outcome
The selected studies should have collected, as a primary or secondary outcome, the intensity of fatigue by means of a self-reported measure for cancer patients [6, 31].
Type of studies
Only randomized controlled trials (RCTs), published in English, between January 1, 2010 (year of publication of the first physical activity guidelines for cancer survivors by the American College of Sports Medicine) [32], and October 19, 2020, were considered. Feasibility trials were excluded. It was also defined the possibility of including studies that, in addition to patients with CRC, integrated participants with other types of cancer, if these studies performed a subgroup analysis that assessed the intensity of CRF specifically for patients with CRC.
Search strategy
Relevant studies were searched using a combination of the following free-text words: exercise OR “physical activity” OR “aerobic training” OR “resistance training” OR “strength training” AND “cancer-related fatigue” OR “cancer related fatigue” OR fatigue AND colorectal OR colon OR rectal, in the electronic databases the Physiotherapy Evidence Database (PEDro), PubMed, Scopus, Web of Science and SPORTDiscus. Searches were limited to articles published in English language. The first and second authors (PM and MM) conducted the search on the platforms within the same week, to ensure that the articles were obtained in the same time period. Detailed information about the search strategy is provided in Online Resource 1.
Identification and selection of studies
Records retrieved by the searches were imported into the software EndNote X8 (Thompson Reuters, San Francisco, CA, USA) and duplicates were removed. Studies’ selection procedure was performed in two phases by two independent reviewers (PM and MM). First, the titles and abstracts were screened using a hierarchical approach for exclusions: study design (RCT), intervention (exercise training), population (CRC patients) and outcome (CRF assessment tool). If one reviewer recognized that a potential article met the inclusion criteria or if there was insufficient information to decide on the inclusion or exclusion, the article was retained to the second screening phase. Subsequently, a full text reading procedure was followed in this screening phase. Studies that had been identified by mutual consent were included in the review. In case of disagreement between the reviewers, an independent third reviewer (JR) appraised the article, and the final decision was a combination of the three evaluations. The Cohen’s kappa coefficient was calculated to evaluate interrater reliability in the initial and full text screenings [33]. Kappa values ≤ 0 suggest no agreement between reviewers, 0.01–0.20 none to slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial and 0.81–1.00 as almost perfect agreement [33].
Data extraction
Data were extracted using a standardized form for each article. The extracted data included the following topics: (1) studies characteristics, (2) participant’s demographics and clinical characteristics, (3) exercise training dose, (4) CRF severity and measurement tool. Data extraction was independently performed by two reviewers (JR and MM) with any discrepancies being resolved through discussion with a third reviewer (PM). When information regarding any of the above topics was unclear, the authors of the original reports were contacted to provide details. Data were recorded in a spreadsheet and results were summarized for comparison between studies.
Assessment of the methodological quality of included studies
Two reviewers (PM and MM) independently appraised the methodological quality of the studies included using PEDro scale [34]. Any disagreement on eligibility was resolved through discussion with another reviewer (CS). PEDro scale comprises 11 items rated with 0 or 1: Eligibility criteria, randomized allocation, hidden allocation, baseline comparison between groups, participants, physiotherapists and blind assessors, adequate follow-up, intention to treat the analysis, comparison between groups and point estimate and variability. Based on these items, a score of 0 to 10 is attributed to the RCTs (eligibility criteria not accountable). According to the PEDro scale, studies with a score of 0 to 3 have a “poor” methodological quality, between 4 to 5 “reasonable”, 6 to 8 “good” and 9 to 10 “excellent” [34].
Data analysis and synthesis of results
To summarize and compare studies, mean and standard deviation (SD) values of CRF scores were directly pooled and analysed with standardized mean differences (SMDs) and 95% confidence intervals (CIs) [35]. Additionally, prediction intervals (PI) were calculated for the purpose of estimating the treatment effect in future clinical trials [25,26,27].
In cases where higher scores represented lower fatigue levels, the mean value was subtracted from the maximum possible value of the scale to ensure that all the scales varied in the same direction throughout the analysis. For interpretation, an SMD of 0.2 represents a “small” effect, an SMD of 0.5 represents a “medium” effect and an SMD of 0.8 represents a “large” effect.
Study‐specific estimates were pooled with random‐effect models. The statistical heterogeneity among studies was assessed using the I2 index [36]. This index represents the percentage of variation in the global estimate that is attributable to heterogeneity (I2 = 25%: low; I2 = 50%: moderate; I2 = 75%: high heterogeneity).
Forest plots were created to visually illustrate the effects in the meta‐analysis of the different studies and the global estimation. Considering that CRF is expected to peak during chemotherapy treatment [10, 37], and that the effect of exercise training is predictively larger in cancer patients with worse baseline fatigue levels [24], a subgroup analysis was performed to differentiate the effectiveness of exercise training in in patients receiving chemotherapy treatment and in the post-treatment phase. R [38] and RStudio [39] were used to perform all analyses. R package meta was used to conduct standard meta-analysis [40]. Statistical significance was defined as a p-value < 0.05.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence [41]. Evidence was downgraded if there were issues with risk of bias across studies, inconsistency of results, publication bias, imprecision and indirectness, according to the recommendations of the GRADE Working Group [42,43,44,45,46].
Results
Study selection
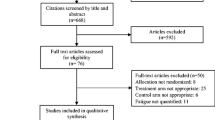
The flowchart of the search, screening and selection of study process is presented in Fig. 1. A total of 663 records were obtained from the electronic databases. After removing duplicates, 513 records remained. Screening based on the title and abstract resulted in the selection of 17 articles. Of these 17 records, 11 articles were excluded following the evaluation of the full text because eligibility criteria were not verified. Six studies met the eligibility criteria and were included in the qualitative and quantitative syntheses [47,48,49,50,51,52]. Agreement between reviewers on title/abstracts (kappa = 0.87) and full text (kappa = 0.72) screenings was strong and moderate, respectively.
Study characteristics
Table 1 describes the characteristics of the included studies. A total of 330 CRC survivors participated in these studies: 170 individuals were allocated to the intervention group (exercise training), and 160 were in the control group. All patients were diagnosed with histologically confirmed non-metastatic CRC (tumour stage I–III).
In three studies (n = 156), exercise training was delivered after CRC treatment [47, 49, 51]. In one study (n = 54), 3.7% of the participants were undergoing adjuvant chemotherapy and the remaining were not receiving any type of treatment [48]. In the other two studies (n = 120), all participants were undergoing adjuvant chemotherapy treatment during the intervention [50, 52]. Control groups received usual care that, in addition to standard CRC treatment, consisted in, advising patients to continuing their usual activities [48, 49, 52] or maintaining their physical activity levels [47], delivering education sessions about CRF [50], distributing information leaflets and calling participants for symptom monitoring [51].
Methodological quality of the studies
The six studies included were evaluated following the 11 items of the PEDro scale (Table 2). Three of the included studies [48, 51, 52] presented a good methodological quality (score of 6 and 7) and the remaining three [47, 50] had a reasonable methodological quality (score of 5). Only two study performed a hidden allocation [48, 52].
Intervention characteristics
A detailed description of the exercise training dose prescribed is presented in Table 3. The total duration of the exercise training interventions varied from 18 to 24 weeks in the two studies conducted in CRC patients undergoing chemotherapy [50, 52] and from 10 to 24 weeks in the studies conducted in patients following completion of CRC treatment [47,48,49, 51]. The type of exercise most commonly prescribed was aerobic exercise (10,000 steps per day or 150–300 min per week at an intensity of 50–75% of the estimated maximum heart rate), which was performed in four of the included studies (n = 100) [47, 49, 51, 52]. The modes of aerobic exercise were walking, biking and unspecified home-based aerobic exercises [47, 49, 51]. In two studies (n = 54), a combination of aerobic and resistance exercise was used (2 to 3 sets of 10–20 repetitions, 45–75% 1-maximum repetition [1-RM]), and in both, a significant reduction in CRF was achieved [49, 52]. One study (n = 39) compared the effect of two different doses of aerobic exercise training on CRF and found that only the group (n = 12) that performed a high exercise dose (300 min per week with an intensity between 50 and 75% of the estimated maximum heart rate) reached significant reduction in CRF [47]. In the remaining two studies, the type of exercise prescribed was Baduanjin qigong (n = 43)[50] and Hatha yoga (n = 27) [48], both characterized by sequences of different body positions in combination with breathing control exercises, being classified as low-intensity exercise training [50]. Of these two types of exercise, only Baduanjin qigong, performed by patients undergoing chemotherapy, achieved significant reductions in CRF, measured at 24 weeks, with no differences between groups at 12 weeks of intervention [50]. The length of the Hatha yoga intervention was 10 weeks and had a low adherence by patients [48].
The progression was identified in three studies [49, 51, 52] and consisted in increasing exercise volume, from 10 min on at least 2 days/week to 30 min on at least 5 days/week [51] or intensity, increasing the time at the ventilatory threshold or progressing from 65 to 75% of 1-RM [52].
Supervision in the intervention programmes varied between weekly telephone-based [51] and presential meetings [48, 50, 52]. In two studies, supervision was carried out by telephone and in-person, during clinical/exercise encounters [47, 49].
The majority of the studies delivered educational sessions and used digital platforms or DVDs to instruct the participants about the exercises to be performed [50,51,52]. A weekly supervision/encounter between the exercise training specialist and the participants was scheduled to monitor the fulfilment of goals, provide positive reinforcement and implement strategies to overcome barriers that occurred during the programmes [47, 51].
Characteristics of outcome measures
Three of the six studies included applied the Functional Assessment of Cancer Therapy: Fatigue (FACT-F) to evaluate CRF [48, 49, 51]. The others applied three different measurement tools, namely the Brief Fatigue Inventory (BFI) [50], the Fatigue Symptom Inventory (FSI) [47] and the (Multidimensional Fatigue Inventory) MFI [52].
Meta-analysis
Low heterogeneity between studies (I2 = 0%) was found both in the global and subgroup analyses (Fig. 2). Globally, a small-to-moderate positive effect of exercise training in patients’ fatigue was observed (SMD = − 0.29: 95% CI: [− 0.53; − 0.06]; p = 0.01). The prediction interval for SMD ranged from − 0.63 to 0.04. Subgroup analysis revealed a moderate-to-large effect of exercise training in patients undergoing chemotherapy (SMD = − 0.63; 95% CI: [− 1.06; − 0.21]; p = 0.003; n = 120) [50, 52]. In the subgroup of post-treatment CRC survivors, there was uncertainty about the effect of exercise training on CRF (SMD = − 0.14; 95% CI: [− 0.43; 0.14]; p = 0.32; n = 180) [47,48,49, 51]. The prediction interval in the latter ranged from − 0.76 to 0.47.
Forest plot of effect of exercise training on cancer-related fatigue. Abbreviations: BFI, brief fatigue inventory; CI, confidence interval; FACT-F, Functional Assessment of Cancer Therapy — Fatigue; FSI, Fatigue Symptom Inventory; MFI-GF, Multidimensional Fatigue Inventory — General Fatigue; SMD, standardized mean difference
GRADE assessment
The evidence about the effect of exercise training on CRF was rated as low-quality because most of the included studies presented serious risk of bias due to the lack of hidden allocation [47, 49,50,51] and unblinded assessors [47,48,49,50, 52]. In addition, inconsistency was downgraded because a wide prediction interval was found (− 0.63 to 0.04) (Table 4).
Considering the insufficient number of studies to meet rigorous criteria for creating a funnel plot [53], the evaluation of publication bias would be speculative and we decided not rating down the evidence, despite all studies presented small sample sizes [54].
Discussion
The aim of this systematic review and meta-analysis was to investigate the effect of exercise training in CRF among CRC survivors. Our findings suggest that exercise is, globally, an effective intervention to reduce fatigue symptoms in these patients (small-to-moderate effect), however, the robustness of results is challenged by the overall low-quality of the evidence.
Our results are partially in accordance with previous systematic reviews that also found a therapeutic benefit of exercise training on fatigue among CRC survivors [19, 20]. However, by complementing summary effect size with prediction intervals, we provide initial evidence of a large variability in the exercise training effects on CRF in future clinical trials, highlighting the need to identify the subgroup of patients that could benefit most from exercise interventions.
The results of our subgroup-analysis in fact suggest that exercise training may be the most beneficial for patients receiving adjuvant chemotherapy. This is probably related with higher levels of CRF of these patients at baseline [50, 52], as consequence of chemotherapy [10] and in accordance with previous research underlining that the effects of exercise interventions are expectably larger in cancer patients with worse baseline levels of fatigue [24]. Clinically, exercise training could be regarded as a powerful supportive therapy for the management of such prevalent problem in CRC patients undergoing chemotherapy, adding also benefits in other adverse events, such as nausea and gastric reflux [55].
With respect to patients following CRC treatment, the effect of exercise training on CRF was marginal and with wide prediction intervals (− 0.76 to 0.47). Hence, the effect of future exercise interventions is unclear at the time. One potential explanation for these unclear results is the fact that the studies included in this subgroup analysis were not designed to target CRC survivors with higher baseline fatigue levels, the subgroup of patients that may obtain stronger benefits from exercise interventions [24]. Additionally, it is likely that higher exercise doses may be required to manage this problem in survivors following CRC treatment. This rationale is supported by one study that compared the effects of two exercise training doses and found that 150 min of weekly aerobic exercise was insufficient to reduce CRF, but a higher exercise volume (300 min per week) significantly improved CRF and quality of life [47]. In another study, 150 min of aerobic exercise per week at 50–70% of peak heart rate was insufficient to improve cardiorespiratory fitness and body composition in CRC survivors, in comparison to a high-intensity exercise training, highlighting the importance of prescribing higher exercise doses in this population to maximise effects [56]. These distinctive effects of exercise training between patients undergoing and after CRC treatment are in agreement with a previous meta-analysis [57]. Grounded mostly in studies conducted in breast and prostate cancer survivors, in that meta-analysis, it was also found beneficial effects of exercise interventions in CRF among patients undergoing active cancer treatment but non-significant differences in patients after anticancer treatment [57]. Therefore, further studies in survivors after CRC treatment are necessary to definitively clarify the effects of exercise training on this symptom. Of great importance, these studies should recruit patients with higher baseline fatigue values [24, 58] and assess the effects of exercise doses superior to 150 min of moderate intensity aerobic exercise to manage this problem.
Based on the results of this review, the dose of exercise training that demonstrated more consistent improvements on CRF was a combination of resistance exercise (2 to 3 sets of 10–20 repetitions, 45–75% 1-maximum repetition [1-RM], for the main muscle groups) with aerobic exercise (10,000 steps per day or 150 min per week at an intensity of 50–75% of the estimated maximum heart rate), in programmes lasting 12 to 24 weeks [49, 52]. This exercise dose is within the standards of the ACSM for the clinical management of CRF [15]. The integration of resistance training on the exercise programmes could be particularly important to mitigate fatigue symptoms in CRC patients because skeletal muscle dysfunction caused by oxaliplatin treatment is possibly involved in the pathogenesis of CRF [59], and resistance training is an effective intervention to improve muscle function among these patients [55].
Other types of exercise training used in two studies integrated in this systematic review [48, 50], such as Hatha yoga and Baduanjin qigong, consisting in performing different postures in combination with breathing control exercises, and categorised as low-intensity exercise, showed contradictory results (Tables 1 and 3). Several factors may have contributed to these findings. Firstly, the exercise programme with Baduanjin qigong, which significantly reduced CRF, was implemented in patients with CRC undergoing chemotherapy, where CRF levels are more severe [10]. It is known that even low-intensity exercise can bring benefits in these patients, as seen in other types of cancer, particularly in women with breast cancer [60]. In the exercise programme of Hatha yoga [48], the low adherence of the participants and the short period of intervention (10 weeks) might have limited the efficacy of this exercise modality. Therefore, at the moment there is an amount of uncertainty that prevents the recommendation of these modalities for the clinical management of CRF in CRC survivors and consequently, additional high-quality research is required to assess the effect of these types of exercise on fatigue symptoms. Finally, it should be emphasized that CRF has many causative elements and is rarely an isolated symptom, occurring most commonly in a symptom cluster [13]. Particularly in CRC patients, the presence of cognitive symptoms, anxiety, depression, increased number of comorbidities and lower haemoglobin was associated with greater fatigue [10]. Therefore, as recommended by clinical guidelines, assess fatigue contributing factors and integrate exercise training in an interdisciplinary approach tailored to the needs of each individual might be clinically relevant to optimally manage this symptom [13].
Strengths and limitations
The strengths of this systematic review rely on the rigorous effort to strictly follow PRISMA guidelines and the clinical relevance in investigating the effect of exercise training on CRF specifically in CRC patients. The selection of studies was performed by two independent reviewers with a strong and moderate agreement on the title/abstract and full text screening, respectively. To eliminate potential confounding factors, studies where exercise was combined with other interventions, like psychological therapy, that also shows beneficial effects on this symptom [61], were excluded. Finally, in addition to traditional effect size statistics, the prediction interval was calculated, which could inform what true effects of exercise training on CRF can be expected in future exercise studies in CRC patients.
Nonetheless, the main findings of this systematic review need to be considered in the context of some key limitations, including the small number of eligible RCTs with small sample sizes which could influence the external validity and increase the possibility of type II error. Although all the included studies were RCTs, only two studies carried out a hidden allocation [48, 52], which could lead to an overestimation of the exercise training effects [62, 63]. Additionally, the heterogeneity of the exercise interventions between the included studies limits more robust recommendations about the exercise training dose that should be prescribed to manage fatigue among CRC survivors.
Clinical implications
Our meta-analysis provided evidence that exercise training is an effective supportive therapy to the clinical management of CRF, especially in patients undergoing chemotherapy. Despite the heterogeneity in the exercise dose prescribed prevents us to recommend a specific amount of exercise to manage CRF, steady improvements were achieved when a combination of aerobic plus resistance exercise was used, in interventions lasting 12 to 24 weeks.
For decision-makers involved in health policies, it should be underlined that exercise training is significantly more effective than the available pharmaceutical options to manage CRF [61] and in addition to its beneficial impact on this problem, the implementation of exercise programs during adjuvant treatment for patients with colon cancer, resulted in a cost saving of 4321 euros, demonstrating to be an effective and cheaper intervention [64].
Conclusion
In CRC survivors, exercise training is an effective intervention to reduce CRF and could be prescribed as a rehabilitation option to the clinical management of this highly prevalent problem, particularly in patients undergoing chemotherapy. Further studies are necessary to clarify the effects of exercise training on CRF after CRC treatment.
This conclusion is based on low-quality evidence; hence, there is a need for more well-designed randomized controlled trials that investigate the effectiveness of exercise training to prevent or reduce CRF in these patients.
Data availability
The data supporting the conclusions of this article are included in the manuscript and in its supplementary files.
Code availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Ait Ouakrim D, Pizot C, Boniol M, Malvezzi M, Boniol M, Negri E, Bota M, Jenkins MA, Bleiberg H, Autier P (2015) Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ 351:h4970. https://doi.org/10.1136/bmj.h4970
Brenner H, Bouvier AM, Foschi R, Hackl M, Larsen IK, Lemmens V, Mangone L, Francisci S (2012) Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer 131(7):1649–1658. https://doi.org/10.1002/ijc.26192
Brenner H, Gondos A, Arndt V (2007) Recent major progress in long-term cancer patient survival disclosed by modeled period analysis. J Clin Oncol 25(22):3274–3280. https://doi.org/10.1200/JCO.2007.11.3431
Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, Sloan J, Wenzel K, Chauhan C, Eppard W et al (2012) Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol 30(34):4249–4255. https://doi.org/10.1200/jco.2012.42.5967
Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, Weis J, Jordan K, Ripamonti CI (2020) Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol 31(6):713–723. https://doi.org/10.1016/j.annonc.2020.02.016
Oliver A, Greenberg CC (2009) Measuring outcomes in oncology treatment: the importance of patient-centered outcomes. Surg Clin North Am 89(1):17–25, vii. https://doi.org/10.1016/j.suc.2008.09.015
Walling AM, Weeks JC, Kahn KL, Tisnado D, Keating NL, Dy SM, Arora NK, Mack JW, Pantoja PM, Malin JL (2015) Symptom prevalence in lung and colorectal cancer patients. J Pain Symptom Manage 49(2):192–202. https://doi.org/10.1016/j.jpainsymman.2014.06.003
Zerillo JA, Schouwenburg MG, van Bommel ACM, Stowell C, Lippa J, Bauer D, Berger AM, Boland G, Borras JM, Buss MK et al (2017) An international collaborative standardizing a comprehensive patient-centered outcomes measurement set for colorectal cancer. JAMA Oncol 3(5):686–694. https://doi.org/10.1001/jamaoncol.2017.0417
Vardy JL, Dhillon HM, Pond GR, Renton C, Dodd A, Zhang H, Clarke SJ, Tannock IF (2016) Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol 27(9):1761–1767. https://doi.org/10.1093/annonc/mdw252
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK et al (2000) Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist 5(5):353–360. https://doi.org/10.1634/theoncologist.5-5-353
Thong MSY, Mols F, Wang XS, Lemmens VEPP, Smilde TJ, Van De Poll-Franse LV (2013) Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry. Eur J Cancer 49(8):1957–1966. https://doi.org/10.1016/j.ejca.2013.01.012
National Comprehensive Cancer Network (2021) NCCN clinical practice guidelines in oncology: cancer-related fatigue. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1424. Accessed 9 December 2021
Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT (2011) Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomark Prev 20(1):123–133. https://doi.org/10.1158/1055-9965.EPI-10-0988
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390. https://doi.org/10.1249/MSS.0000000000002116
Cramp F, Byron-Daniel J (2012) Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 11:Cd006145. https://doi.org/10.1002/14651858.CD006145.pub3
Kelley, G.A.; Kelley, K.S. (2017) Exercise and cancer-related fatigue in adults: a systematic review of previous systematic reviews with meta-analyses. BMC Cancer 17 (1). https://doi.org/10.1186/s12885-017-3687-5.
Cramer H, Lauche R, Klose P, Dobos G, Langhorst J (2014) A systematic review and meta - analysis of exercise interventions for colorectal cancer patients. Eur J Cancer Care 23(1):3–14. https://doi.org/10.1111/ecc.12093
McGettigan, M.; Cardwell, C.R.; Cantwell, M.M.; Tully, M.A. 2020 Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. 2020, https://doi.org/10.1002/14651858.CD012864.pub2.
Singh B, Hayes SC, Spence RR et al (2020) Exercise and colorectal cancer: a systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int J Behav Nutr Phys Act 17(1):122–122. https://doi.org/10.1186/s12966-020-01021-7
Bennett S, Pigott A, Beller EM, Haines T, Meredith P, Delaney C (2016) Educational interventions for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 11(11):Cd008144. https://doi.org/10.1002/14651858.CD008144.pub2
Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME (2005) Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J Clin Oncol 23(7):1431–1438. https://doi.org/10.1200/JCO.2005.02.054
Zick SM, Colacino J, Cornellier M, Khabir T, Surnow K, Djuric Z (2017) Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res Treat 161(2):299–310. https://doi.org/10.1007/s10549-016-4070-y
Buffart LM, Sweegers MG, May AM, Chinapaw MJ, van Vulpen JK, Newton RU, Galvão DA, Aaronson NK, Stuiver MM, Jacobsen PB et al (2018) Targeting exercise interventions to patients with cancer in need: an individual patient data meta-analysis. J Natl Cancer Inst 110(11):1190–1200. https://doi.org/10.1093/jnci/djy161
Graham PL, Moran JL (2012) Robust meta-analytic conclusions mandate the provision of prediction intervals in meta-analysis summaries. J Clin Epidemiol 65(5):503–510. https://doi.org/10.1016/j.jclinepi.2011.09.012
Higgins JPT, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 172(1):137–159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
IntHout J, Ioannidis JP, Rovers MM, Goeman JJ (2016) Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6(7):e010247. https://doi.org/10.1136/bmjopen-2015-010247
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097–e1000097. https://doi.org/10.1371/journal.pmed.1000097
Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. (2007) Utilization of the PICO framework to improve searching PubMed for clinical questionsBMC Med Inform Decis Mak 7https://doi.org/10.1186/1472-6947-7-16
Denlinger CS, Carlson RW, Are M, Baker KS, Davis E, Edge SB, Friedman DL, Goldman M, Jones L, King A et al (2014) Survivorship: introduction and definition: clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw 12(1):34–45. https://doi.org/10.6004/jnccn.2014.0005
Fisher, M.I.; Davies, C.; Lacy, H.; Doherty, D. 2018 Oncology section EDGE task force on cancer: measures of cancer-related fatigue - a systematic teview. 36, 93-105https://doi.org/10.1097/01.REO.0000000000000124
Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors EXPERT PANELhttps://doi.org/10.1249/MSS.0b013e3181e0c112
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochemia Medica 22(3):276–282. https://doi.org/10.11613/bm.2012.031
Cashin AG, McAuley JH (2020) Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother 66(1):59. https://doi.org/10.1016/j.jphys.2019.08.005
Faraone, S.V. (2008) Interpreting estimates of treatment effects: implications for managed care. P & T : a peer-reviewed journal for formulary management 33 (12): 700-711
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Berger, A.M.; Grem, J.L.; Visovsky, C.; Marunda, H.A.; Yurkovich, J.M. (2010) Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncology nursing forum 37 (6). https://doi.org/10.1188/10.onf.e359-e369.
Team, R.C. (2020).R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria.
Team, R.S. (2020).RStudio: integrated development for R, RStudio, PBC, Boston, MA.
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22(4):153–160. https://doi.org/10.1136/ebmental-2019-300117
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G et al (2011) GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol 64(12):1283–1293. https://doi.org/10.1016/j.jclinepi.2011.01.012
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G et al (2011) GRADE guidelines: 8. rating the quality of evidence–indirectness. J Clin Epidemiol 64(12):1303–1310. https://doi.org/10.1016/j.jclinepi.2011.04.014
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA et al (2011) GRADE guidelines: 7. rating the quality of evidence–inconsistency. J Clin Epidemiol 64(12):1294–1302. https://doi.org/10.1016/j.jclinepi.2011.03.017
Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y et al (2011) GRADE guidelines: 5. rating the quality of evidence–publication bias. J Clin Epidemiol 64(12):1277–1282. https://doi.org/10.1016/j.jclinepi.2011.01.011
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y et al (2011) GRADE guidelines: 4. rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 64(4):407–415. https://doi.org/10.1016/j.jclinepi.2010.07.017
Brown JC, Damjanov N, Courneya KS, Troxel AB, Zemel BS, Rickels MR, Ky B, Rhim AD, Rustgi AK, Schmitz KH (2018) A randomized dose-response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psychooncology 27(4):1221–1228. https://doi.org/10.1002/pon.4655
Cramer H, Pokhrel B, Fester C, Meier B, Gass F, Lauche R, Eggleston B, Walz M, Michalsen A, Kunz R et al (2016) A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psychooncology 25(4):412–420. https://doi.org/10.1002/pon.3927
Kim JY, Lee MK, Lee DH, Kang DW, Min JH, Lee JW, Chu SH, Cho MS, Kim NK, Jeon JY (2019) Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: a randomized controlled trial. Support Care Cancer 27(8):2933–2940. https://doi.org/10.1007/s00520-018-4588-0
Lu Y, Qu HQ, Chen FY, Li XT, Cai L, Chen S, Sun YY (2019) Effect of Baduanjin qigong exercise on cancer-related fatigue in patients with colorectal cancer undergoing chemotherapy: a randomized controlled trial. Oncol Res Treat 42(9):431–438. https://doi.org/10.1159/000501127
Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N (2013) Home-based physical activity intervention for colorectal cancer survivors. Psychooncology 22(1):54–64. https://doi.org/10.1002/pon.2047
Van Vulpen JK, Velthuis MJ, Bisschop CNS, Travier N, Van den Buijs BJW, Backx FJG, Los M, Erdkamp FLG, Bloemendal HJ, Koopman M et al (2016) Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc 48(5):767–775. https://doi.org/10.1249/mss.0000000000000855
Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ (Clinical research ed) 333(7568):597–600. https://doi.org/10.1136/bmj.333.7568.597
Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, Atkins D, Kunz R, Montori V, Jaeschke R et al (2013) GRADE guidelines: 11. making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 66(2):151–157. https://doi.org/10.1016/j.jclinepi.2012.01.006
Hong Y, Wu C, Wu B (2020) Effects of resistance exercise on symptoms, physical function, and quality of life in gastrointestinal cancer patients undergoing chemotherapy. Integr Cancer Ther 19:153473542095491–153473542095491. https://doi.org/10.1177/1534735420954912
Devin JL, Sax AT, Hughes GI, Jenkins DG, Aitken JF, Chambers SK, Dunn JC, Bolam KA, Skinner TL (2016) The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv 10(3):467–479. https://doi.org/10.1007/s11764-015-0490-7
Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R (2015) Effects of supervised multimodal exercise interventions on cancer-related fatigue: systematic review and meta-analysis of randomized controlled trials. Biomed Res Int 2015:328636. https://doi.org/10.1155/2015/328636
Barsevick AM, Irwin MR, Hinds P, Miller A, Berger A, Jacobsen P, Ancoli-Israel S, Reeve BB, Mustian K, O’Mara A et al (2013) Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst 105(19):1432–1440. https://doi.org/10.1093/jnci/djt242
Yang S, Chu S, Gao Y, Ai Q, Liu Y, Li X, Chen N (2019) A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells 8(7):738–738. https://doi.org/10.3390/cells8070738
Zetzl T, Renner A, Pittig A, Jentschke E, Roch C, van Oorschot B (2021) Yoga effectively reduces fatigue and symptoms of depression in patients with different types of cancer. Support Care Cancer 29(6):2973–2982. https://doi.org/10.1007/s00520-020-05794-2
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF et al (2017) Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 3(7):961–968. https://doi.org/10.1001/jamaoncol.2016.6914
Armijo-Olivo S, Saltaji H, Da Costa BR, Fuentes J, Ha C, Cummings GG (2015) What is the influence of randomization sequence generation and allocation concealment on treatment effects of physical therapy trials? A meta-epidemiological study. BMJ Open 5(9):e008562–e008562. https://doi.org/10.1136/bmjopen-2015-008562
Pildal J, Hróbjartsson A, Jörgensen KJ, Hilden J, Altman DG, Gøtzsche PC (2007) Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 36(4):847–857. https://doi.org/10.1093/ije/dym087
May AM, Bosch MJ, Velthuis MJ, van der Wall E, Steins Bisschop CN, Los M, Erdkamp F, Bloemendal HJ, de Roos MA, Verhaar M et al (2017) Cost-effectiveness analysis of an 18-week exercise programme for patients with breast and colon cancer undergoing adjuvant chemotherapy: the randomised PACT study. BMJ Open 7(3):e012187. https://doi.org/10.1136/bmjopen-2016-012187
Acknowledgements
Authors acknowledge the financial support provided by the Portuguese Foundation for Science and Technology to their research unit (REF UIDB/05704/2020)
Funding
Pedro Machado holds a PhD fellowship supported by the Portuguese Foundation for Science and Technology (REF UIDB/05704/2020).
Author information
Authors and Affiliations
Contributions
All the authors contributed to conceived and the designed the study. PM, MM, JR and MM conducted the screening of studies and data extraction. CS and NM provided statistical expertise and contributed to the analysis, discussion and preparation of manuscript. All authors participated in the revision and final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Machado, P., Morgado, M., Raposo, J. et al. Effectiveness of exercise training on cancer-related fatigue in colorectal cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 30, 5601–5613 (2022). https://doi.org/10.1007/s00520-022-06856-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06856-3