Abstract
Purpose
There was no optimal risk assessment tool to stratify the risk of peripherally inserted central catheter-related venous thromboembolism (PICC-RVT) in cancer patients. We currently use the Caprini risk assessment model for thrombotic risk assessment, but no evidence exists on the effectiveness of Caprini in such patients. This study was to assess the validity of the Caprini in Chinese cancer patients with PICCs.
Methods
We conducted a prospective study of 468 participants. Following calculating the Caprini score, color Doppler ultrasonography was performed every 7 days for 3 weeks to confirm PICC-RVT.
Results
There was a correlation between PICC-RVT and the Caprini score. Compared with scores of 5, the risk was 2.089-fold greater (95% CI 1.165–3.743, P = 0.012) in patients with a score of 6 and 7, and 7.156-fold greater (95% CI 3.157–16.217, P < 0.001) in patients with scores ≥8. The area under the receiver-operating characteristic curve was 0.636 (95% CI 0.590–0.680; P < 0.001). 6 was the best cutoff point for Caprini, with a sensitivity of 0.76 and a specificity of 0.44.
Conclusions
The Caprini can be used for high-risk screening of the PICC-RVT in cancer patients, and classification of the highest risk level using a score of 6 can be more clinically significant compared to 5 as recommended. The results provide evidence for the practitioner's early use of the Caprini to assess the thrombotic risk in patients with PICCs and take timely prevention measures. But pharmacological prevention should be considered seriously for its low specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peripherally inserted central catheters (PICCs) have been frequently used in patients with most cancers to supply extended chemotherapy [1]. Several reasons had made PICC among the most common type of central venous catheter in medical practice, including its simplicity of insertion by nurses, perceived safety, cost-effectiveness, and medium and long-term access for drug administration (such as irritants and foaming agents) in comparison to central venous catheters (CVC) [2,3,4]. Despite these benefits, they are with some complications including PICC-related venous thrombosis (PICC-RVT) [5]. PICCs are associated with an up to 2.5-fold greater incidence of venous thrombosis compared with CVCs [3]. The formation of PICC-RVT is greatly harmful and can lead to delays in intravenous therapy, more medical costs, and even mortality [6]. Rates of clinically manifest PICC-RVT have been reported at 2% to 11%[7], while rates in patients with cancers had been 6% to 15% [1, 8]. However, a wide variety of patients have no apparent clinical manifestations after developing thrombosis. The incidence of PICC-RVT reported in the literature that covered asymptomatic thrombus was between 35% and 71.9% [9, 10]. Many previous studies mainly focused on symptomatic venous thrombosis, and many researchers did Doppler-ultrasonography only if symptoms or signs suggested thrombosis [1, 3, 11]. So, patients' asymptomatic thrombi were not treated promptly and effectively. A risk assessment instrument is crucial to improve the situation.
The implementation of scientific and effective prophylaxis can reduce the incidence of venous thrombosis by 30%–60% [9, 12]. And most guidelines recommended the use of prophylaxis for patients with an increased risk of developing venous thrombus [13]. So, early assessment or high-risk screening is of great significance. Several risk assessment models have been used to forecast and evaluate the venous thrombosis risk clinically. However, studies related to PICC-RVT are rare [14]. Since there is currently no recognized PICC-RVT risk prediction tool, we mainly use some deep venous thrombosis risk assessment tools, such as the Caprini risk assessment model (RAM) in these patients. Caprini RAM, which is perhaps the most widely used risk prediction tool, has been well-validated in many studies [15]. It has been integrated into electronic medical records at many institutions[16]. Still, whether Caprini RAM is suitable for the risk prediction in patients with PICCs requires further study. To the best of our knowledge, the most recent version of the Caprini RAM has not been validated in cancer patients with PICCs. In this study, we want to (a) assess the validity of the Caprini risk assessment model in Chinese cancer patients with PICC and (b) determine the incidence and the influence factors related to PICC-RVT.
Methods
Study design and patients
A prospective study of cancer patients who underwent PICC insertions from January 2018 to January 2019 was conducted in a comprehensive teaching hospital. Each hospitalized patient should receive a series of blood sampling such as blood routine, biochemical routine, and coagulation function, as well as limb color Doppler ultrasonography before PICC catheterization. Patients were enrolled if they (1) had been diagnosed with a malignant tumor and (2) were planned to receive intravenous chemotherapy and needed PICC catheterization. Patients were excluded if they (1) had a history of anticoagulation use 1 week before catheterization, (2) received anticoagulant therapy, (3) dropped out, or (4) removed the PICC during follow-up.
Basic information and clinical data of the patients such as demographics, history of present illness, physical examination, laboratory results, and relevant information on PICC placement were collected from the electronic medical record. Risk factors used to calculate the Caprini risk score were also captured. Our nurses would observe whether the catheter was functioning normally during hospitalization. They would also measure the arm circumference and ask patients if they had symptoms such as pain, swelling, fever, increased skin temperature, and so on. If a patient was suspected to have a venous thrombosis, color Doppler ultrasonography should be used promptly to diagnose the presence of a thrombus.
According to the literature [17], about 28.1% of PICC-RVT occurs within 1 week after catheterization, and 65.6% of venous thrombosis occurs at 2–3 weeks. Therefore, we will follow the subjects for three weeks (21 days). The discharged patients came back for PICC maintenance by our nurses on Day 7, Day 14, and Day 21 after catheterization. Color Doppler ultrasonography was also performed on these days. If the patients had symptoms such as pain, swelling, and increased skin temperature, they came to the hospital immediately for color Doppler ultrasonography to check whether there was thrombosis.
PICC insertion and maintenance
All PICC catheterizations had been operated on with the guidance of ultrasound by our skilled vascular access nurse. And all PICCs used in our study were single-lumen catheters (Bard Access Systems, USA) with a length of 60 cm and a model of 4-French. The preferred puncture vessel was the basilic vein of the right arm. However, if the precatheterization color Doppler ultrasound confirmed thrombosis, the brachial or cephalic veins or the left arm would be a substitution. Arm circumference and length of the catheter had been measured before catheterization. The chest X-ray films (PA position) were carried out to determine the position of the catheter tip to make certain that the catheter tip position was correct.
Our trained nurses provided the preservation of care for all PICCs. The first maintenance was required 24 h after PICC catheterization, and then, it was generally maintained weekly [18, 19]. If there had been a curling edge of the dressing, errhysis, and exudation at the puncture site, or extreme cough, difficult defecation ensuing in catheter blocking, we would maintain immediately. All PICCs were flushed with 10-ml saline and then locked using 10-ml 25-IU/ml heparin saline once a week normally [18]. Nurses would examine whether or not the catheter was in normal function throughout maintenance. They would additionally measure the arm circumference and ask patients if they had signs and symptoms such as pain, swelling, fever, and increased skin temperature.
Caprini risk assessment model
The Caprini risk assessment model (see Table 1) is originally developed for medical and surgical patients by Caprini and his colleges in 1991 [20], and it has been adopted and modified by the American College of Chest Physicians (ACCP) guideline in 2012 [21]. The modified version estimates risk by adding points for 39 risk factors weighted of 1–5 points each. By calculating a risk score, patients were stratified into 4 categories: “very low risk” (0 and 1 point), “low risk” (2 points), “moderate risk” (3 and 4 points), or “high risk” (≥5 points) [21]. ACCP-9 recommended the application of Caprini RAM in nonorthopedic surgical patients [21]. Studies have also confirmed [13, 22, 23] its validity in medical patients. Due to the lack of a suitable instrument for screening the thrombus risk in patients with PICC, many hospitals in China currently use Capirni risk assessment model for the PICC-RVT risk screening in medical patients.
Statistical analysis
In our study, continuous variables with a normal distribution were summarized as mean and standard deviation (SD). A t test was used to compare the difference between groups. Data not normally distributed were expressed as median (interquartile range), and nonparametric tests were used to compare the difference between groups. Categorical variables were presented as frequencies and percentages, group comparisons were carried out using the chi-squared test. But for some of those who have low expected counts, a Fisher exact test was used instead. Receiver-operating characteristic curves were plotted according to the sensitivity and specificity of Caprini RAM, and the area under the curve (AUC) and 95% CI were also calculated. Then, MEDCALC version 19.2 (https://www.medcalc.org) was used to provide the sensitivity and specificity with confidence limits at every observed cut point for Caprini RAM.
Binary logistic regression analyses were used to identify factors associated with PICC-RVT and to estimate the adjusted odds ratio and 95% confidence intervals. The Box-Tidwell method was used to test whether the continuous-independent variable was linear with the logit transformed value of the dependent variable. A total of 10 items were included when testing the linear model, and the significance level was 0.0005 after the Bonferroni correction. The results of the linearity test yielded a linear relationship between the continuous independent variable and the logit transformed value of the dependent variable. 27 observations had studentized residuals greater than 2.5 times the standard deviation but were retained in the analysis. All statistical tests were two-tailed, and P < 0.05 was considered statistically significant. Data were performed using SPSS version 26.0 (IBM, Armonk, NY, USA).
Ethical approval and informed consent
Approval was obtained from the ethics committee of the hospital. Written informed consent was obtained from all the participants included in this study. The procedure used in this study adheres to the tenets of the Declaration of Helsinki.
Results
Participant characteristics
We recruited cancer patients in a comprehensive teaching hospital from January 2018 to January 2019. 468 cancer patients were enrolled in this study. There were 351 men (75.0%) and 117 women (25.0%), with a mean age of 57.43 years, ranging from 18 to 79 years. The vast majority of subjects had PICC placed in the right arm (91.7%), basilic vein selected (85.0%). Most patients (98.5%) had a body mass index (BMI) ≤ 30 kg/m2, with 80.6% of patients in the category of ≤25 kg/m2. Table 2 shows the detailed characteristics of PICC-RVT cases and no PICC-RVT subjects. There were statistically significant differences between the group PICC-RVT and no PICC-RVT concerning gender, age, BMI, history of deep venous thrombosis, and placement attempts >1 (all P < 0.05).
Incidence rate of PICC-RVT and risk stratification of Caprini RAM
A total of 82 (17.5%) cancer patients were identified as having PICC-RVT, including 10 (2.1%) symptomatic thrombosis and 72 (15.4%) asymptomatic thrombosis. There were 56 (68.3%) PICC-RVT that occurred within 7 days, 16 (19.5%) of 82 cases occurred between 7 and 14 days, and 10 of them (12.2%) occurred between 15 and 21 days.
Among all participants, the mean Caprini score was 5.89 (SD = 1.04), ranging from 4 to 11. The incidence of PICC-RVT for each score was presented in Fig. 1 and Table 3. As the score increases, the incidence of thrombosis is generally on the rise. The median cumulative risk score of Caprini in participants who developed PICC-RVT was significantly higher than that in patients with no PICC-RVT (median score 6.0 [5.8–7.0] vs. 6.0 [5.0–6.0], P < 0.001). We continued risk stratification in patients with a score ≥5, and the chi-square test showed a statistically significant difference in the incidence of PICC-RVT among three groups (χ2 = 25.355, P < 0.001). Compared with the group with a score of 5, the risk was 2.089-fold greater (95% confidence interval (CI) 1.165–3.743, P = .012) in patients with a score of 6 and 7, and 7.156-fold greater (95% CI 3.157–16.217. P < 0.001) in the group with scores ≥8.
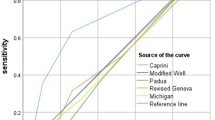
ROC curve analysis
Analysis of ROC curves (Fig. 2) indicated that the area under the curve (AUC) was 0.636 (95% CI, 0.590–0.680: P < 0.001). A cumulative risk score of 6 was the best cutoff point for Caprini RAM, with a sensitivity of 0.76 (95% CI 0.65–0.84), and a specificity of 0.44 (95% CI 0.39–0.49) (Table 4).
Related factors of PICC-RVT in the study population
In the univariate analysis, 5 factors (male gender, age, BMI ≥ 25 kg/m2, history of DVT, and placement attempts >1) were related statistically significantly (all P < 0.05) with an increased risk of PICC-RVT in cancer patients. Variables that had a P value < 0.10 in univariable analysis were selected for inclusion in the multivariable model.
The final logistic model was statistically significant (χ2 = 52.040, P < 0.001). The model was able to correctly classify 83.0% of the study subjects. Of the 9 variables included, history of DVT (OR = 4.475, 95% CI 1.992–10.055), placement attempt >1 (OR = 5.244, 95% CI 2.434–11.298), history of chemotherapy (OR = 2.014, 95% CI 1.038–3.910), and decreased PT (OR = 3.898, 95% CI 1.265–12.013) were statistically significant (All P < 0.05) (Table 5).
Discussion
Cancer patients with PICCs have high risks of venous thrombosis. Our study found 82 (17.5%) patients with PICC-RVT among 468 cancer patients, only 10 (2.1%) developed symptomatic thrombosis. In a previous retrospective study in 522 PICCs, venous thrombosis was at a rate of 2.9% [24], which was similar to our study. However, a large number of patients have no obvious clinical manifestations after developing thrombosis and many previous studies mainly focused on symptomatic venous thrombosis, and most researchers did Doppler-ultrasonography only if symptoms or signs suggested thrombosis [1, 3, 11]. So, patients' asymptomatic thrombi were not treated promptly and effectively. Besides, clinicians have no way to identify patients who might be at high risk for VTE when considering PICC placement. A simple but effective risk assessment instrument is crucial to improve the situation.
Caprini RAM was developed originally for surgical and medical patients, and there is convincing evidence of its validity in surgical patients [22, 25]. It has also been proved to be a utility in medical patients [13, 23, 26]. Bo et al. [15] assessed risks for VTE generally in a large sample (N = 24,524) of Chinese medical and surgical inpatients from 25 hospitals. They reported an AUC of 0.74 (95% CI [0.71, 0.77]) for the Caprini score overall, 0.77 [0.74, 0.81] for surgical and 0.66 [0.60, 0.71] for medical inpatients. Their results indicated that the Caprini RAM had lower accuracy for predicting VTE in nonsurgical patients compared to surgical patients. Their AUC for medical inpatients is similar to our results (AUC 0.64 95% CI 0.59–0.68).
In our study, each of our subjects met the two items of “Malignancy (score 2)” and “Central Venous Access (score 2)” and had a score of at least 4 (moderate risk) as assessed using Caprini. 458 (97.9%) of them had a score of ≥5 (Pharmacological prophylaxis is obligatory if there is a low risk of bleeding, recommended by the American College of Chest Physicians guideline, 9th edition.) while only 80 patients developed PICC-RVT. So, if we use Caprini RAM to evaluate the PICC-RVT risk and guide prophylaxis in cancer patients with PICC as recommended, nearly 82.5% of patients may have limited benefit from it. Therefore, reconsideration of the classification of thrombotic risk in this population and the risks and benefits encouraging the use of VTE prophylaxis appears necessary. By further dividing the highest risk category in our study, we found that risk was 2.09-fold greater in the 'High risk with a score of 6 and 7 group and 7.16-fold greater in the group with scores ≥8 compared with the group with a score of 5. The finding is consistent with the results of the study by Bo et al. [15] that further stratification of the highest risk category is necessary to determine the exact extent of risk. In the ROC curve analysis, the sensitivity of the Caprini was 0.76 and the specificity was only 0.44 at the cutoff value of 6. Nearly 90% of the participants scored between 5 and 7, so the Caprini could not accurately distinguish these patients. The low specificity may lead to increased “misdiagnosis” which can lead to overtreatment. A prospective study had also found the possibility of overtreatment with Caprini [27].
In addition to what the Caprini evaluated, we also collected some other variables that may be related to PICC-RVT. We found that the history of DVT, placement attempt >1, history of chemotherapy, and decreased PT were statistically significantly correlated with PICC-RVT in the final logistic model. However, only the “history of DVT” was included in the Caprini model. Other items of this assessment tool did not become independent risk factors in our logistic model, which may be related to the good general conditions of the target population during sampling. For example, we did not have patients such as “continuous bed rest >72 h”, “history of prior major surgery (<1 month)”, “stroke (<1 month)”, and so on. Literature reported [28] that patients with obesity (BMI > 30 kg/m2) and a recent history of surgery had a higher risk of PICC-RVT compared with none obese patients without a recent history of surgery. The incidence of thrombosis also increases with the effective catheter diameter and thus the number of lumens used [1, 3]. We only used single lumen catheters with 4Fr in our study. Some previous studies indicated that a high D-dimer level before PICC placement was associated with the development of PICC-RVT, especially for patients with D-dimer level >0.55mg/L [29, 30]. We did not get such results. The study of Kang et al. [31] was similar to ours that the D-dimer level before PICC insertion could not predict PICC-RVT. This is possibly because the catheter-related coagulation mechanism had not been activated [31].
It could be noted that unlike the population in which Caprini RAM was validated, patients with PICCs had an increased risk of upper extremity venous thromboembolism (VTE), and endothelial damage during catheterization [1] was greatly related in addition to the blood stasis and hypercoagulable state [15] to be assessed by Caprini RAM. PICC insertion could cause mechanical injury to the vascular wall and thrombosis. And the catheter itself could injure the vein constantly because of its pressure against the vein wall and the rubbing caused by respiratory and body movements [32]. That said, it is important to develop a risk assessment tool in this distinct population. Caprini does not discriminate degrees of elevated risk, given the joint presence of a cancer diagnosis and a PICC line. However, the amount of work involved in developing a risk assessment tool is substantial, and there may be ways an existing instrument can be modified to make it suitable for use in this distinct population.
Our study still had some limitations. Firstly, this was a single-center study with a small sample size. We only followed patients for 21 days. Catheter-related venous thrombosis occurring after 21 days has been reported in the literature [11]. So, there may be some bias in the statistics of the incidence of PICC-RVT. Secondly, the predictive assessment was only performed once after catheterization, and no dynamic assessment was performed thereafter. When the patients' various indicators changed, whether it needed to be reassessed, and when to reassess was not considered. It should be improved and supplemented in future studies. Finally, our subjects were mainly patients with lung cancer and esophageal cancer, which may have some impact on the results.
Despite some limitations, our study validates the effectiveness and feasibility of the Caprini risk assessment model in cancer patients with PICCs and shows relatively satisfactory results. It can help clinicians to identify patients who might be at high risk for VTE when considering PICC placement. And can also help doctors and nurses perform timely thromboprophylaxis for their patients after catheterization to ensure the patients' safety. However, for which risk level people should use anticoagulant therapy preventively, there should be more studies with a large sample size to provide evidence. Also, future studies should consider constructing new thrombosis risk assessment models for patients with PICC or modifying and refining the existing mature models like Caprini RAM to apply to this population.
Conclusion
We found that the Caprini RAM can be used for high-risk screening of the PICC-RVT in cancer patients, and classification of the highest risk level using a cumulative score of 6 can be more clinically significant. Patients with higher scores had a higher risk of thrombosis, but pharmacological prevention should be considered carefully for its low specificity.
Data and materials availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Chopra V, Kaatz S, Conlon A, Paje D, Grant PJ, Rogers MAM, Bernstein SJ, Saint S, Flanders SA (2017) The Michigan risk score to predict peripherally inserted central catheter-associated thrombosis. J Thromb Haemost 15(10):1951–1962. https://doi.org/10.1111/jth.13794
Tejedor SC, Tong D, Stein J, Payne C, Steinberg JP (2012) Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol 33(1):50–57
Chopra V, Anand S, Hickner A, Buist M, Rogers MAM, Saint S, Flanders SA (2013) Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 382(9889):311–325. https://doi.org/10.1016/S0140-6736(13)60592-9
Bhargava M, Broccard S, Bai Y, Wu B, Broccard A (2020) Risk factors for peripherally inserted central catheter line–related deep venous thrombosis in critically ill intensive care unit patients. SAGE Open Med 8:205031212092923
Verma AA, Kumachev A, Shah S, Guo Y, Jung HY, Rawal S, Lapointe-Shaw L, Kwan JL, Weinerman A, Tang T, Razak F (2020) Appropriateness of peripherally inserted central catheter use among general medical inpatients: an observational study using routinely collected data. BMJ Qual Saf 0:1-7. doi:https://doi.org/10.1136/bmjqs-2019-010463, 29
Koo CM, Vissapragada R, Sharp R, Nguyen P, Ung T, Solanki C, Esterman A (2018) ABO blood group related venous thrombosis risk in patients with peripherally inserted central catheters. Br J Radiol 91(1082):20170560. https://doi.org/10.1259/bjr.20170560
Austin RE, Shahriar S, Siavash B, Jeschke MG (2015) Peripherally inserted central venous catheter safety in burn care: a single-center retrospective cohort review. J Burn Care Res 36(1):111–117. https://doi.org/10.1097/BCR.0000000000000207
Ahn DH, Illum HB, Wang DH, Sharma A, Dowell JE (2013) Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract 9(1):e8–e12. https://doi.org/10.1200/JOP.2012.000595
Paauw JD, Borders H, Ingalls N, Boomstra S, Lambke S, Fedeson B, Goldsmith A, Davis AT (2008) The incidence of PICC line-associated thrombosis with and without the use of prophylactic anticoagulants. JPEN J Parenter Enteral Nutr 32(4):443–447. https://doi.org/10.1177/0148607108319801
Maxim I, Mondshein JI, William S, Shlansky-Goldberg RD, Soulen MC, Trerotola SO (2014) Peripherally inserted central catheter thrombosis—reverse tapered versus nontapered catheters: a randomized controlled study. J Vasc Interv Radiol 25(1):85–91.e81. https://doi.org/10.1016/j.jvir.2013.10.009
Chen Y, Chen H, Yang J, Jin W, Fu D, Liu M, Xu Y, Tao Z, Li Y, Lu L, Wang M, Zhu C, Chen Y (2020) Patterns and risk factors of peripherally inserted central venous catheter-related symptomatic thrombosis events in patients with malignant tumors receiving chemotherapy. J Vasc Surg 8(6):919–929. https://doi.org/10.1016/j.jvsv.2020.01.010
Monreal M, Alastrue A, Rull M, Mira X, Muxart J, Rosell R, Abad A (1996) Upper extremity deep venous thrombosis in cancer patients with venous access devices—prophylaxis with a low molecular weight heparin (Fragmin). Thromb Haemost 76(02):251–253. https://doi.org/10.1055/s-0038-1650254
Zhou H, Hu Y, Li X, Wang L, Wang M, Xiao J, Yi Q (2018) Assessment of the risk of venous thromboembolism in medical inpatients using the Padua prediction score and Caprini risk assessment model. J Atheroscler Thromb 25(11):1091–1104. https://doi.org/10.5551/jat.43653
Liu S, Zhang F, Xie L, Wang Y, Xiang Q, Yue Z, Feng Y, Yang Y, Li J, Luo L, Yu C (2019) Machine learning approaches for risk assessment of peripherally inserted central catheter-related vein thrombosis in hospitalized patients with cancer. Int J Med Inform 129:175–183. https://doi.org/10.1016/j.ijmedinf.2019.06.001
Bo H, Li Y, Liu G, Ma Y, Wu X (2020) Assessing the risk for development of deep vein thrombosis among Chinese patients using the 2010 Caprini risk assessment model: a prospective multicenter study. J Atheroscler Thromb 27(8):801–808
Caprini JA (2010) Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg 199(1 Suppl):S3–S10. https://doi.org/10.1016/j.amjsurg.2009.10.006
Liu Y, Gao Y, Wei L, Chen W, Ma X, Song L (2015) Peripherally inserted central catheter thrombosis incidence and risk factors in cancer patients: a double-center prospective investigation. Ther Clin Risk Manag 11:153–160. https://doi.org/10.2147/TCRM.S73379
Liang YJ, He Y, Li JM, Chen LM, Chen LP, Wang C, Ji L, Li ZX, Tang LQ, Chen QY, Fan YY, Hu W (2018) The incidence and predictors of symptomatic venous thromboembolism associated with peripherally inserted central catheters in patients with nasopharyngeal carcinoma. Onco Targets Ther 11:3119–3127
Liu K, Zhou Y, Xie W, Gu Z, Jin Y, Ye X, Chen X, Fan B, Wang H, Cui Y (2018) Handgrip exercise reduces peripherally-inserted central catheter-related venous thrombosis in patients with solid cancers: a randomized controlled trial. Int J Nurs Stud 86:99–106. https://doi.org/10.1016/j.ijnurstu.2018.06.004
Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F (1991) Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost 17(Suppl 3):304–312. https://doi.org/10.1055/s-2007-1002590
Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM (2012) Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2, Supplement):e227S–e277S. https://doi.org/10.1378/chest.11-2297
Pannucci CJ, Swistun L, Macdonald JK, Henke PK, Brooke BS (2017) Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg 265(6):1094–1103. https://doi.org/10.1097/SLA.0000000000002126
Haixia Z, Lan W, Xiaoling W, Yongjiang T, Jing Y, Bo W, Yu Y, Binmiao L, Ke W, Xuemei O (2014) Validation of a venous thromboembolism risk assessment model in hospitalized chinese patients: a case-control study. J Atheroscler Thromb 21(3):261–272. https://doi.org/10.5551/jat.20891
Mielke D, Wittig A, Teichgraber U (2020) Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support Care Cancer 28(10):4753–4760. https://doi.org/10.1007/s00520-019-05276-0
Stroud W, Whitworth JM, Miklic M, Schneider KE, Finan MA, Scalici J, Reed E, Bazzett-Matabele L, Straughn JM, Rocconi RP (2014) Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol 134(1):160–163. https://doi.org/10.1016/j.ygyno.2014.04.051
Liu X, Liu C, Chen X, Wu W, Lu G (2016) Comparison between Caprini and Padua risk assessment models for hospitalized medical patients at risk for venous thromboembolism: a retrospective study. Interact Cardiovasc Thorac Surg 23:538–543. https://doi.org/10.1093/icvts/ivw158
Grant PJ, Greene MT, Chopra V, Bernstein SJ, Hofer TP, Flanders SA (2016) Assessing the Caprini score for risk assessment of venous thromboembolism in hospitalized medical patients. Am J Med 129(5):528–535. https://doi.org/10.1093/icvts/ivw158
Blom JW, Doggen CJM, Osanto S, Rosendaal FR (2005) Old and new risk factors for upper extremity deep venous thrombosis. J Thromb Haemost 3(11):2471–2478. https://doi.org/10.1111/j.1538-7836.2005.01625
Chen P, Wan G, Zhu B (2020) Incidence and risk factors of symptomatic thrombosis related to peripherally inserted central catheter in patients with lung cancer. J Adv Nurs 77:1284–1292. https://doi.org/10.1111/jan.14666
Longfang P, Qianru Z, Xiangmei Y (2014) Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med 7(12):5814
Kang J, Sun W, Li H, Ma E, Chen W (2020) Variable D-dimer thresholds in predicting peripherally inserted central catheter-related vein thrombosis in patients with hematological malignancies: a pilot study. Thromb Res 190:8–10. https://doi.org/10.1016/j.thromres.2020.03.022
Xiang DZ, Verbeken EK, Lommel ATV, Stas M, Wever ID (1998) Composition and formation of the sleeve enveloping a central venous catheter. J Vasc Surg 28(2):260–271. https://doi.org/10.1016/S0741-5214(98)70162-4
Acknowledgments
We would like to thank all the medical staff in the thoracic oncology department as well as the physicians in the ultrasound department for their help and support in this study. Thanks to all the patients who participated in this study.
Funding
This work was supported by the Department of Science and Technology of Sichuan Province, China (grant number 2018SZ0203).
Author information
Authors and Affiliations
Contributions
Yue Feng: methodology; investigation; formal analysis; writing-original draft;
Rujun Zheng: formal analysis; wiring-review and editing;
Yan Fu: resources; supervision;
Qiufen Xiang: investigation; resources;
Zhiying Yue: methodology; investigation;
Junying Li: conceptualization; methodology; writing-review and editing; funding acquisition;
Chunhua Yu: conceptualization; resources;
Yan Jiang: conceptualization; resources.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Medical Ethics Committee of our hospital (2018-345). All participants involved in this study gave their informed consent.
Consent for publication
Patients signed informed consent regarding publishing their data.
Conflict of interests
The authors declare that no conflict of interest could be perceived as prejudicing the impartiality of the research reported.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, Y., Zheng, R., Fu, Y. et al. Assessing the thrombosis risk of peripherally inserted central catheters in cancer patients using Caprini risk assessment model: a prospective cohort study. Support Care Cancer 29, 5047–5055 (2021). https://doi.org/10.1007/s00520-021-06073-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06073-4