Abstract
Purpose
This study is aimed at examining the buffering effect of sense of coherence (SOC) on symptom distress during cancer drug therapy, which thereby affects health-related quality of life (QoL), and obtaining suggestions for promoting supportive care.
Methods
We investigated health-related QoL (SF-8), symptom distress (using the Symptom Distress Scale (SDS)), and SOC (the SOC 13-item Scale) in 66 patients receiving adjuvant chemotherapy for non-small cell lung cancer. We employed descriptive statistics to seek the correlation of each variable; then, a hierarchical multiple regression analysis was conducted with SF-8 score as the dependent variable.
Results
Results showed that significant changes in bodily pain showed a buffering effect on the SDS and sense of comprehensibility (β = − 0.658, p < 0.01, β = − 0.319, p < 0.05), sense of manageability (β = − 0.658, p < 0.01, β = 0.398, p < 0.01), and meaningfulness (β = − 0.658, p < 0.01, β = − 0.257, p < 0.05). Significant changes in general health perception showed a buffering effect on the SDS and sense of manageability (β = − 0.406, p < 0.01, β = 0.329, p < 0.05). As a result of the simple inclination test, SOC proved to be effective under high levels of symptom distress; the buffering effect of sense of manageability was reversed regarding bodily pain; and when meaningfulness was lower, it had a positive effect on QoL.
Conclusion
This study revealed that SOC exerts a buffering effect in situations where symptoms are highly painful. It also revealed that the effect of SOC was reversed for bodily pain and that a high SOC had a negative effect on QoL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in chemotherapy mean that cancer patients will live longer; in fact, a study showed that more patients are being able to live owing to pharmacotherapy [1]. However, a recent Japanese report showed that the proportion of adverse reactions, complications, and sequelae of cancer treatment regarding chemotherapy have increased from 19.2% (in 2003) to 44.3% (in 2013) [2]. Supportive therapy is a type of therapy that can ensure enhanced quality of life (QoL) by focusing on how patients experience and perceive symptoms. Since symptoms are sensations specific to the patients who experience them, supportive therapy may be required for patients during recovery from some treatments, such as chemotherapy.
Moreover, most lung cancer patients experience stress [3, 4] and depression, both of which can reduce their health-related quality of life (HRQoL) and adversely affect the treatment of physical illnesses [5]. Depression and indication disorders have been associated with cancer patients and have shown a high prevalence (i.e., ranging from 9 to 42%), and an association with lower QoL, in this population [6,7,8]. Accordingly, research have analyzed potential early palliative care interventions to improve depression and QoL in cancer patients [9], albeit the mechanisms of this process are not yet known. Nevertheless, screening and specialized nursing care for distress and adverse reactions are important tasks requiring attention and funding.
Additionally, although we know that patients’ subjective perception of chemotherapy-related symptoms differs from that of medical personnel [10,11,12], the individual characteristics affecting these perceptions remain unclear. The Integrated Approach to Symptom Management (IASM) [13] was developed to ensure the clinical application of the Model of Symptom Management (MSM) [14]. Studies show the effectiveness of patient-centered management based on the IASM vis-à-vis patients’ experience of symptoms, mainly because it provides knowledge, skills, and nursing support that are aligned with patients’ self-care ability [15,16,17].
Sense of coherence (SOC) is a personal characteristic related to patients’ HRQoL. SOC is a key concept in Antonovsky’s health generation theory (salutogenesis), as it is an individual characteristic that affects patients’ perception of their symptoms [18]. It refers to people’s ability, when exposed to stressful events and situations, to protect their mental and physical health by effectively mobilizing internal and external resources. SOC is an important predictor of QoL because it is effective; studies have shown that patients who had a disease and a high SOC also had a QoL that was considered good [19]. Although we know that SOC is highly associated with anxiety and depression as an indicator of pain in cancer patients [20], the mechanisms have not been clarified.
SOC has both direct and moderating effects [21]; evidence on its direct effect showed that health and well-being vary by SOC level, regardless of the presence of a stressor. Thus, since the buffering effect of SOC on physical and mental health differs by SOC levels when encountering a similar stressor, we can predict that SOC will have different effects on mental and physical health depending on patients’ SOC levels for the stressor amid cancer chemotherapy.
In this study, we included outpatients who were diagnosed with a non-small cell lung cancer in the postoperative stage IA T1bN0M0 or stage IB, receiving standardized treatment with postoperative adjuvant chemotherapy, and using tegafur/uracil therapy (250 mg/m2/day, 1–2 years’ oral administration) [22, 23].
Purpose
This study is aimed at clarifying the moderating effect of SOC on the relationship between symptom distress and HRQoL in patients during cancer chemotherapy; we hope that these examinations can help stakeholders design a nursing care regime that is more efficient at dealing with the HRQoL of these patients.
Conceptual framework and definition of terms
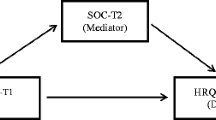
Conceptual framework (Fig. 1)
Our conceptual model captures the moderating effect of SOC [24]: similar stressors have different effects with different levels of SOC. Figure 1 represents the relationship among pharmacotherapy-induced symptom severity, HRQoL, and SOC.
Definition of terms
Symptom is not an objective index, but individuals’ subjective experience of symptoms.
Distress is an unpleasant experience that widens the difference between the ideal state and reality.
Symptom management is the process used by support staff to help patients deal with symptom distress.
HRQoL is individuals’ subjective judgments regarding their HRQoL (both mental and physical), including symptom experience factors.
Methods
Study design
This was an exploratory study, which was conducted as a pilot study as there are no prior studies that could be referenced regarding this specific approach. Nonetheless, its reliability and validity were confirmed using a major assessment scale in our examination. Therefore, we calculated the 95% confidence interval at 80% power and set it to 0.5 (Cohen’s d medium).
The first author got in touch with the head manager of medical facilities in Japan and asked for their cooperation with this study. Then, the attending physician at those facilities that agreed to participate introduced the first author to potential participants based on our selection. To avoid biases because of differences in treatment time and drugs (e.g., in treatment duration and in the time when side effects were strong owing to different treatment characteristics), we included only patients receiving standardized treatments as study participants.
Participants and period
Study participants were undergoing care at cancer treatment hospitals that were designated by the Ministry of Health, Labour, and Welfare or the prefectural governments in Japan. The following were the inclusion criteria:
-
1.
Diagnosed with non-small cell lung cancer after complete resection; postoperative stage IA T1bN0M0 or IB; and outpatients currently treated with postoperative adjuvant chemotherapy using tegafur and uracil combination therapy (UFT 250 mg/m2/day, 1–2 years’ oral administration)
-
2.
Aged 20 years or older; understood written Japanese; could complete a 15–20 min survey
-
3.
Started chemotherapy more than 3 months prior to study participation
-
4.
Judged by the attending physician to be able to cooperate with the study
The exclusion criteria were having serious mental or severe chronic illnesses.
Survey content and methods
Survey content
We asked participants about their age, gender, family status, occupation, income after treatment, problems during treatment, and sources of information about their illness and treatment from time of diagnosis. The following indices were used:
-
(a)
HRQoL
The Short Form-8 Health Survey, Japanese version (SF-8) [25], an internationally standardized, comprehensive, nonspecific survey with norm-based scoring adapted for Japanese people—and which is interpreted by comparing results with national standards—was used. It has eight items on the following topics: physical functioning (PF), bodily pain (BP), general health perception (GH), vitality (VT), social functioning (SF), role emotional (RE), role physical (RP), and mental health (MH). Each item is rated on either a 5- or 6-point subscale; the higher the score, the better the HRQoL. Results are calculated using a standardized scoring algorithm and two summary scores, the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores are also calculated. Each lower scale is rated out of 100; since previous studies have provided evidence on the standard value and standard deviation of the scores of average Japanese people at the national level, we compared participants’ scores with the 2010 national standard value. This also allowed for the examination of the representativeness of our sample. In this study, its Cronbach’s alpha was 0.919, indicating stability (Fig. 2).
-
(b)
Symptom distress during cancer chemotherapy
The Symptom Distress Scale (SDS) [26], Japanese version [27], was used to evaluate symptom distress (max score range 11–33). It has 11 factors—Nausea (range 1–9), Appetite (range 1–4), Insomnia (range 1–4), Pain (range 1–9), Fatigue (range 1–4), Bowel Patterns (range 1–4), Concentration (range 1–4), Appearance (range 1–4), Breathing (range 1–4), Outlook (range 1–4), and Cough (range 1–4)—and 13 more items, which were evaluated on a 7-point scale. Specifically, the scales of nine of these 13 items ranged from “normal or no distress for a given symptom” (1) to “representing extensive distress” (5), while that of four items (concerning the frequency and intensity of pain and nausea) ranged from “almost never/mild” (1) to “almost constantly/unbearable” (5). The higher the score, the greater the symptom distress. Its Cronbach’s alpha was 0.76, denoting that its criterion-related validity was verified. For this study, its Cronbach’s alpha was 0.678, indicating stability.
-
(iii)
SOC
SOC capacity was measured using the Japanese version [28] of the 13-item SOC Scale (SOC-13), a shortened version of the SOC-29 [29]. It includes the following three subscales: Sense of Comprehensibility (CO) (range 12–35), Sense of Manageability (MA) (range 10–26), and Meaningfulness (ME) (range 12–24); the subscales are rated on a 7-point scale. The higher the SOC score, the higher the capacity to maintain health conditions (max score range 46–79). Its Cronbach’s alpha coefficient for the total scale and its subscales ranged from 0.72–0.89, denoting that its criterion-related validity and predictive validity were verified.
Its three subscales measure the capacity to formulate an explanation for, and comprehend the source of, problems faced in daily life, as well as to project what may happen with those problems. Specifically, MA assesses whether people have sufficient resources to handle problems; ME assesses whether the problems people face are deemed as worthy of attempts to resolve, despite the potential hardships. In this study, its Cronbach’s alpha coefficient was 0.671, indicating stability.
Data collection
The survey was conducted between July 2016 and March 2017 on patients who consented to participate. Anonymous self-administered questionnaires were distributed to participants and received by post.
Analysis method
Descriptive statistics were calculated for each questionnaire item. The Mann–Whitney U test and Spearman’s rank correlation coefficient were used to determine participants’ characteristics and SF-8. Correlations among variables were examined between the SF-8, SDS, and SOC indices using Spearman’s rank correlation coefficient, with each scale item score set as a variable. Thereafter, hierarchical multiple regression analysis was performed to examine the direct and moderating effects of SOC.
Specifically, Step 1 included SF-8 as the dependent variable, and the control variables were age, time from surgery to start of chemotherapy, chemotherapy history, and the number of problems during treatment; in Step 2, symptom distress (i.e., the SDS) during cancer chemotherapy was included; in Step 3, SOC (i.e., the SOC-13) was included; finally, in Step 4, the interaction terms for SDS and SOC were included. A significance test of change in R2 (ΔR2) was conducted for each step, and the extent and direction of the effect on HRQoL was confirmed. The analysis used centralized variable scores to avoid multicollinearity between independent variables. Simple slope analysis was performed for significant interaction terms, using mean ± 1SD (standard deviation). SPSS ver. 26 for Windows was used for statistical analysis. Significance was set at p < 0.05.
Compliance with ethical standards
The bioethics review committee of each participating hospital approved this study. The survey was conducted at facilities that agreed to cooperate, and approval from the appropriate ethics committees of the target facilities was secured.
Candidates were selected by the attending physician, and after that physician introduced me to the candidate, I explained the purpose, methods, and confidentiality of the study both verbally and in writing. It was explained that the survey would be anonymous and that consent would be deemed granted by voluntary submission of the questionnaire via post. Participants were informed that their completed questionnaires would not be disclosed, that they would not be disadvantaged in any way regarding their medical treatment and nursing owing to participation, and that no details enabling identification would be released.
Results
Participants’ characteristics (Table 1)
The analysis included data from 66 participants (87 questionnaires were distributed, but 21 were not returned) participants who responded to the survey. Mean age was 69.45 ± 8.42 years, with 39 men (59.1%) and 27 women (40.9%; Table 1). Mean time from diagnosis to surgery was 1.97 ± 2.29 months, from surgery to chemotherapy was 1.48 ± 1.77 months, and chemotherapy history was 18.9 ± 8.9 months.
Overall, 57 (86.4%) participants lived with family members, and four (6.1%) reported that their economic situation had changed after their diagnosis. The most common problem during treatment was “burden on the spouse” (n = 17; 25.8%), followed by “distress regarding treatment-related symptoms” (n = 16; 24.2%), and “treatment and medical expenses” (n = 15; 22.7%). The mean number of problems during treatment was 1.70 ± 1.61. The most common source of information about their illness and treatment was “doctors, nurses, and pharmacists” (n = 55; 83.3%), followed by “family, friends, and acquaintances” (n = 25; 37.9%) and “books and journals” (n = 23; 34.8%).
HRQoL, SDS, and SOC indices (Table 1)
In the SF-8 (i.e., the HRQoL scale), the mean score was 48.61 ± 5.22 for the PCS and 49.46 ± 6.58 for the MCS (scores for BP, VT, and MH are listed in Table 1). The mean score for the SDS was 18.29 ± 5.03, with high scores in the Pain (2.48 ± 1.86), Outlook (2.02 ± 0.85), and Appetite (1.88 ± 0.90) subscales. The mean total score for the SOC-13 was 63.28 ± 8.98, and the score for the subscales was 26.90 ± 5.56 for CO, 18.32 ± 3.35 for MA, and 17.83 ± 2.84 for ME.
Correlation between participants’ basic attributes and HRQoL (SF-8)
A significant negative correlation was seen between age and PF (ρ = − 0.269, p < 0.05, Supplementary Table 1). Time from surgery to chemotherapy had a significantly negative correlation with PCS scores (ρ = − 0.295, p < 0.05). We also noticed significantly positive correlations of chemotherapy history with BP (ρ = 0.352, p < 0.01), GH (ρ = 0.320, p < 0.05), and PCS scores (ρ = 0.287, p < 0.05). The number of problems during treatment had a significantly negative correlation with GH (ρ = − 0.303, p < 0.05), VT (ρ = − 0.317, p < 0.01), MH (ρ = − 0.457, p < 0.01), and MCS scores (ρ = − 0.368, p < 0.01). The number of sources of information about their illness and treatment was not significantly correlated with any scale or subscale. There was also no significant correlation between any SF-8 item and whether the patient was living with family members or whether there was a decline in income after falling ill (Supplementary Table 2).
Correlation between SOC, SDS, and SF-8 (Table 2)
The scores for SDS were found to be strongly and negatively correlated to all SF-8 items. Moreover, the SOC-13 was positively correlated with the following subscales of the SF-8: MCS (ρ = 0.420, p < 0.01), general health perception (ρ = 0.467, p < 0.01), VT (ρ = 0.324, p < 0.05), SF (ρ = 0.346, p < 0.01), and MH (ρ = .470, p < 0.01).
Regarding SOC-13 subscales, SOC was positively correlated with the following subscales of the SF-8: MCS (ρ = 0.413, p < 0.01), Overall Sense of Health (ρ = 0.451, p < 0.01), VT (ρ = 0.330, p < 0.05), and MH (ρ = 0.441, p < 0.01). The MA subscale was weakly and positively correlated with the MCS (ρ = 0.298, p < 0.05), Overall Sense of Health (ρ = 0.345, p < 0.01), SF (ρ = 0.310, p < 0.05), and MH (ρ = 0.360, p < 0.01) subscales. Finally, the ME subscale was weakly and positively correlated with the MCS (ρ = 0.330, p < 0.01), PF (ρ = 0.308, p < 0.05), RP (ρ = 0.248, p < 0.05), VT (ρ = 0.273, p < 0.05), SF (ρ = .369, p < 0.01), and MH subscales (ρ = 0.279, p < 0.05).
Relationship between SDS and SOC with SF-8 (Table 3, 4)
Substantial changes were observed for the GH subscale of SF-8 in Step 1 (ΔR2 = 0.109, p < 0.01), Step 2 (ΔR2 = 0.303, p < 0.01), Step 3 (ΔR2 = 0.402, p < 0.01), and Step 4 (ΔR2 = 0.496, p < 0.01); these changes had a buffering effect on SDS and the MA subscale of the SOC (β = − 0.406, p < 0.01, β = 0.329, p < 0.05). Regarding interactions between the SDS and SOC-13, a simple slope analysis was performed, substituting the following adjusted variable: value ± 1SD.
SOC was effective even when SDS scores were low; however, when SDS scores were high, SOC became higher or else the HRQoL worsened further. For the MA subscale, the effects of SOC were reversed; MA was effective when SDS was high, whereas when SDS was low, the MA became lower and HRQoL became higher. For the ME subscale, SOC was effective even when SDS was low; however, when SDS was high, the ME became higher or else the HRQoL worsened further (Fig. 2).
Discussion
This study showed that the SDS had a strong native correlation with all items of SF-8 and that the SOC exerted a buffering effect in participants with high levels of pain; these results provide in-depth data on the HRQoL of cancer patients. Additionally, this study showed that the effect of SOC was reversed for BP and that high meaningfulness on SOC had a negative effect on HRQoL.
Since study participants were receiving chemotherapy as a postoperative aid and there were no adverse events related to pain owing to drug use, BP was considered chronic post-surgical pain (CPSP). Thus, we inferred that BP was a compound symptom, being associated with the side effects of cancer chemotherapy.
The International Pain Society [30] is defined CPSP as “pain lasting at least 3 months after surgery”; it develops in 10–50% of surgical patients and interferes in the daily life activities of 2–10% [31]; these surgical complications, thereby, cause the loss of QoL in many patients. The cause may relate to patients’ complex disease states, in which surgery-related factors (e.g., the type and degree of surgical invasion) associate with patient-related factors (e.g., mental and psychological factors and individual differences). In fact, many cancer patients who received active treatment have also experienced multiple symptoms, and the occurrence of multiple symptoms is associated with functional conditions and poor QoL [32]. A study examining the relationship between symptomatic experiences and QoL in patients undergoing outpatient chemotherapy has shown that the more surgeries patients undergo, the stronger their symptoms are [33]; another reported the importance of considering pain, malaise, and depression symptoms as being interrelated, not separated [34]. In addition to chemotherapy-specific symptoms, study participants seemed to recognize symptom experiences that reflected treatment history.
Significant interactions between SDS and SOC on BP and overall health were also observed, with SOC subscales exerting buffering effects under high SDS conditions. Specifically, the effect of people’s assessment on the manageability of a condition (i.e., the MA subscale) was reversed for body pain, and a lower meaningfulness for SOC had a positive effect on HRQoL. Since the sense of meaning (i.e., in this study, the ME subscale) is internally oriented and less susceptible to external environmental factors [35], it may affect people’s use of surrounding resources to deal with BP. During people’s cancer chemotherapy, people’s CPSP is not one of the side effects that often subjected to evaluation, so they may often not be a subject approached by medical care. Although SOC is considered a force to mobilize surrounding resources and cope well, it is possible that the pain does not improve and the QoL is low because patients cannot cope without the concern of medical personnel.
A previous study also revealed the symptom experience of, and the strategies used by, patients who experience chemotherapy-induced peripheral neuropathy; the authors reported that, although patients may not be able to respond to mental stress, they are prone to mental stress owing to the characteristics of the symptoms (i.e., they have no effective solution, as the symptoms cannot be easily relieved) [36]. This cited study further remarked the need for appropriate stress management interventions by medical personnel for cancer patients undergoing chemotherapy. In this regard, a study in patients with metastatic lung cancer showed that early palliative care intervention regarding coping and communication support may be the key to effective interventions [37]; another study found that patients with advanced cancer who received early palliative care showed a better coping approach and QoL and reported reduced depression [38]. These studies also remarked that medical personnel needed to be able to recognize grade 1–2 side effects of cancer chemotherapy, to note that there is no care solution that is effective at relieving such symptoms and to thereby ensure that proper supportive therapies (e.g., stress management, coping, and communication support) are provided to these patients. Thus, our results and prior research highlight that medical personnel could improve the accuracy of their future pain screenings by adding patients’ characteristics (i.e., patients’ SOC and HRQoL) into the assessment, alongside the evaluations regarding the side effects of cancer chemotherapy.
Limitations of the study and future directions
The first limitation is that this was a cross-sectional study; thus, the causal relationship between SOC and HRQoL has not been elucidated. Generally, a person’s SOC stabilizes around the age of 30 [39]; however, it can change through life experiences, and it is possible that patients’ experiences since the onset of cancer therapy increase their SOC. Second, study participants were patients who continued their therapy; thus, the SOC of patients who had to discontinue their therapy was not investigated. Hence, future longitudinal surveys are warranted to explore the relationship between cancer patients’ HRQoL and SOC, and the SOC characteristics of patients who discontinue their treatment; investigations regarding the relationship between HRQoL and SOC in patients undergoing cancer treatment with larger samples are also warranted.
Conclusions
We identified a moderating effect of SOC on the relationship between patients’ symptom distress and HRQoL during cancer chemotherapy. Thus, patients with low SOC need interactions with medical personnel that promote their SOC, as this may support their symptom management; moreover, medical staff should proactively engage with patients with high ME and provide them with social support accordingly. Since the method of approaching such patients has not been clarified in this study or past research, this topic is left for future examination.
Data availability
The manuscript has no associated data, or the data will not be deposited.
References
Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T (2015) 全国がん罹患モニタリング集計 2006-2008年生存率報告 [Monitoring of cancer incidence in Japan – survival 2006–2008 report]. Center for Cancer Control and Information Services, National Cancer Center, Tokyo. https://ganjoho.jp/reg_stat/statistics/dl/index.html#survival. Accessed 30-06-2020
Ishikawa M, Yamaguchi K (2016) 2013 がんと向き合った4054人の声(がん体験者の悩みや負担等に関する実態調査 報告書)[Voices of 4,054 people who faced cancer (survey on problems and burdens of cancer survivors: report)]. Shizuoka Cancer Center, Nagaizumi, Shizuoka
Mukai M, Oishi F, Onishi K (2012) 外来通院中の進行肺がん患者のストレス-コーピングとソーシャル・サポートの検討 [The study of the stress-coping of outpatients with advanced lung cancer and the social supports for them]. Mie Nurs J 14(1):29–39
Narisawa K, Sato F, Kashiwagura E, Sato N (2014) 外来で分子標的治療を受けるがん患者の症状体験とQOLの関連 [Relationship between symptom experience and QOL for cancer outpatients receiving molecular targeted therapy]. J Jpn Soc Cancer Nurs 28(3):5–12. https://doi.org/10.18906/jjscn.2014-28-3-5
Block SD (2000) Assessing and managing depression in the terminally ill patient. Ann Intern Med 132(3):209–2018. https://doi.org/10.7326/0003-4819-132-3-200002010-00007
Atalay NS, Selçuk ST, Akkaya N, Konukcu S, Kaya V, Şahin F (2011) Anxiety, depression and quality of life among breast cancer patients. J Rheumatol Med Rehabil 22(4):71–77
Ander M, Grönqvist H, Cernvall M, Engvall G, Hedström M, Ljungman G, Lyhagen J, Mattsson E, von Essen L (2016) Development of health-related quality of life and symptoms of anxiety and depression among persons diagnosed with cancer during adolescence: a 10-year follow-up study. Psychooncology 25(5):582–589. https://doi.org/10.1002/pon.3965
Lewandowska A, Rudzki G, Lewandowski T, Prochnicki M, Rudzki S, Laskowska N, Brudniak J (2020) Quality of life of cancer patients treated with chemotherapy. Int J Environ Res Public Health 17(19):6938. https://doi.org/10.3390/ijerph17196938
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ (2010) Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 363:733–742. https://doi.org/10.1056/nejmoa1000678
Uchino J, Hirano R, Tashiro N, Yoshida Y, Ushijima S, Matsumoto T, Ohta K, Nakatomi K, Takayama K, Fujita M, Nakanishi Y, Watanabe K (2012) Efficacy of aprepitant in patients with advanced or recurrent lung cancer receiving moderately emetogenic chemotherapy. Asian Pac J Cancer Prev 13(8):4187–4190. https://doi.org/10.7314/apjcp.2012.13.8.4187
Tamura K, Aiba K, Saeki T et al (2015) Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol 20(5):855–865. https://doi.org/10.1007/s10147-015-0786-7
Williams LA, Bohac C, Hunter S, Cella D (2016) Patient and health care provider perceptions of cancer-related fatigue and pain. Support Care Cancer 24(10):4357–4363. https://doi.org/10.1007/s00520-016-3275-2
Larson PJ, Uchinuno A, Izumi S, Kawano A, Takemoto A, Shigeno M, Yamamoto M, Shibata S (1999) An integrated approach to symptom management. Nurs Health Sci 1(4):203–210. https://doi.org/10.1046/j.1442-2018.1999.00027.x
The University of California, San Francisco School of Nursing Symptom Management Faculty Group (1994) A model for symptom management. Image J Nurs Sch 26(4):272–276. https://doi.org/10.1111/j.1547-5069.1994.tb00333.x
Arao H (2002) 症状マネジメントにおけるIASMの有効性の検討—がん性疼痛の症状マネジメントにおける比較から [Investigation of efficacy of IASM in symptom management—from the comparison in cancer pain symptom management]. Jpn J Nurs Res 35(3):213–227. https://doi.org/10.11477/mf.1681900673
Nishitani Y, Yuasa S, Hosomi Y, Kitayama N, Isomoto A, Nakano H, Uchinuno A (2017) 分子標的薬による皮膚障害の症状マネジメントの実態 [Clarification of patients’ skin reaction experience, strategies and outcomes associated with EGFR inhibitor treatment]. Univ Hyogo Coll Nurs Art Sci Res Inst Nurs Care People Commun Bull 24(1):93–103
Kikuta M, Shizu K, Adachi M, Oouchi S, Wakiguchi Y, Nakano H, Uchinuno A (2017) 薬物療法を受ける造血器腫瘍患者の口腔トラブルの実態とそのマネジメント [Actual condition and symptom management of oral complications in patients with hematologic malignancies receiving chemotherapy]. J Jpn Soc Cancer Nurs 31:155–164. https://doi.org/10.18906/jjscn.31_kikuta_20170925
Antonovsky A (1979) Health, stress, and coping. Jossey-Bass Publishers, San Francisco
Eriksson M, Lindström B (2007) Antonovsky’s sense of coherence scale and its relation with quality of life: a systematic review. J Epidemiol Community Health 61(11):938–944. https://doi.org/10.1136/jech.2006.056028
Winger JG, Adams RN, Mosher CE (2016) Relations of meaning in life and sense of coherence to distress in cancer patients: a meta-analysis. Psychooncology 25(1):2–10. https://doi.org/10.1002/pon.3798
Takayama T, Asano Y, Yamazaki Y, Yoshii K, Nagasaka Y, Fukada J, Furusawa Y, Takahashi S, Seki Y (1999) ストレスな生活出来事が首尾一貫感覚 (sense of coherence: SOC) と精神健康に及ぼす影響 [Sense of coherence, stressful life events and psychological health]. Nihon Koshu Eisei Zasshi [Jpn J Public Health] 46(11):965–976
Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, Ohta M, Japan Lung Cancer Research Group on Postsurgical Adjuvant Chemotherapy (2004) A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350(17):1713–1721. https://doi.org/10.1056/NEJMoa032792
Okimoto N, Soejima R, Teramatsu T (1996) 非小細胞肺がんに対する術後補助化学療法の検討 [A randomized controlled postoperative adjuvant chemotherapy trial of CDDP+VDS+UFT and UFT alone in comparison with operation only for non-small cell lung carcinomas-second study]. Haigan 36(7):863–871. https://doi.org/10.2482/haigan.36.863
Vahtera J, Pentti J, Uutela A (1996) The effect of objective job demands on registered sickness absence spells; do personal, social and job-related resources act as moderators? Work Stress 10(4):286–308. https://doi.org/10.1080/02678379608256809
Fukuhara T, Suzukamo Y (2004) SF-8 日本語版マニュアル第2版 [Japanese SF-8 manual version 2]. Institute for Health Outcomes & Process Evaluation Research, Tokyo
McCorkle R, Young K (1978) Development of a symptom distress scale. Cancer Nurs 1(5):373–378. https://doi.org/10.1097/00002820-197810000-00003
Okawa A, Muraki A, Ishikawa M, Onishi K (2004) 症状の苦痛尺度日本語版の信頼性・妥当性の検討 [Examination of the reliability and validity of the Japanese version of the McCorkle Symptom Distress Scale]. Mie Nurs J 6(1):49–55
Antonovsky A (1993) The structure and properties of the sense of coherence scale. Soc Sci Med 36(6):725–733. https://doi.org/10.1016/0277-9536(93)90033-Z
Yamazaki Y, Togari T, Sakano J (2008) Introduction to the sense of coherence in the salutogenic model. Yushindo-Kobunsya, Tokyo (In Japanese)
Task Force on Taxonomy of the International Association for the Study of Pain (1986) Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. IASP Press, Seattle
Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. Lancet 367(9522):1618–1625. https://doi.org/10.1016/s0140-6736(06)68700-x
Kim JEE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C (2009) A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manag 37(4):715–736. https://doi.org/10.1016/j.jpainsymman.2008.04.018
Narisawa K, Sato F, Kashiwagura E, Sato N (2014) Relationship between symptom experience and QOL for cancer outpatients receiving molecular targeted therapy. J Jpn Soc Cancer Nurs 28(3):5–12. https://doi.org/10.18906/jjscn.2014-28-3-5
Laird BJA, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KCH, Fallon MT (2011) Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manag 42(1):1–11. https://doi.org/10.1016/j.jpainsymman.2010.10.261
Takahashi K, Kato A, Igari T, Sase E, Shibanuma A, Kikuchi K, Nanishi K, Jimba M, Yasuoka J (2015) Sense of coherence as a key to improve homebound status among older adults with urinary incontinence. Geriatr Gerontol Int 15(7):910–917. https://doi.org/10.1111/ggi.12353
Nakano H, Takeda M, Matsuoka K (2020) Symptom management strategies chemotherapy-induced peripheral neuropathy in cancer patients. Univ Hyogo Coll Nurs Art Sci Res Inst Nurs Care People Commun Bull 27:25–38
Temel JS, Greer JA, El-Jawahri A et al (2017) Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 35(8):834–841. https://doi.org/10.1200/jco.2016.70.5046
Greer JA, Jacobs JM, El-Jawahri A et al (2018) Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. J Clin Oncol 36(1):53–60. https://doi.org/10.1200/jco.2017.73.7221
Antonovsky A (1987) Unravelling the mystery of health: how people manage stress and stay well. Jossey-Bass Publishers, San Francisco
Funding
This study was funded by the Yasuda Medical Foundation research grant (No. 2016Y-27).
Author information
Authors and Affiliations
Contributions
Conceptualization: Kaori Asaba, Akiko Okawa. Data collection: Kaori Asaba. Analyzing and interpreting data: Kaori Asaba. Writing–original draft preparation: Kaori Asaba. Writing–reviewing and editing: Kaori Asaba and Akiko Okawa. Approval of final draft: Kaori Asaba and Akiko Okawa.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
The Nagoya University Graduate School of Medicine and Hospital Bioethics Review Committee approved this study. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 287 kb)
Rights and permissions
About this article
Cite this article
Asaba, K., Okawa, A. Moderating effect of sense of coherence on the relationship between symptom distress and health-related quality of life in patients receiving cancer chemotherapy. Support Care Cancer 29, 4651–4662 (2021). https://doi.org/10.1007/s00520-021-06003-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06003-4