Abstract
Purpose
To assess the prevalence of bowel dysfunctions after treatment for gynaecological cancer and the impact on the quality of life.
Methods
We identified a cohort of 217 eligible women treated with radiotherapy (RT) with curative intention, alone or as combined treatment, for gynaecological malignancies at three institutions in Catalonia (Spain). Demographic, diagnosis and treatment modality were reviewed. Patients were sent validated questionnaires to assess bowel function and a set of questions asking on the changes after RT in bowel function, urinary function, sexuality, pain and lymphoedema.
Results
Questionnaires were returned by 109 patients (50.2%) with a mean age of 65 ± 11 years. Of them, 71.8% had been treated for endometrial cancer and 28.2% for cervical cancer. Overall, 42.7% of patients reported bowel dysfunction, affecting their quality of life in 36% of cases. Symptoms were more frequent in patients who had undergone external beam RT compared to brachytherapy. The most common symptom was defecatory urgency which was reported by more than 40% of patients according to the St Mark’s score, although it was less common in other questionnaires. Overall, faecal incontinence ranged between 10 and 15%, and usual loose stools and diarrhoea were reported by 13.5% and 5.1%, respectively.
Conclusion
Prevalence of bowel symptoms after treatment of gynaecological malignancies is high. A systematic evaluation using validated questionnaires should be performed in order to allow the decision-making process and also because there are a number of treatments available to improve the quality of life of cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few decades, the implementation of screening programmes and the improvements in the diagnosis and treatment, together with the increase in the life expectancy, have led to an increase in cancer survivors. The Institute of Medicine has defined survivorship as a separate phase in the treatment of cancer and has recommended following a survivorship care plan to improve medical treatment and outcomes in survivors [1].
A significant percentage of cancer survivors experience negative physical, social and emotional effects as a result of their cancer and its treatment. Many symptoms are transient, but others may become chronic and significantly affect the quality of life. Therefore, long-term follow-up is essential to provide treatment to improve symptoms and quality of life.
Outcomes for patients diagnosed with gynaecologic cancer have improved in line with the improvements in other cancers. For instance, a 5-year survival for cervical cancer jumped from 47% in 2000 to 56% in 2010 [2] and for uterine cancer from 52% in 1970 to 80% now [3]. There are studies investigating the psychosocial impact of treatment for these cancers, but there are few reports about the frequency, severity and range of physical issues that these patients may experience [4]. The PORTEC-2 trial [5] compared the outcomes after external beam radiotherapy (EBRT) and vaginal brachytherapy (BT) in patients with endometrial cancer concluding that brachytherapy was associated with better social functioning and lower symptom scores for diarrhoea, faecal leakage, the need to stay close to the toilet and limitation in daily activities because of bowel symptoms. In the EMBRACE study on MRI-guided BT in cervical cancer, grade 1–2 bowel morbidity was 28–33% during follow-up, with diarrhoea, flatulence and incontinence being the most prevalent symptoms [6].However, these studies did not evaluate bowel function using detailed and sensitive questionnaires, relying only on the EORTC-QLQ C30 questionnaire and subscales, and the physician-reported morbidity (CTCAE v.3.0.) in the second one.
Symptoms will depend on the treatments that the patient has undergone, and a proportion of them will receive pelvic radiotherapy which may induce pathophysiological changes in the organs within the radiation field. In a Swedish study [7], patients with gynaecologic cancer treated with pelvic RT between 1991 and 2003 experienced more bowel and urinary symptoms, lymphedema, sexual dysfunction and pelvic pain, when compared with a control group of non-irradiated women. However, more studies are required in view of the technological improvements that have been implemented the last years.
Gynaecological cancer treatment may include multiple modalities alone or in combination which may cause pelvic floor disorders. Bowel symptoms may be secondary to denervation after a radical hysterectomy, but RT may also play an important role. Among all the potential long-term side effects after pelvic RT, bowel dysfunction is the one with a greatest impact on quality of life [8, 9]. Most patients will experience a permanent change in their bowel habit after radiotherapy, which does not affect quality of life in a proportion of them, but in other patients may be more severe and interfere with daily activities [10]. Symptoms may include diarrhoea, mucus discharge, defecatory urgency, pain, tenesmus, faecal incontinence (FI) and bleeding. After treatment for gynaecological cancer, the need for assessment of a potential bowel dysfunction after the treatment is particularly relevant, given that women who may have had obstetric trauma in the past are at a higher risk for defecatory urgency and FI. In addition to the bowel symptoms, patients treated with pelvic RT may also experience urinary dysfunction [7, 11], sexual dysfunction [12,13,14,15], lymphedema [7, 16], bone fractures [17] and chronic pain [7, 18].
The prevalence of bowel symptoms in patients treated for gynaecological cancer may be underestimated for three reasons: (a) patients may not report their problems if they are not questioned directly about them because they may assume that are unavoidable consequences of cancer treatments (b) the RTOG scales, widely used by radiation oncologists may unintentionally minimize the impact of gastrointestinal symptoms on quality of life as they are classified as grade I or II toxicity, and (c) women may have pre-existing bowel symptoms which may worsen after radiotherapy. However, it is important to point out that the identification of the specific needs of this group of patients could contribute to develop a follow-up strategy to provide treatment if required. Moreover, the potential adverse effects after the treatment should be discussed with the patients as part of the informed consent process.
Therefore, the aim of our study was to assess the prevalence of bowel dysfunctions after treatment for gynaecological cancer and the impact on the quality of life.
Materials and methods
Study design
In December 2016, questionnaires were sent to patients treated for gynaecological malignancies whose treatment included radiotherapy between January 2010 and December 2013 at three institutions (Consorci Sanitari de Terrassa, Hospital Universitari Sant Joan de Reus and Hospital General de Catalunya). However, treatment potentially also included surgery and/or chemotherapy. Patients were sent a letter by mail explaining the purpose of the study along with the questionnaires and a prepaid return envelope. A second questionnaire set was sent 2 months later to those patients who did not respond.
Participants
The inclusion criteria were patients with gynaecological cancer (endometrial, cervical, vulvar, ovarian, vaginal) treated with radiotherapy (external beam radiotherapy and/or brachytherapy), with or without surgery and/or associated chemotherapy with curative intention.
The exclusion criteria were patients treated with palliative intention or disseminated disease.
Radiotherapy technique
Radiation therapy (RT) was performed according to the site of the primary disease and if RT was planned as radical or adjuvant.
In cervical cancers, the treatment was mainly performed in a radical way and consisted in concurrent chemoradiation including weekly cisplatin. Radiation consisted in a first step of EBRT to the pelvis ± lumbo-aortic lymph node area to a dose of 46–50.4 Gy (at 2–1.8 Gy/fr), followed by high-dose-rate BT in 4–5 fractions of 7 Gy or pulsed-dose-rate BT to a cumulative dose (EBRT+BT) of 80 to 90 Gy EQD2 according to the treatment institution.
In endometrial cancers, radiation treatment was mainly indicated in the adjuvant setting. After total hysterectomy or adjuvant chemotherapy, patients were treated with endocavitary high-dose-rate BT alone (usually 15–25 Gy in 3–5 fractions three times a week) or with a first step of EBRT to the pelvis (45 Gy at 1.8 Gy/fr), followed by a high-dose-rate BT boost to the vaginal cuff (13.5–15 Gy in 3 fractions). Chemotherapy was performed in patients with high-risk endometrial cancer or non-endometrioid histology.
For vulvar cancer, the pelvis was treated with EBRT in the adjuvant setting (45 Gy at 1.8 Gy/fr) according FIGO stage when there were positive nodes. For less frequent tumours such as vaginal cancer, the treatment was extrapolated from cervical cancer.
Demographic and clinical data
Patient charts were reviewed to collect data on age, time since the end of treatment, type of cancer and modalities of treatment.
Medical history that could contribute to symptoms of FI such previous deliveries, previous conditions causing diarrhoea such as irritable bowel syndrome, cholecystectomy, neurological conditions, diabetes mellitus, and anal, colorectal or pelvic floor surgery were also recorded.
Symptom and quality of life assessments
Selected questionnaires were agreed with five patients previously treated for gynaecological cancer who chose those that were considered more appropriate:
-
a)
Gastrointestinal Symptom Rating Scale (GSRS) [19]: a disease-specific instrument, including 15 items which combine into five symptom clusters: (reflux, abdominal pain, indigestion, diarrhoea and constipation). The original questionnaire was modified to become a self-administered questionnaire. Symptoms were rated in four possible categories: never, occasionally, frequently affecting QoL and causing important changes in QoL. Only items related to lower tract symptoms were analysed.
-
b)
Memorial Sloan-Kettering Cancer Center Bowel Instrument [20]: a validated questionnaire to assess bowel function after rectal resection. Each question has five possible answers: always, most of the time, sometimes, rarely and never. In our study, patients filled in the full score, but we analysed selected questions.
-
c)
St Mark’s incontinence score [21]: a validated questionnaire to assess the severity of FI. Each question has five possible answers: never, rarely, sometimes, weekly and daily.
-
d)
Faecal Incontinence Quality of Life (FIQL) [22]: a disease-specific tool designed to evaluate the impact of FI on four aspects of quality of life (lifestyle, coping behaviour, depression and self-perception and level of embarrassment).
-
e)
EORTC-QLQ C30 (validated version in Spanish) [23]: a tool designed to assess quality of life (QoL) in patients with cancer.
-
f)
EORTC-QLQ EN24 (validated version in Spanish) [24]: a disease-specific tool designed to assess QoL in patients with endometrial cancer.
-
g)
EORTC-QLQ CX24 (validated version in Spanish) [25]: a disease-specific tool designed to assess QoL in patients with cervical cancer. Patients with ovarian and vaginal cancer also filled in this questionnaire.
-
h)
FACT-V (validated version in Spanish) [26]: a disease-specific tool designed to assess QoL in patients with vulvar cancer.
-
i)
Visual analogue scale measuring the QoL ranging from 0 (worst) to 10 (best).
-
j)
Visual analogue scale measuring the impact on bowel symptoms on QoL ranging from 0 (nothing) to 10 (very much).
In addition, a set of questions was added to the validated questionnaires asking on the changes after RT in bowel function, urinary function, sexuality, pain and lymphedema (annex 1).
Regarding the responses of the first three questionnaires, only clinically relevant symptoms with an impact on QoL were taken into account to overcome the limitations of studies based on questionnaires. Therefore, data presented only includes those patients who reported their symptoms as follows:
-
a)
Gastrointestinal Symptom Rating Scale: patients rating symptoms as “frequently affecting QoL” or “causing important changes in QoL”.
-
b)
Memorial Sloan-Kettering Cancer Center Bowel Instrument: patients rating the symptom as “always” or “most of the time”.
-
c)
St Mark’s incontinence score: patients experiencing the symptom “weekly” or “daily”.
Results are presented using mean, SD and range for quantitative variables, and frequencies for qualitative variables. No statistical analysis was performed.
Ethical approval
The study was approved by the Ethics Committee of the Consorci Sanitari de Terrassa and the Hospital Universitari Sant Joan de Reus. Informed consent was not required by the first institution according to Spanish law but was required by the Ethics Committee from the second institution and obtained for their patients.
Results
Participants
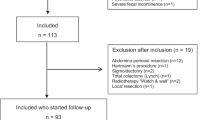
Between January 2010 and December 2013, 372 patients were treated with RT with curative intention for gynaecological malignancies at three institutions. Of them, 131 (35%) had died and 24 (6%) were lost to follow-up at the time of sampling. Therefore, the questionnaires were sent to 217 patients who fulfilled the inclusion criteria. Questionnaires were returned by 109 patients, giving a response rate of 50.2%. Clinical data of patients who returned the questionnaire as well as treatment modalities are shown in Table 1. Given that there were very few patients with vulvar, ovarian and vaginal cancer (three, two and two cases respectively), only data from patients with endometrial and cervical cancer were finally analysed.
Bowel dysfunction
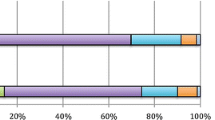
Bowel symptoms assessed by the GSRS questionnaire revealed that almost 27% of patients presented defecatory urgency “frequently affecting” or “causing important changes” in their QoL. More than 20% of patients complained of abdominal pain, almost 10% feeling of incomplete evacuation and FI (Fig. 1). Overall, the percentage of patients who rated the symptoms as “frequently affecting” or “causing important changes” in their QoL was higher in patients who had undergone EBRT with or without BT, compared with those who received BT alone. As an exception, the feeling of incomplete evacuation was higher in patients treated with BT.
When assessed by the MSKCC questionnaire, 15% altered their activities “always” or “most of the time” because of bowel function (Fig. 2). Defecatory urgency (“unable to wait 15 minutes to get to the toilet”) was the most common symptom (31%) followed by gas incontinence (20.6%), the sensation of incomplete emptying (19.8%) and fragmented defecation (9.3%). Regarding stool consistency, loose stools and diarrhoea “most of the time” or “always” were reported by 13.5% and 5.1% respectively. Similarly, the percentage of patients rating their symptoms as activities “always” or “most of the time” was lower in the group treated with BT alone.
The St Mark’s score (Table 2) showed defecatory urgency (defined as not being able to defer defecation more than 15 min) in 40.7% of cases. Overall, 28.9%, 11% and 14.5% of patients experienced daily or weekly gas incontinence, incontinence to liquid stool and incontinence to solid stool respectively. Defecatory urgency was higher in the BT group, but the percentage of patients with FI was higher in the EBRT group. The St Mark score was 6.0 ± 5.5 (0–24) for the overall series but increased to 13.6 ± 5.1 (8–24) for patients with weekly or daily incontinence to solid stools and to 14.7 ± 5.6 (8–24) for patients with weekly or daily incontinence to liquid stools.
Overall functional outcomes
Table 3 shows the subjective evaluation of changes after radiotherapy. It should be noted that more than 40% of patients reported bowel or sexual dysfunction, affecting their QoL in more than 35% of cases. Changes in urinary function, bone pain or lymphedema were over 30%.
Quality of life
Data on QoL measured by the EORTC-QLQ C30, the EORTC-QLQ EN24 and the EORTC-QLQ CX24 is shown in Table 4. Mean value of QoL on a visual scale ranging was 6.8 ± 2.2 (0–10). Mean value of the impact on bowel symptoms on QoL was 3.9 ± 3.4 (0–10), but 24.3% of patients scored between 7 and 10.
Discussion
Our main finding was that almost half of the patients treated for gynaecologic malignancies reported changes in their bowel function, with one in three rating these changes “affect their quality of life”.
The most common symptoms were defecatory urgency, feeling of incomplete evacuation, loose stools and FI. Our figures are similar to the results of a survey [15] completed by 1029 gynaecological cancer survivors who experienced bowel complaints in 42% of cases, and listed sexual, bowel and urinary dysfunctions as the three most important health concerns. A recent systematic review [27] concluded that the prevalence of pelvic floor disorders in gynaecological cancer population is very high. However, rates vary enormously due to differences in reported measures and timings. In patients treated for cervical cancer, the rate of FI and faecal urgency respectively varied between 2–34% and 3–49%. Concerning endometrial cancer, outcomes of the PORTEC-2 trial [28] reported higher FI and faecal urgency rates in patients who received EBRT (24% and 55%, respectively) than in those who were treated with BT (15 and 32%, respectively).
Studies [29, 30] have found that prevalence of FI was significantly higher in gynaecological cancer survivors compared to controls. However, both studies assessed anal continence by the Wexner score which does not take defecatory urgency into account, a very common symptom after radiotherapy.
Dunberger et al. [31] compared 616 women treated with pelvic radiotherapy alone or as combined treatment for gynaecological cancer, with 344 controls randomly recruited. Survivors experienced a higher occurrence of defecatory urgency and FI when compared to controls. Prevalence of defecatory urgency with faecal leakage was 49% among cancer survivors and 12% among controls. Another prospective study [32] reported 69% and 18% of women experiencing defecatory urgency and FI, respectively, 3 years after radiotherapy for gynaecological cancer using the LENT-SOMA scales.
The great variation of tools to assess bowel function and the use of non-validated questionnaires complicate the assessment of prevalence of bowel dysfunctions after gynaecological cancer treatment. This was the conclusion of a previous study of our group assessing bowel symptoms after prostate cancer treatment in which defecatory urgency remained undetected after radiotherapy treatment [33]. It should be noted that questionnaires frequently used, such as those from the EORTC, do not fully assess the bowel function. The EORTC-QLQ-C30 includes questions on bowel consistency but not on FI or defecatory urgency. The EORTC EN 24 contains questions about FI but not urgency, and the EORTC CX 24 does not include any question on bowel symptoms. Others such as the LENT-SOMA include enough questions about bowel function, and very few studies use specific questionnaires that are usually used in coloproctology such as the St Mark’s score which includes a question on defecatory urgency in addition to several on FI. In our study, there were also differences according to the different questionnaires that were used. Defecatory urgency was 40.7% in the overall group and more than 62% in the BT group assessed by the St Mark’s score, a specific score for FI. However, rates were lower when the GSRS and the MSKCC questionnaires where used (27–31% in the overall series). A potential explanation is that in the two last questionnaires we only considered the symptoms that were rated by the patients as “frequently affecting” or “causing important changes on QoL” and symptoms experienced “always” or “most of the time”. The percentage of patients with FI was also higher according to the St Mark’s score, in which symptoms are asked in greater detail. Other questions with a binary response such as wearing pads were similar in all questionnaires.
Women undergoing treatment for gynaecological cancer may present pre-existing bowel, urinary and/or sexual disorders which may worsen after the treatments. A cross-sectional study [34] in 186 women scheduled for surgery for gynaecological malignancy revealed urinary incontinence in 40.9% cases, FI in 3.9%, abdominal pain in 47.4% and diarrhoea in 20.1%. Barraclough et al. [32] reported defecatory urgency and FI in 25% and 4% respectively at baseline before any treatment. In a study [35] in which 43% of gynaecological cancer survivors reported anal incontinence, figures were surprisingly high in control patients (32%). More than 87% of women in our study had previous deliveries, with over 60% having obstetric risk factors for FI. Consequently, previous conditions together with ageing may contribute to the bowel dysfunction, specially defecatory urgency and FI. Therefore, FI in this patient group may be a consequence of different events that happen along life and underscore the importance of assessing bowel function before and after treatment for gynaecological cancer.
Our study has several limitations. Firstly, due to the retrospective nature of our study we lack a detailed baseline assessment, and therefore, it is impossible to be sure whether any of the bowel dysfunction reported was caused by the treatment. Another limitation is that the study population is heterogeneous, including patients with different cancers and treated with different modalities. Moreover, we did not correlate the mean radiation dose to organs at risk or the type of treatment with the dysfunctions, but the objective of our study was not to assess the risk factors in detail but to raise awareness of the need to proactively ask the patients. Finally, the response rate means we do not know how the 50% of non-responders had fared. It has been reported that patients in Spain historically have low response rates to questionnaires. However, due to the limitations of studies with questionnaires, only symptoms clinically relevant with an impact on QoL were taken into account in an attempt to identify those patients really affected by bowel dysfunction.
We would like to emphasize the importance of assessing bowel symptoms in cancer survivors. It has been previously reported that the prevalence may be higher than expected [32, 36], and they may have a significant impact on daily life and social functioning. A study [37] among gynaecological cancer survivors reported that patients with loose stools (37% experienced loose stools at least once a week) were more likely to experience defecatory urgency and FI compared to survivors without loose stools. This finding is consistent with reports in patients from general population in which diarrhoea was identified as the main independent risk factor for FI [38]. Assessing faecal consistency in these patients provides an opportunity to identify those patients who might benefit from medical treatment with methylcellulose and/or loperamide [39] or who may suffer from small intestine bacterial overgrowth and/or bile salt malabsorption which have potential specific treatments [40]. In the UK, practice guidance to manage gastrointestinal problems after treatment for cancer [40] have been endorsed by all gastrointestinal professional societies and shown to be beneficial in a randomized clinical trial [41].
Independently, Henson et al. [42] have shown that a structured gastroenterological evaluation benefits patients with gastrointestinal symptoms after pelvic RT. They concluded that a gastroenterological assessment identifies potentially treatable diagnoses in patients with chronic gastrointestinal symptoms leading to a significant improvement of symptoms. Moreover, patients with urgency and/or FI may be offered additional treatments such as biofeedback and/or neuromodulation.
Conclusions
The high prevalence of bowel symptoms after treatment of gynaecological malignancies suggests the need for systematic assessments. Moreover, given the high prevalence of pelvic floor symptoms in women, a baseline evaluation using validated questionnaires should be included before the treatment and taken into account for decision-making. Bowel dysfunctions, and pelvic floor problems in general, should be discussed openly with the patients because there are a number of treatments available to improve the quality of life of patient after cancer treatment.
References
Earle CC (2006) Failing to plan is planning to fail: improving the quality of care with survivorship care plans. J Clin Oncol 24:5112–5116. https://doi.org/10.1200/JCO.2006.06.5284
Vale CL, Tierney JF, Davidson SE, Drinkwater KJ, Symonds P (2010) Substantial improvement in UK cervical cancer survival with chemoradiotherapy: results of a Royal College of Radiologists’ audit. Clin Oncol R Coll Radiol G B 22:590–601. https://doi.org/10.1016/j.clon.2010.06.002
(2015) Uterine cancer survival statistics. In: Cancer Res. UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/survival. Accessed 24 Jul 2019
Salani R (2013) Survivorship planning in gynecologic cancer patients. Gynecol Oncol 130:389–397. https://doi.org/10.1016/j.ygyno.2013.05.022
Nout RA, Putter H, Jürgenliemk-Schulz IM et al (2009) Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol 27:3547–3556. https://doi.org/10.1200/JCO.2008.20.2424
Jensen NBK, Pötter R, Kirchheiner K, Fokdal L, Lindegaard JC, Kirisits C, Mazeron R, Mahantshetty U, Jürgenliemk-Schulz IM, Segedin B, Hoskin P, Tanderup K, EMBRACE Collaborative Group (2018) Bowel morbidity following radiochemotherapy and image-guided adaptive brachytherapy for cervical cancer: physician- and patient reported outcome from the EMBRACE study. Radiother Oncol 127:431–439. https://doi.org/10.1016/j.radonc.2018.05.016
Lind H, Waldenstrom AC, Dunberger G et al (2011) Late symptoms in long-term gynaecological cancer survivors after radiation therapy: a population-based cohort study. Br J Cancer 105:737–745. https://doi.org/10.1038/bjc.2011.315
Bergmark K, Avall-Lundqvist E, Dickman PW et al (2002) Patient-rating of distressful symptoms after treatment for early cervical cancer. Acta Obstet Gynecol Scand 81:443–450
Andreyev HJ, Wotherspoon A, Denham JW, Hauer-Jensen M (2011) “Pelvic radiation disease”: new understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol 46:389–397. https://doi.org/10.3109/00365521.2010.545832
Andreyev HJ (2007) Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol R Coll Radiol 19:790–799. https://doi.org/10.1016/j.clon.2007.08.011
Wit EM, Horenblas S (2014) Urological complications after treatment of cervical cancer. Nat Rev Urol 11:110–117. https://doi.org/10.1038/nrurol.2013.323
Frumovitz M, Sun CC, Schover LR, Munsell MF, Jhingran A, Wharton JT, Eifel P, Bevers TB, Levenback CF, Gershenson DM, Bodurka DC (2005) Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol 23:7428–7436. https://doi.org/10.1200/JCO.2004.00.3996
Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G (1999) Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 340:1383–1389. https://doi.org/10.1056/NEJM199905063401802
Jensen PT, Froeding LP (2015) Pelvic radiotherapy and sexual function in women. Transl Androl Urol 4:186–205. https://doi.org/10.3978/j.issn.2223-4683.2015.04.06
Westin SN, Sun CC, Tung CS, Lacour RA, Meyer LA, Urbauer DL, Frumovitz MM, Lu KH, Bodurka DC (2016) Survivors of gynecologic malignancies: impact of treatment on health and well-being. J Cancer Surviv 10:261–270. https://doi.org/10.1007/s11764-015-0472-9
Beesley V, Janda M, Eakin E, Obermair A, Battistutta D (2007) Lymphedema after gynecological cancer treatment : prevalence, correlates, and supportive care needs. Cancer 109:2607–2614. https://doi.org/10.1002/cncr.22684
Higham CE, Faithfull S (2015) Bone health and pelvic radiotherapy. Clin Oncol R Coll Radiol G B 27:668–678. https://doi.org/10.1016/j.clon.2015.07.006
Waldenstrom AC, Olsson C, Wilderang U et al (2011) Pain and mean absorbed dose to the pubic bone after radiotherapy among gynecological cancer survivors. Int J Radiat Oncol Biol Phys 80:1171–1180. https://doi.org/10.1016/j.ijrobp.2010.04.007
Svedlund J, Sjödin I, Dotevall G (1988) GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 33:129–134. https://doi.org/10.1007/BF01535722
Temple LK, Bacik J, Savatta SG et al (2005) The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Colon Rectum 48:1353–1365. https://doi.org/10.1007/s10350-004-0942-z
Vaizey CJ, Carapeti E, Cahill JA, Kamm MA (1999) Prospective comparison of faecal incontinence grading systems. Gut 44:77–80
Rockwood TH, Church JM, Fleshman JW et al (2000) Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Colon Rectum 43:7–9
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Greimel E, Nordin A, Lanceley A et al (2011) Psychometric validation of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Endometrial Cancer Module (EORTC QLQ-EN24). Eur J Cancer Oxf Engl 1990 47:183–190. https://doi.org/10.1016/j.ejca.2010.08.014
Greimel ER, Kuljanic Vlasic K, Waldenstrom A-C et al (2006) The European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer 107:1812–1822. https://doi.org/10.1002/cncr.22217
Janda M, Obermair A, Cella D, Perrin LC, Nicklin JL, Ward BG, Crandon AJ, Trimmel M (2005) The functional assessment of cancer-vulvar: reliability and validity. Gynecol Oncol 97:568–575. https://doi.org/10.1016/j.ygyno.2005.01.047
Ramaseshan AS, Felton J, Roque D, Rao G, Shipper AG, Sanses TVD (2017) Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J 29:459–476. https://doi.org/10.1007/s00192-017-3467-4
de Boer SM, Nout RA, Jürgenliemk-Schulz IM et al (2015) Long-term impact of endometrial cancer diagnosis and treatment on health-related quality of life and cancer survivorship: results from the randomized PORTEC-2 trial. Int J Radiat Oncol 93:797–809. https://doi.org/10.1016/j.ijrobp.2015.08.023
Neron M, Bastide S, de Tayrac R et al (2019) Impact of gynecologic cancer on pelvic floor disorder symptoms and quality of life: an observational study. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-38759-5
Rutledge TL, Heckman SR, Qualls C et al (2010) Pelvic floor disorders and sexual function in gynecologic cancer survivors: a cohort study. Am J Obstet Gynecol 203:514.e1–514.e7. https://doi.org/10.1016/j.ajog.2010.08.004
Dunberger G, Lind H, Steineck G et al (2010) Self-reported symptoms of faecal incontinence among long-term gynaecological cancer survivors and population-based controls. Eur J Cancer 46:606–615. https://doi.org/10.1016/j.ejca.2009.10.023
Barraclough LH, Routledge JA, Farnell DJJ, Burns MP, Swindell R, Livsey JE, Davidson SE (2012) Prospective analysis of patient-reported late toxicity following pelvic radiotherapy for gynaecological cancer. Radiother Oncol 103:327–332. https://doi.org/10.1016/j.radonc.2012.04.018
Bonet M, Cayetano L, Nunez M et al (2017) Assessment of acute bowel function after radiotherapy for prostate cancer: is it accurate enough? Clin Transl Oncol 20:576–583. https://doi.org/10.1007/s12094-017-1749-4
Bretschneider CE, Doll KM, Bensen JT, Gehrig PA, Wu JM, Geller EJ (2016) Prevalence of pelvic floor disorders in women with suspected gynecological malignancy: a survey-based study. Int Urogynecol J 27:1409–1414. https://doi.org/10.1007/s00192-016-2962-3
Rutledge TL, Heckman SR, Qualls C et al (2010) Pelvic floor disorders and sexual function in gynecologic cancer survivors: a cohort study. Am J Obstet Gynecol 203:514.e1–514.e7. https://doi.org/10.1016/j.ajog.2010.08.004
Andreyev J (2007) Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol 8:1007–1017. https://doi.org/10.1016/S1470-2045(07)70341-8
Dunberger G, Lind H, Steineck G, Waldenström AC, Onelöv E, Avall-Lundqvist E (2011) Loose stools lead to fecal incontinence among gynecological cancer survivors. Acta Oncol 50:233–242. https://doi.org/10.3109/0284186X.2010.535013
Bharucha AE, Zinsmeister AR, Schleck CD, Melton LJ 3rd (2010) Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology 139:1559–1566. https://doi.org/10.1053/j.gastro.2010.07.056
Ribas Y, Muñoz-Duyos A (2018) Conservative treatment of severe defecatory urgency and fecal incontinence: minor strategies with major impact. Tech Coloproctol 22:673–682. https://doi.org/10.1007/s10151-018-1855-5
Andreyev HJ, Davidson SE, Gillespie C, Allum WH, Swarbrick E, British Society of Gastroenterology, Association of Colo-Proctology of Great Britain and Ireland, Association of Upper Gastrointestinal Surgeons, Faculty of Clinical Oncology Section of the Royal College of Radiologists (2012) Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 61:179–192. https://doi.org/10.1136/gutjnl-2011-300563
Andreyev HJ, Benton BE, Lalji A et al (2013) Algorithm-based management of patients with gastrointestinal symptoms in patients after pelvic radiation treatment (ORBIT): a randomised controlled trial. Lancet. https://doi.org/10.1016/S0140-6736(13)61648-7
Henson CC, Davidson SE, Ang Y, Babbs C, Crampton J, Kelly M, Lal S, Limdi JK, Whatley G, Swindell R, Makin W, McLaughlin J (2013) Structured gastroenterological intervention and improved outcome for patients with chronic gastrointestinal symptoms following pelvic radiotherapy. Support Care Cancer 21:2255–2265. https://doi.org/10.1007/s00520-013-1782-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. We have full control of all primary data and we agree to allow the journal to review our data if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ribas, Y., Bonet, M., Torres, L. et al. Bowel dysfunction in survivors of gynaecologic malignancies. Support Care Cancer 28, 5501–5510 (2020). https://doi.org/10.1007/s00520-020-05402-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05402-3