Abstract
Purpose
Fatigue is a common problem among rectal cancer patients and can affect their quality of life. This study conducted a systematic review to better understand changes in fatigue severity in rectal cancer patients before, during, and after they undergo therapy.
Methods
We used preset keywords to search the Cochrane Library, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PubMed, and ProQuest databases for relevant studies published between 2000 and 2018, and data analysis was performed using Comprehensive Meta-Analysis (CMA) software (version 2.2.048) and SPSS software (version 19.0). In total, nine articles with complete data were included in our meta-analysis.
Results
Fatigue conditions were compared before the start of therapy (baseline) and at 1 month (time 1), 3 months (time 2), 6 months (time 3), and 12 months (time 4) after the start of therapy. The standardized mean differences (SMDs) of the pooling effects size were 1.013 (95% confidence interval (CI) 0.217–1.810), − 0.551 (95% CI − 0.647 to − 0.456), − 0.330 (95% CI − 0.427 to − 0.233), and − 0.149 (95% CI − 0.221 to − 0.078), respectively. Subsequent analysis with a linear mixed effect model revealed that the estimate of the time variable was − 0.226 (p = 0.047), which indicates that the severity of fatigue varies over time and over the course of treatment. The results reveal that fatigue affects rectal cancer patients even before they start therapy.
Conclusion
Although fatigue worsened during the first month after cancer therapy, it gradually improved thereafter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue is a physical and mental symptom that often affects cancer patients during the course of their disease or treatment. Over 75% of cancer patients suffer from fatigue [1], including feeling weak or heavy-limbed and/or being unable to execute daily activities or maintain focus. This cancer-related fatigue is not simply the feeling of fatigue; it may not be directly associated with the patient’s activity level, and it cannot be relieved with rest or sleep. As a result, fatigue can be even more troubling for cancer patients than pain or nausea [2]. At present, many types of cancer are not immediately fatal, but patients may nonetheless suffer from a morbid chronic fatigue known as cancer-related fatigue, which can disrupt or delay therapy [3, 4]. Despite the seriousness of the condition, fatigue has been overlooked as a quality of life indicator in the past, and fatigue can even severely affect cancer prognosis [5].
Rectal cancer patients also often commonly experience fatigue, which can lead to vertigo, tiredness, or exhaustion during therapy [6]. Nevertheless, for the most part, fatigue in rectal cancer patients remains underestimated and improperly treated. When fatigue is detected early and treated properly, the discomfort experienced by cancer patients can be relieved and their quality of life can be improved [7].
The forms of treatment for rectal cancer are manifold and complex and can have a strong impact on fatigue [7]. The conventional first-line treatment for non-metastatic rectal cancer is surgical resection. Tumors that are smaller in size and located higher in the rectum can be successfully resected, at which point the rectum can be directly reconnected to the colon. However, rectal cancers that are within 7 cm of the anus require abdominal-perineal resection, which means that a colostomy, a surgical procedure that creates a stoma through the abdominal wall, will be required afterwards. The latest treatment methods allow tumors which are located approximately 5 cm from the anus to be resected with a higher chance of retaining the anus [8]. Furthermore, neoadjuvant concurrent chemoradiotherapy (CCRT) can be administered before surgical resection to shrink the tumor and increase the chance of success. Patients receiving neoadjuvant CCRT must undergo radiotherapy and concurrent chemotherapy for about 5 weeks. Surgical resection is then performed following assessment, between 1 and 2 months after the patient receives neoadjuvant CCRT [9]. A complete round of treatment generally includes four rounds of chemotherapy [10]. Post-surgical adjuvant CCRT is available to patients who cannot receive radical surgery due to poor physical condition or multiple comorbidities. Depending on the pathologic stage and other risk factors, patients that did not undergo adjuvant radiotherapy before surgery may be eligible for chemotherapy and radiotherapy to reduce the chance of local recurrence [11].
Only by understanding the changes in the fatigue of rectal cancer patients throughout their treatment can we assist patients with the discomfort brought on by fatigue as well as provide more active fatigue care treatments to improve their quality of life. The objective of this meta-analysis was to not only gain an in-depth understanding of the severity of fatigue that rectal cancer patients suffer from at different points before and after therapy but also to explore the severity of fatigue during each stage of therapy.
Materials and methods
Empirical literature search
We performed a systematic literature search of articles published between 2000 and 2018 on four databases: the Cochrane Library, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PubMed, and ProQuest. Our search focused on four groups of keywords: rectal cancer/cancer of the rectum, before neoadjuvant therapy/neoadjuvant chemoradiotherapy/neoadjuvant chemotherapy/neoadjuvant radiotherapy/surgery, after neoadjuvant therapy/neoadjuvant chemoradiotherapy/neoadjuvant chemotherapy/neoadjuvant radiotherapy/surgery, and fatigue/tiredness/lack of energy. Our search was limited to studies that were published in English and involved humans. Studies were excluded from our meta-analysis if they (1) involved subjects aged 18 or under, (2) involved subjects with recurrent rectal cancer, or (3) were argumentative or retrospective in nature. Finally, we used retrospective literature and our search results to look for other study papers that fit our criteria.
Evaluating the quality of literature
The articles identified through the search were evaluated by two reviewers using appraisal criteria for non-randomized experimental studies from the Joanna Briggs Institute [12]. The appraisal criteria included nine assessment items: whether the sample was representative of patients in the population as a whole, whether the patients were at a similar point in the course of their condition/illness; whether bias had been minimized in selecting cases and controls, whether confounding factors had been identified and whether the strategies used to address them had been explained; whether outcomes were assessed using objective criteria; whether follow-up was carried out over a sufficient time period; whether the outcomes of people who withdrew were described and included in the analysis, whether outcomes were measured in a reliable way; and whether appropriate statistical analysis was performed. The results of each assessment were either yes, no, unclear, or not applicable. An article was only awarded 1 point if the result of an assessment item was yes; no points were awarded for any other result. Only articles with a total score of 4 or higher were included in our analysis. To determine the degree of consistency between the assessments of the two reviewers, the Kappa coefficient of agreement was calculated using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
Data analysis
Data analysis was performed using Comprehensive Meta-Analysis (CMA) software, version 2.2.048, and SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). Before analyzing the results, we tested the heterogeneity of the collected articles using the Cochran’s Q test to determine whether a fixed or random effect model should be used in calculating pooling effect sizes. We then conducted a sensitivity analysis to determine whether the elimination of any article would have an impact on the overall results. We used standardized mean differences (SMDs) and 95% confidence intervals to determine the statistical effects. If the 95% confidence intervals did not include 0, then the results were deemed to have statistical meaning. We also used a forest plot to display pooling effect sizes and 95% confidence intervals. Finally, we used a linear mixed effect model to examine changes in the severity of fatigue at different time points, as follows. The baseline time point was before the start of therapy; the other time points were 1 month after the start of therapy (time 1), 3 months after the start of therapy (time 2), 6 months after the start of therapy (time 3), and at the end of therapy (time 4; i.e., 12 months after the start of therapy).
Results
Number and quality of studies included in the meta-analysis
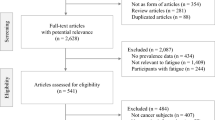
We obtained a total of 831 studies from the four databases. Repetition led to the elimination of 432 studies. After reading the titles and abstracts of the remaining 399 studies, we selected and read 100 candidate studies in their entirety. Upon so doing, 91 more studies were eliminated for the following reasons: 24 already involved interventions to deal with fatigue, 13 focused on non-rectal or multiple cancers, five used a cross-sectional design with single time points, 38 involved cancer therapies that had already begun, two examined patients with recurrent rectal cancer, six were systematic reviews, and three were not written in English or their full text could not be accessed. In the end, our meta-analysis included nine articles (Table 1), each of which had a quality assessment score between 6 and 8 points (Table 2). The Kappa coefficient of consistency between the two reviewers was 0.821 (p < 0.001). Table 3 displays the complete data presented by studies included in the meta-analysis. Although a total of nine studies were included in our meta-analysis, the time points at which each study collected their data varied. There were four groups for the comparison between the baseline and time 1, 3 groups for the comparison between the baseline and time 2, 12 groups for the comparison between the baseline and time 3, and eight groups for the comparison between the baseline and time 4.
Heterogeneity test, sensitivity analysis, and pooling effect size of baseline and time 1
The heterogeneity test revealed statistically significant differences (p < 0.001) among the four studies, with the percentage of variation due to heterogeneity (I2) calculated at 96.78%. This indicates that these four studies share a high degree of heterogeneity; thus, we adopted the random effect model. Sensitivity analysis results (Figs. 1 and 2) revealed that eliminating the data from Pucciarelli [15] led to a significant change in the mean effect value; it soared to 1.013. This indicates that Pucciarelli [15] was significantly different from the other studies, and that the data contains influential extreme values. As a result, we eliminated Pucciarelli [15] and then re-analyzed the pooling effect size to enhance the accuracy of our meta-analysis. Overall, the SMD of the pooling effect size was 1.013, and the 95% confidence interval ranged from 0.217 to 1.810, which reveals that the fatigue experienced by patients at time 1 was much more severe than the fatigue that they experienced at baseline (p = 0.013) (Fig. 3).
Sensitivity analysis of fatigue data before, during, and after therapy. Blue square SMD of single study; — 95% CI; red diamond combined effect size after meta-analysis. SMD, standardized mean difference; 95% CI, 95% confidence interval; CT, chemotherapy; RT, radiotherapy; AR, anterior resection; APE, abdominoperineal extirpation; APR, abdominoperineal resection; LAR, low anterior resection
Forest plots illustrating differences in fatigue before, during, and after therapy. Blue square SMD of single study; — 95% CI; red diamond combined effect size after meta-analysis. SMD, standardized mean difference; 95% CI, 95% confidence interval; CT, chemotherapy; RT, radiotherapy; AR, anterior resection; APE, abdominoperineal extirpation; APR, abdominoperineal resection; LAR, low anterior resection
Heterogeneity test, sensitivity analysis, and pooling effect size of baseline and time 2
The heterogeneity test did not reveal any statistically significant differences (p = 0.689) among the three studies, with the percentage of variation due to heterogeneity (I2) calculated at 0.00%. This indicates that no heterogeneity exists among these three studies; thus, we adopted the fixed effect model. Sensitivity analysis results (Fig. 2) showed that the research data did not contain extreme values that impacted the mean effect value; therefore, all three studies were included in pooling effect size analysis. The SMD of the pooling effect size was − 0.551, and the 95% confidence interval ranged from − 0.647 to − 0.456, which reveals that the fatigue experienced by patients at time 2 was much less severe than the fatigue experienced by patients at baseline (p < 0.001) (Fig. 3).
Heterogeneity test, sensitivity analysis, and pooling effect size of baseline and time 3
The heterogeneity test revealed statistically significant differences (p = 0.001) among the 12 studies, with the percentage of variation due to heterogeneity (I2) calculated at 65.94%. This indicates that heterogeneity exists among these 12 studies; thus, we adopted the random effect model. Sensitivity analysis results (Fig. 2) showed that the research data did not contain extreme values which significantly impacted the mean effect value; thus, all of the studies were included in pooling effect size analysis. The SMD of the pooling effect size was − 0.330, and the 95% confidence interval ranged from − 0.427 to − 0.233, which reveals that the fatigue experienced by patients at time 3 was greatly improved compared to the fatigue that patients experienced at baseline (p < 0.001) (Fig. 3).
Heterogeneity test, sensitivity analysis, and pooling effect size of baseline and time 4
The heterogeneity test did not reveal any statistically significant differences (p = 0.574) among the eight studies, with the percentage of variation due to heterogeneity (I2) calculated at 0.00%. This indicates that no heterogeneity exists among these studies; thus, we adopted the fixed effect model. Sensitivity analysis results (Fig. 2) showed that the research data did not contain extreme values which significantly impacted the mean effect value, so all eight of these studies were included in pooling effect size analysis. The SMD of the pooling effect size was − 0.149, and the 95% confidence interval ranged from − 0.221 to − 0.078, which reveals that the fatigue experienced by patients at time 4 was greatly improved compared to the fatigue that patients experienced at baseline (p < 0.001) (Fig. 3).
Results of pooling effect size and linear mixed effect model
As described earlier, comparing fatigue conditions at time 1, time 2, time 3, time 4, and baseline revealed significant differences in the SMDs of pooling effect sizes. As shown in Fig. 4, the severity of fatigue changed significantly over time. In considering the heterogeneity among the different studies, we weighted studies according to their importance and then used a linear mixed effect model to analyze the severity of fatigue experienced by rectal cancer patients at different time points before, during, and after therapy. The dependent variable of this model was the comparative effect size of fatigue scores reported by the various studies at different time points before and during therapy, while the independent variable was time. The residual weights were the weighted percentages of the various time points in the studies. In considering the heterogeneity among the different studies, we weighted studies according to their importance and then used a linear mixed effect model to analyze the severity of fatigue experienced by rectal cancer patients at different time points before, during, and after therapy. The estimate of the time variable was − 0.226 and was statistically significant (p = 0.047), which indicates that, overall, the severity of fatigue changed over the course of therapy.
Discussion
The results of our meta-analysis indicate that rectal cancer patients experience different fatigue conditions before, during, and after therapy. Although fatigue became more severe in the first month after the start of cancer therapy, it gradually improved and, at 3 months, 6 months, and 1 year after the start of therapy, was less severe than it had been before the start of therapy.
In the past, researchers speculated that the invasion of cancer cells in the rectum of rectal cancer patients caused bleeding, which led to anemia. Pre-treatment anemia prevented the blood from transporting oxygen normally, thereby keeping the bodies of these patients in an oxygen-deficient state that made them tire easily and suffer from poor physical strength [22]. Although the mechanisms which underlie fatigue in cancer patients are still not completely clear, it is certain that the fatigue these patients experience is highly associated with three processes induced by the pro-inflammatory signals and cytokines of tumors: the hypothalamic–pituitary–adrenal (HPA) axis suppressing cortisol secretion via important endocrine systems [23], hematopoietic stem cells being affected and causing anemia [24], and the metabolism of neurotransmitters (5-HT) changing [25]. A number of other major factors also directly and indirectly cause fatigue in cancer patients, such as the range of the tumor itself, the physical and mental stress caused by cancer treatment, and any pre-existing pain, sleep disorders, or depression. All of these may cause the body to generate immune inflammation and neuroendocrine hormone responses, which lead to fatigue-related symptoms [26]. Our meta-analysis revealed that although rectal cancer patients experienced a temporary increase in fatigue during the first month of their treatment, the fatigue that they experienced at 3 months, 6 months, and 12 months after the start of therapy was less severe than the fatigue experienced before the start of therapy.
Many studies have investigated cancer-related fatigue and its factors, and they unanimously point out that fatigue is common during cancer treatment. In addition to being a side effect of cancer treatment, fatigue may also be associated with the physiological and psychosocial state of the patient during the treatment process [27,28,29]. In contrast, little research has been done on post-diagnosis, pre-treatment fatigue. Goedendorp et al. [30] evaluated fatigue in 179 cancer patients before the start of treatment and discovered that approximately 1/4 of these patients already suffered from severe fatigue caused by low physical activity, depression, and impaired sleep and rest. These patients expressed that their fatigue symptoms had existed as long as a year prior to their diagnosis. These and the immune inflammation and neuroendocrine hormone responses generated by the body are highly associated with the symptoms of cancer-related fatigue [31].
When cancer patients are just starting cancer therapy, they often display obvious fatigue, depression, and deteriorated physical activity and function [32]. Research has shown that the symptoms which result from a patient’s first round of chemotherapy are often the most severe [33]. Surgery is the primary treatment for rectal cancer; however, stage II and stage III patients may also undergo pre-surgical CCRT to reduce the chance of recurrence, increase the resection rate, and lower the likelihood that a permanent artificial anus will be required. Pre-surgical CCRT can also reduce the likelihood that post-surgical radiotherapy will cause acute intestinal toxicities such as intestinal fibrosis or intestinal stenosis [34]. When rectal cancer patients are just starting cancer therapy, they experience various types of physical and mental stress, which adds to their fatigue [35]. Our meta-analysis confirmed that patients feel increased fatigue during the first month of treatment, which is then significantly reduced later. Some studies have also reported that, as cancer patients undergo therapy, the generation or increased accumulation of metabolic waste from damaged cells causes fatigue. However, this fatigue is temporary and gradually dissipates during the course of treatment [14, 36].
As fatigue is a common and serious problem for cancer patients, methods which can effectively evaluate and treat this condition would be extremely beneficial [37]. During treatment, cancer patients often display symptoms of both fatigue and depression. Although the mechanisms by which fatigue and depression develop are different, fatigue can severely affect the ability of cancer patients to enjoy everyday life. Fatigue is often accompanied by depression, and a positive correlation exists between the two. Depression also affects the ability of patients to perform daily activities, which makes it difficult to differentiate fatigue from depression. Thus, only with comprehensive assessments can symptoms be confirmed to be caused by cancer-related fatigue [38]. Consequently, the fatigue in cancer patients is currently assessed using questionnaires with good credibility and validity. One assessment tool specifically for fatigue in our meta-analysis was the Cancer Fatigue Scale (CFS) [39], which is a self-reported assessment scale for multiple aspects of fatigue and its symptoms. Containing 15 assessment items divided into three subscales (namely physical, affective, and cognitive), the CFS is a simple assessment scale and takes only 2 min to complete. It has only a small number of question items, is easy to use, and has high internal consistency with Cronbach’s α = .88 as well as good construct validity and content validity [40]. However, the CFS was developed for Japanese cancer patients, and it cannot assess the degree to which fatigue interferes with the daily activities of cancer patients. More cross-cultural studies will be needed for verification [41].
Various quality of life questionnaires also encompass fatigue in their subscales to examine physiological and psychological aspects of health. The studies examined in our meta-analysis used the fatigue score from the MD Anderson Symptom Inventory (MDASI) and the European Organization for Research and Treatment of Cancer (EORTC). The MDASI was developed by the MD Anderson Cancer Center to gauge the severity to which cancer symptoms agitated cancer patients and affected their functionality and activities of daily life. It is a multidimensional symptom assessment tool containing 13 symptom items, including pain, fatigue, nausea, disturbed sleep, distress/feeling upset, shortness of breath, difficulty remembering, a lack of appetite, drowsiness, dry mouth, sadness, vomiting, and numbness/tingling. The MDASI has high internal consistency (Cronbach’s α 0.76 to 0.91) and construct validity [42]. While it does not have many question items and is easy to read, it only has one question item regarding fatigue (“Your fatigue (tiredness) at its WORST?”) and lacks a multi-aspect assessment of fatigue. The EROTC uses the QLQ-C30 questionnaire, which is a 30-item scale regarding quality of life. The QLQ-C30 contains five functional scales and three symptom scales, the latter of which includes fatigue, pain, and nausea and vomiting. The internal consistency of the questionnaire is 0.72 [43], and there are three question items involving fatigue (“Were you tired?”, “Have you felt weak?”, and “Do you need rest?”). Knobel et al. found a high correlation between the item scores in the fatigue scale of the QLQ-C30 and the Fatigue Questionnaire (FQ) (r = 0.67–0.75). However, for cancer patients receiving palliative care, they observed a ceiling effect that may prevent the detection of cancer patients suffering from severe fatigue [44].
Aside from the scales used in this meta-analysis, clinical studies have also used a linear scale called the visual analogue fatigue scale (VAFS) to evaluate the fatigue conditions of cancer patients. This scale involves a straight horizontal line 10 cm in length. The left end of the line is 0, indicating a complete absence of fatigue, whereas the right end of the line represents the severest fatigue imaginable. Patients mark where the level of fatigue they feel on the line. It is the simplest method of measurement and can be used to monitor a patient’s fatigue throughout the day, thereby giving an understanding of the changes in the patient’s fatigue at any time [45]. Another scale is the multidimensional fatigue inventory (MDI), which uses five dimensions, namely, general fatigue, physical fatigue, reduced motivation, reduced activity, and mental fatigue, to perform a comprehensive and effective evaluation of the severity of fatigue symptoms in cancer patients and the severity of its impact on the daily activities and abilities of cancer patients [46]. Other scales include the Piper Fatigue Scale (PFS), the Fatigue Symptom Inventory (FSI), and the Functional Assessment of Cancer Therapy-Fatigue scale (FACT-F), which have been employed by different studies to assess the severity of fatigue in cancer patients [47].
Limitations
The process from initial diagnosis to treatment is very lengthy for rectal cancer patients. The causes of fatigue in these patients are numerous and complex, and our meta-analysis did not control all of the factors that may exacerbate fatigue. Malnutrition and anemia are particularly common in rectal cancer patients during therapy and may contribute to fatigue. In another aspect, whether rectal cancer patients undergo Enhanced Recovery after Surgery (ERAS) before they receive therapy may also affect the severity of fatigue during therapy. However, none of the nine articles included in our meta-analysis mentioned whether any of the research participants underwent ERAS or how the participants were nutrition-wise, and only one article had a rectal cancer patient with anemia. Thus, it was difficult to control the malnutrition or anemia conditions of the samples in the meta-analysis. We therefore suggest that more articles be collected in future studies to understand the crucial factors of heterogeneity and that subgroup analysis be performed to control the malnutrition and anemia conditions of the samples in the meta-analysis. This will give a better understanding of the fatigue conditions in rectal cancer patients prior to therapy. Furthermore, due to the co-existence and mutual influence of fatigue and depression in cancer patients, we could not verify whether the rectal cancer patients involved in our meta-analysis had been correctly assessed as having cancer-related fatigue.
Conclusions
The results of our meta-analysis indicate that fatigue may appear in rectal cancer patients at the time of diagnosis and also during the cancer treatment period. Most notably, the severity of fatigue peaks around 1 month after the start of therapy. We must give fatigue the attention it deserves and use a standardized treatment process to effectively reduce its severity and improve the quality of life for cancer patients.
References
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ (2000) Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist 5:353–360. https://doi.org/10.1634/theoncologist.5-5-353
Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A (2007) Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manag 34:94–104. https://doi.org/10.1016/j.jpainsymman.2006.10.015
Charalambous A, Kouta C (2016) Cancer related fatigue and quality of life in patients with advanced prostate cancer undergoing chemotherapy. Biomed Res Int 2016:3989286. https://doi.org/10.1155/2016/3989286
Chen HL, Liu K, You QS (2018) Self-efficacy, cancer-related fatigue, and quality of life in patients with resected lung cancer. Eur J Cancer Care (Engl) 27:e12934. https://doi.org/10.1111/ecc.12934
Fabi A, Falcicchio C, Giannarelli D, Maggi G, Cognetti F, Pugliese P (2017) The course of cancer related fatigue up to ten years in early breast cancer patients: what impact in clinical practice? Breast 34:44–52. https://doi.org/10.1016/j.breast.2017.04.012
Grenon NN, Chan J (2009) Managing toxicities associated with colorectal cancer chemotherapy and targeted therapy: a new guide for nurses. Clin J Oncol Nurs 13:285–296. https://doi.org/10.1188/09.CJON.285-296
Wang XS, Janjan NA, Guo H, Johnson BA, Engstrom MC, Crane CH, Mendoza TR, Cleeland CS (2001) Fatigue during preoperative chemoradiation for resectable rectal cancer. Cancer 92:1725–1732. https://doi.org/10.1002/1097-0142(20010915)92:6+<1725::aid-cncr1504>3.0.co;2-d
Yeom SS, Park IJ, Jung SW, Oh SH, Lee JL, Yoon YS, Kim CW, Lim SB, Kim N, Yu CS, Kim JC (2017) Outcomes of patients with abdominoperineal resection (APR) and low anterior resection (LAR) who had very low rectal cancer. Medicine 96:e8249. https://doi.org/10.1097/MD.0000000000008249
Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, Hui B, Liu R, Ma H, Ren J (2016) A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci 12:1022–1031. https://doi.org/10.7150/ijbs.15438
Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S (2012) Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 3:CD004078. https://doi.org/10.1002/14651858.CD004078.pub2
Kim CG, Ahn JB, Shin SJ, Beom SH, Heo SJ, Park HS, Kim JH, Choe EA, Koom WS, Hur H, Min BS, Kim NK, Kim H, Kim C, Jung I, Jung M (2017) Role of adjuvant chemotherapy in locally advanced rectal cancer with ypT0-3N0 after preoperative chemoradiation therapy and surgery. BMC Cancer 17:615. https://doi.org/10.1186/s12885-017-3624-7
Joanna Briggs Institute (2011) Module 3-the appraisal, extraction and pooling of quantitative data. http://www.joannabriggs.edu.au.pdf. Accessed 30 August 2019
Li SX, Liu BB, Lu JH (2014) Longitudinal study of cancer-related fatigue in patients with colorectal cancer. Asian Pac J Cancer Prev 15:3029–3033. https://doi.org/10.7314/apjcp.2014.15.7.3029
Park HC, Janjan NA, Mendoza TR, Lin EH, Vadhan-Raj S, Hundal M, Zhang Y, Delclos ME, Crane CH, Das P, Wang XS, Cleeland CS, Krishnan S (2009) Temporal patterns of fatigue predict pathologic response in patients treated with preoperative chemoradiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 75:775–781. https://doi.org/10.1016/j.ijrobp.2008.11.027
Pucciarelli S, Del Bianco P, Efficace F et al (2011) Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg 253:71–77. https://doi.org/10.1097/SLA.0b013e3181fcb856
Couwenberg AM, Burbach JPM, van Grevenstein WMU, Smits AB, Consten ECJ, Schiphorst AHW, Wijffels NAT, Heikens JT, Intven MPW, Verkooijen HM (2018) Effect of neoadjuvant therapy and rectal surgery on health-related quality of life in patients with rectal cancer during the first 2 years after diagnosis. Clin Colorectal Cancer 17:e499–e512. https://doi.org/10.1016/j.clcc.2018.03.009
Schmidt CE, Bestmann B, Küchler T, Longo WE, Kremer B (2005) Prospective evaluation of quality of life of patients receiving either abdominoperineal resection or sphincter-preserving procedure for rectal cancer. Ann Surg Oncol 12:117–123. https://doi.org/10.1245/ASO.2005.12.036
Zhang JK, Fang LL, Zhang DW, Jin Q, Wu XM, Liu JC, Zhang CD, Dai DQ (2016) Type D personality is associated with delaying patients to medical assessment and poor quality of life among rectal cancer survivors. Int J Color Dis 31:75–85. https://doi.org/10.1007/s00384-015-2333-4
Grumann MM, Noack EM, Hoffmann IA, Schlag PM (2001) Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg 233:149–156. https://doi.org/10.1097/00000658-200102000-00001
Monastyrska E, Hagner W, Jankowski M, Głowacka I, Zegarska B, Zegarski W (2016) Prospective assessment of the quality of life in patients treated surgically for rectal cancer with lower anterior resection and abdominoperineal resection. Eur J Surg Oncol 42:1647–1653. https://doi.org/10.1016/j.ejso.2016.07.007
Herrle F, Sandra-Petrescu F, Weiss C, Post S, Runkel N, Kienle P (2016) Quality of life and timing of stoma closure in patients with rectal cancer undergoing low anterior resection with diverting stoma: a multicenter longitudinal observational study. Dis Colon Rectum 59:281–290. https://doi.org/10.1097/DCR.0000000000000545
Väyrynen JP, Tuomisto A, Väyrynen SA, Klintrup K, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ, Mäkinen MJ (2018) Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep 8:1126. https://doi.org/10.1038/s41598-018-19572-y
Bondurant KL, Lundgreen A, Herrick JS, Kadlubar S, Wolff RK, Slattery ML (2013) Interleukin genes and associations with colon and rectal cancer risk and overall survival. Int J Cancer 132:905–915. https://doi.org/10.1002/ijc.27660
Macciò A, Madeddu C, Gramignano G et al (2015) The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica 100:124–132. https://doi.org/10.3324/haematol.2014.112813
Manocha M, Khan WI (2012) Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol 3:e13. https://doi.org/10.1038/ctg.2012.8
Coleman EA, Goodwin JA, Coon SK, Richards K, Enderlin C, Kennedy R, Stewart CB, McNatt P, Lockhart K, Anaissie EJ, Barlogie B (2011) Fatigue, sleep, pain, mood, and performance status in patients with multiple myeloma. Cancer Nurs 34:219–227. https://doi.org/10.1097/NCC.0b013e3181f9904d
Bower JE (2014) Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11:597–609. https://doi.org/10.1038/nrclinonc.2014.127
Johnson RL, Amin AR, Matzo M (2012) Cancer-related fatigue. Am J Nurs 112:57–60. https://doi.org/10.1097/01.NAJ.0000413462.26668.96
O'Higgins CM, Brady B, O'Connor B, Walsh D, Reilly RB (2018) The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer 26:3353–3364. https://doi.org/10.1007/s00520-018-4318-7
Goedendorp MM, Gielissen MF, Verhagen CA, Peters ME, Bleijenberg G (2008) Severe fatigue and related factors in cancer patients before the initiation of treatment. Br J Cancer 99:1408–1414. https://doi.org/10.1038/sj.bjc.6604739
Silverman MN, Heim CM, Nater UM, Marques AH, Sternberg EM (2010) Neuroendocrine and immune contributors to fatigue. PM R 2:338–346. https://doi.org/10.1016/j.pmrj.2010.04.008
Gil F, Costa G, Hilker I, Benito L (2012) First anxiety, afterwards depression: psychological distress in cancer patients at diagnosis and after medical treatment. Stress Health 28:362–367. https://doi.org/10.1002/smi.2445
Turgay AS, Khorshid L, Eser I (2008) Effect of the first chemotherapy course on the quality of life of cancer patients in Turkey. Cancer Nurs 31:E19–E23. https://doi.org/10.1097/01.NCC.0000339248.37829.c2
Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, Hu CY, Chang GJ (2012) Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 30:1770–1776. https://doi.org/10.1200/JCO.2011.39.7901
Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, Wojcik C (2002) Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum 29:949–956. https://doi.org/10.1188/02.ONF.949-956
Saber MM, Al-Mahallawi AM, Nassar NN, Stork B, Shouman SA (2018) Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer 18:822. https://doi.org/10.1186/s12885-018-4727-5
Banipal RPS, Singh H, Singh B (2017) Assessment of cancer-related fatigue among cancer patients receiving various therapies: a cross-sectional observational study. Indian J Palliat Care 23:207–211. https://doi.org/10.4103/IJPC.IJPC_135_16
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR (2007) Cancer-related fatigue: the scale of the problem. Oncologist 12:4–10. https://doi.org/10.1634/theoncologist.12-S1-4
Piper BF, Borneman T, Sun VC, Koczywas M, Uman G, Ferrell B, James RL (2008) Cancer-related fatigue: role of oncology nurses in translating National Comprehensive Cancer Network assessment guidelines into practice. Clin J Oncol Nurs 12:37–47. https://doi.org/10.1188/08.CJON.S2.37-47
Okuyama T, Akechi T, Kugaya A et al (2000) Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manag 19:5–14. https://doi.org/10.1016/s0885-3924(99)00138-4
Wu HS, McSweeney M (2001) Measurement of fatigue in people with cancer. Oncol Nurs Forum 28:1371–1384
Cleeland CS, Mendoza TR, Wang XS et al (2000) Assessing symptom distress in cancer patient: the M. D. Anderson Symptom Inventory. Cancer 89:1634–1646. https://doi.org/10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v
Greimel ER, Kuljanic Vlasic K, Waldenstrom AC, Duric VM, Jensen PT, Singer S, Chie W, Nordin A, Bjelic Radisic V, Wydra D, European Organization for Research and Treatment of Cancer Quality-of-Life Group (2006) The European Organization for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer 107:1812–1822. https://doi.org/10.1002/cncr.22217
Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S (2003) The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Palliat Med 17:664–672. https://doi.org/10.1191/0269216303pm841oa
Glaus A (1993) Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer 1:305–315. https://doi.org/10.1007/bf00364968
Munch TN, Strömgren AS, Pedersen L, Petersen MA, Hoermann L, Groenvold M (2006) Multidimensional measurement of fatigue in advanced cancer patients in palliative care: an application of the multidimensional fatigue inventory. J Pain Symptom Manag 31:533–541. https://doi.org/10.1016/j.jpainsymman.2005.11.012
Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR (2007) Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 12:11–21. https://doi.org/10.1634/theoncologist.12-S1-11
Acknowledgments
We thank the anonymous reviewers and editor for their comments.
Author information
Authors and Affiliations
Contributions
Conceptualization: Wen-Pei Chang; Hsiu-Ju Jen.
Data curation: Wen-Pei Chang.
Formal analysis: Wen-Pei Chang; Hsiu-Ju Jen.
Methodology: Wen-Pei Chang.
Project administration: Wen-Pei Chang.
Resources: Wen-Pei Chang.
Software: Wen-Pei Chang; Hsiu-Ju Jen.
Supervision: Wen-Pei Chang.
Validation: Wen-Pei Chang; Hsiu-Ju Jen.
Visualization: Wen-Pei Chang.
Writing, original draft: Wen-Pei Chang.
Writing, review and editing: Wen-Pei Chang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wen-Pei, C., Hsiu-Ju, J. Changes in fatigue in rectal cancer patients before and after therapy: a systematic review and meta-analysis. Support Care Cancer 28, 2513–2522 (2020). https://doi.org/10.1007/s00520-020-05325-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05325-z