Abstract
Purpose
Exercise is beneficial for prostate cancer patients’ physical functioning; however, effects on social and cognitive functioning are inconsistent. This meta-analysis of exercise interventions for prostate cancer patients had two aims: the primary aim was to evaluate the effects of exercise interventions on social functioning; the secondary aim was to consider additional outcomes of cognitive functioning as well as adverse events.
Methods
Electronic databases (Embase, MEDLINE, PubMed, PsycINFO, and the Chinese database Airti Library) were searched for relevant papers (1987–2019), which included hand searching. After careful inspection, 10 relevant randomized controlled trials were analyzed using Comprehensive Meta-Analysis software; pooled means determined social and cognitive functioning.
Results
Meta-analysis of summary scores (fixed-effects model) showed an overall beneficial effect of exercise on social functioning (Hedges’ g = 0.35, 95% CI [0.193, 0.515], p < 0.001) and cognitive functioning (Hedges’ g = 0.35, 95% CI [0.123, 0.575], p < 0.01) in men with prostate cancer when compared to controls. Intervention durations of 12–16 and 24–48 weeks that provided supervised aerobic exercise combined with resistance exercise sessions had a small to medium effect on social functioning compared to controls. One exercise group experienced one serious, but non-fatal, adverse event due to a higher exercise intensity (50–75% VO2max).
Discussion and recommendations
To the best of our knowledge, this is the first meta-analysis to examine the effects of exercise interventions on cognitive functioning among prostate cancer patients. We suggest further research be conducted to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is ranked second in terms of incidence rate among males. Approximately 1.3 million men were diagnosed with prostate cancer worldwide in 2018 [1]. The majority of prostate cancer patients are successfully treated and have a longer life expectancy than other cancer patients [2]; 96% of patients in the USA have a 15-year relative survival rate. However, numerous side effects can appear after receiving anticancer therapies such as androgen deprivation therapy (ADT), which can cause increased body composition, bone mineral density reduction, increased risk of cardiovascular diseases, and psychological distress [3]. Cognitive impairment can also result from ADT or radiotherapy [4, 5].
A meta-analysis showed that patients with prostate cancer had a high incidence of depression and anxiety across the pre- and post-treatment period [6]. Compared to women, men are less likely to discuss their physical or psychosocial concerns with health professionals [7]. This failure to discuss health concerns includes men with prostate cancer, who often avoid seeking psychological support because of gender image [8].
Quantitative and qualitative studies demonstrated that prostate cancer patients who participated in an exercise intervention arm experienced physical and psychological benefits [9,10,11,12,13]. The use of exercise as an intervention for cancer patients is an established method of reducing overall mortality and cancer-specific mortality and improving survival outcomes after a cancer diagnosis (e.g., breast and colon cancer) [14,15,16]. Previous systematic reviews and a meta-analysis which demonstrated exercise interventions for prostate cancer patients improved cardiovascular fitness, fatigue, and quality of life, which positively affected physical well-being [17,18,19,20,21]. However, among prostate cancer patients, findings of whether exercise interventions can improve social functioning were inconsistent, and relatively few studies have examined whether exercise training improves cognitive functioning. Therefore, this meta-analysis of exercise interventions for prostate cancer patients after diagnosis had two aims: the primary aim was to evaluate the effects of exercise interventions on social functioning; the secondary aim was to consider additional outcomes in regard to cognitive functioning and adverse events.
Methods
Identification and selection of studies
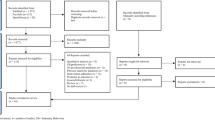
This literature review included the process of identification, screening, eligibility, and inclusion, shown in Fig. 1. The checklist of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol [22] was used to ensure the rigor of this meta-analysis.
A search of studies in peer-reviewed journals published between 1987 and May 3, 2019, was conducted using five electronic databases: Embase, MEDLINE, PubMed, PsycINFO, and the Chinese database Airti Library. All of the references in the identified articles were searched, and when an article was not indexed in the electronic databases, it was retrieved by hand. The literature search was performed by two independent reviewers (the first and third authors). The search used the terms “prostate cancer,” “exercise or physical activity,” and “intervention, training, or strategies” to identify articles. For example, in the PubMed search, keywords were employed such as prostate cancer and exercise (“Physical activity”) and intervention (“Training,” “Strategies”). The same search method was employed for the four other electronic databases.
The inclusion criteria of the studies are shown in Table 1. Briefly, included studies employed a randomized controlled trial (RCT) with participants who were adult men (age ≥ 20 years) with prostate cancer at either early or advanced stages. Selected studies assessed the effects of an intervention that was exclusively aerobic exercise or strength training (any type), and outcome measures were determined by a pretest-posttest design with a control group and a post-intervention assessment. To ensure comparisons were made according to the same benchmark, the control group received only standard oncology care (routine care with or without health education). Social functioning was the primary outcome variable. The exclusion criteria are shown in Table 1. We excluded interventions that were multimodal or involved qigong or yoga because these result in heterogeneous effects. Additionally, if one or more publications identified originated from the same exercise program, we excluded all but the most recent publication.
Quality assessment of the included studies
The quality of the studies was assessed by three team members (the second, third, and fifth author). Two critical appraisal tools, the Jadad quality scale [23] and the Cochrane Collaboration’s tool [24], were used to assess the risk of bias in each RCT study. When the appraisal of an item was inconsistent, the reviewers discussed the item and sought assistance from a third reviewer (the first author); the reviewers then continued to discuss the item until consensus was reached.
Data extraction
All searched studies were imported into Endnote X8 and then the duplicates were removed. After the previous step was completed, the studies were screened by title and abstract. Studies were screened by inclusion and exclusion criteria and applicability. The first and second authors extracted information based on the description of the intervention provided in the review studies (e.g., sample size, type of intervention, and questionnaires used to measure social functioning).
Outcome measures
Three self-report questionnaires most commonly used to measure the outcome of social functioning were the 36-Item Short Form Health Survey (SF-36) [25,26,27,28], the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) [29, 30], and the Functional Assessment of Cancer Therapy–General (FACT-G) [31,32,33,34]. A higher score represents better social functioning.
Four of the ten studies reported on the effect of an exercise intervention for the outcome of cognitive functioning [29, 30, 33, 34], which allowed us to address one of our secondary aims. All four studies used the EORTC QLQ-C30 self-report questionnaire; a lower score indicates a poorer level of cognitive functioning. In this report, we pooled the analyses to determine the level of cognitive functioning. Seven of the ten articles reported on the outcome of adverse effects of exercise interventions [9, 26,27,28, 30, 31, 34]; we used a narrative synthesis to examine this outcome.
Data analysis
Comprehensive Meta-Analysis (CMA) software, version 3.3.070, was used to calculate effect size [35]. For the outcome of social functioning, we used summary scores of the three different questionnaires. For cognitive functioning, we pooled data from the four studies reporting this outcome [29, 30, 33, 34]. We computed means and standard deviations (SDs) or the mean differences (MDs) and SDs to compare differences between the exercise intervention and the standard oncology care groups. If the above values were not available, we used the appropriate formula (e.g., F, p, or t) to determine the effect size for the data. Because the prostate cancer patients in the selected studies were recruited in different areas and were assessed by different questionnaires to detect their social or cognitive functioning, variation in the outcomes of the studies was expected. To report our results, Hedges’ g was used to standardize the between-group effect size and 95% confidence interval (95% CI).
To detect publication bias, funnel plots were constructed using Egger’s regression analysis and Begg’s rank test [36]. Statistical heterogeneity was determined with chi-squared (Cochran’s Q test) and the I2 test; the higher value of I2 indicates greater heterogeneity between the studies. Heterogeneity can be divided into three levels, low (0–25%), moderate (25–50%), and high heterogeneity (50–75%) [35, 37]. If studies showed high heterogeneity, the overall effectiveness was calculated by the random effect method to manage the heterogeneity [38, 39]. Thus, if there was no significant heterogeneity, the fixed-effect method was used to present the final result.
After performing data synthesis, there were significant findings overall among the patients with prostate cancer. Subgroup analyses were performed [40] for differences in exercise intervention effects between subgroups based on their treatment status (categorized as ongoing or completed), intervention design (categorized as supervised or supervised combined with home-based exercise), exercise type (categorized into aerobic, resistance, or aerobic combined with resistance exercise), exercise session (categorized into small group based or individual), and intervention duration (categorized as equal to or less than 8 weeks, 12–16 weeks, or 24–48 weeks). To detect suitability of inclusion and adjustment in effect size with time [41], we used sensitivity analysis and cumulative meta-analysis to investigate the impact of each study and time effect on the pooled results.
Results
Flow of studies
The process of the search and selection of studies is shown in Fig. 1. A total of 1501 relevant studies were published from 1987 to 2019. After removal of duplicates (n = 1085) and exclusion based on titles and abstracts (n = 205), 211 articles remained for further screening. Full-text analysis in the last step resulted in the exclusion of 201 articles. A total of ten full-text RCT studies were retained and selected for this analysis.
Quality of the studies
The Jadad quality scale [23] was used to assess the ten studies (Table 2). All ten selected studies had a Jadad score of three points, indicating they were of high quality. Risk-of-bias judgments, determined with Cochrane Collaboration’s tool [24], are shown in Fig. 2. All studies had a high risk of performance bias. None of the studies were described as double-blind; however, four studies described study coordinators, exercise physiologists, statisticians, or data managers who were blinded to the group assignment of the participants [26, 31, 33, 34]. Six studies included allocation concealment, and 8 studies reported random sequence generation. Of the 10 studies, 70% (n = 7) had a low risk of reporting bias, and 3 studies had a low risk of other sources of bias.
Participants
The 10 RCT studies selected for analysis represented a total 639 patients with prostate cancer (study sample sizes ranged from 20 to 147) (Table 3). Five studies were conducted in Australia [25,26,27,28,29]; two were undertaken in Poland [33, 34]; and three were conducted in Canada [31], the USA. [32], or Norway [30]. Eight studies involved participants undergoing cancer treatment with androgen deprivation therapy (ADT) [25, 26, 28], radiotherapy (RT) [32,33,34], and ADT or RT [27] and treatment with ADT after completion of RT [30]. Two studies reported on participants who had already completed anticancer treatments [29, 31].
The age of participants in the intervention groups ranged from 58 to 73.1 years (median, 67.7 years) and in the control group from 61 to 71.5 years (median, 68.9 years). The median age was not statistically significant between groups.
Exercise interventions and exercise adherence
The type of exercise intervention and adherence for the included studies is shown in Table 3. For most studies, the intervention was supervised; the other three studies used a combination of supervised and home-based exercise interventions [25, 29, 31]. The intensity level for most studies was moderate (n = 7), and these combined aerobic exercise with resistance exercise [25,26,27,28,29, 33, 34]. Two studies examined aerobic exercise alone (e.g., walking) [31, 32]; one examined resistance training [30]. The duration of the intervention programs ranged from 8 to 48 weeks; five studies were 12 to 16 weeks [25, 26, 28, 30], three studies were 24 to 48 weeks [27, 31, 34], and the other two studies were 8 weeks [32, 33]. Exercise frequency varied from 2 to 5 times per week, and total training time per week ranged from 40 to 275 min. Five studies recorded total weekly exercise volume; three employed a weekly self-report exercise diary [29, 33, 34]; and two combined an accelerometer or pedometer with a weekly self-report exercise diary [26, 27]. Of these five studies, four confirmed exercise intensity and also monitored heart rate during the exercise intervention [26, 27, 31, 32].
Exercise adherence rates were not reported for five of the studies [25, 27, 28, 32, 33]. For the five studies reporting adherence rates [26, 29,30,31, 34], rates ranged from 72% to 93.2%. Three of these studies supervised the exercise intervention, and adherence rates ranged from 84% to 93.2% [26, 30, 34]; the two other studies used a combination of supervised and home-based exercise for the intervention and rates ranged from 72% to 85% [29, 31].
Summary of reported outcomes
Outcome measures reported in the selected studies are shown in Table 3. Only three of the ten studies reported that the exercise intervention improved social functioning compared to the control group [25, 27, 32]; one of the studies reported that social functioning also improved within the exercise group [32]. The remaining seven studies found no effect of the exercise intervention on social functioning [26, 28,29,30,31, 33, 34]. Four of the studies also included an outcome measure for cognitive functioning, measured with the EORTC QLQ-C30 [29, 33, 34]; all but one [30] showed that the exercise intervention improved cognitive functioning for the intervention group compared with controls.
Seven of the ten studies provided information on the presence or absence of adverse events [9, 26,27,28, 30, 31, 34] and involved 420 men with prostate cancer (Table 3). Two studies reported no adverse effects during testing or the exercise intervention [25, 28]. For studies reporting the occurrence of adverse events, these events were not life-threatening, and any occurrence of death was not attributed to exercise [9, 26, 28, 30, 31, 34]. Adverse events included a fractured rib, which resulted from a fall while dressing at home [26]; a non-fatal myocardial infarction (MI) during exercise [27], which occurred with high-intensity aerobic exercise (exercise intensity range, 70–85 maximum heart rate); overuse injuries in the lower extremities [34]; and training-induced leg cramps, back pain, or knee pain [30, 31].
Meta-analysis of included studies on the effect of exercise interventions
Forest plots were used to determine the effects of the exercise intervention among the included studies on social functioning (Fig. 3a) and cognitive functioning (Fig. 3b). Only two of the ten studies had a significant improvement on overall social functioning (p values < 0.05) [25, 27]. None of the four studies that examined the outcome of cognitive functioning showed a significant improvement (p values > 0.05) [29, 33, 34]. However, using the fixed-effects model, summary scores showed an overall beneficial effect of exercise on social functioning (Hedges’ g = 0.35, 95% CI [0.193, 0.515], p < 0.001; heterogeneity, Chi2 = 10.94; p = 0.28; I2 = 17.77%) and cognitive functioning (Hedges’ g = 0.35, 95% CI [0.123, 0.575], p = 0.003; heterogeneity, Chi2 = 1.50; p = 0.68; I2 = 0.0%).
Risk of bias across studies
The funnel plot (Fig. 4) and the Egger and Begg tests suggested that the meta-analysis of social functions (coefficient = 0.16, p > 0.05, and Z = 0.18, p > 0.05, respectively) had a small publication bias. As less than ten studies reported on the effects of exercise on cognitive functioning, this was not analyzed in the funnel plots or the Egger or Begg tests.
Subgroup analysis
The fixed-effects model (Fig. 3a) showed that exercise interventions had a positive effect on outcomes compared to patients receiving standard oncology care; however, the effect size was small. Therefore, we performed subgroup analyses (Table 4) with a fixed-effects model to determine if there were significant differences in social functioning. For the subgroup of treatment status, patients undergoing treatment had better social functioning (Hedges’ g = 0.43, 95% CI [0.25, 0.62], p < 0.001) than those who had completed treatment. Both supervised exercise (Hedges’ g = 0.40, 95% CI [0.20, 0.60], p < 0.01) and a combination of supervised and home-based exercise designs (Hedges’ g = 0.27, 95% CI [0.01, 0.54], p < 0.05) had a significant positive effect on social functioning compared to controls. Programs that employed an exercise type of aerobics combined with resistance exercise attained statistical significance for social functioning (Hedges’ g = 0.39, 95% CI [0.21, 0.57], p < 0.01). Interventions with durations of 12–16 weeks and 24–48 weeks had significant benefits for subjective measures of social functioning (Hedges’ g = 0.25, 95% CI [0.03, 0.47], p < 0.05, and Hedges’ g = 0.51, 95% CI [0.23, 0.79], p < 0.01, respectively). The studies with an exercise intervention that combined a supervised and home-based design had low levels of homogeneity (I2 = 23.20%). No significant effect was found for any subgroup using the random effects model.
Sensitivity and cumulative meta-analysis
Sensitivity analyses (Fig. 4) showed that no individual study had an impact on the pooled results for social functioning; when any one of the ten studies was excluded, the pooled results did not change. Cumulative meta-analysis displayed a beneficial effect of an exercise intervention on social functioning (Fig. 4).
Discussion
Our meta-analysis of the effects of an exercise intervention on social and cognitive functioning included ten RCTs involving 639 patients with prostate cancer. All studies examined outcome variables of social functioning; four of the ten studies examined cognitive functioning. Using the fixed-effect model, summary scores showed improvements in social as well as cognitive functioning, suggesting that providing an exercise intervention to prostate cancer patients has beneficial effects for these outcomes.
Anticancer therapy, radiotherapy, or ADT causes short-term and long-term health problems, such as fatigue, muscular atrophy, and cognitive impairment for prostate cancer patients [3,4,5, 42, 43]. Although previous meta-analyses showed that exercise interventions can significantly improve fatigue, muscle mass, muscle strength (e.g., upper or lower body strength) and physical performance for prostate cancer patients [17, 44,45,46,47], there was limited information on whether these interventions improved cognitive functioning. Our study found a small to medium effect on self-reported cognitive functioning (range from 0.12 to 0.58) for prostate cancer patients who received an exercise intervention during and after cancer treatments. To the best of our knowledge, this is the first meta-analysis to examine the effects of exercise interventions on cognitive functioning among prostate cancer patients. We suggest further research be conducted to confirm these findings.
Prostate cancer patients are less likely than breast cancer patients to seek medical and psychological help [7]. This can result in more physical- and or psychosocial-related unmet needs [48,49,50,51,52], which can cause a profound sense of isolation [53] and is associated with poor social functioning [54]. Although physical and psychological difficulties have short- and long-term effects on the survival of cancer patients [54], quantitative evaluations of social functioning are often overlooked. A few qualitative studies have reported the social benefits of exercise for prostate cancer patients, especially when there are interactions with other patients facing similar health difficulties [9,10,11]. This meta-analysis found that prostate cancer patients who participated in an exercise program had significantly better social functioning than controls receiving standard oncology care.
The design and duration of the exercise intervention were found to significantly benefit social function, with close to a medium effect size. This meta-analysis suggests that patients should be provided with an exercise intervention when undergoing treatment, because they are more likely to show clinical improvements in social functioning rather than after treatment is completed. Our meta-analysis also indicated patients who received exercises which were supervised aerobic combined with resistance and group-based and which had a duration from 12 to 16 weeks or 24 to 48 weeks had better social functioning among men with prostate cancer than patients in the usual care control group. The exercise design described above pertains to interventions with small to medium effect size. In a previous study by Cormie, Oliffe, Wootten, Galvao, Newton, and Chambers [49], it was also suggested that prostate cancer patients should undertake exercise as a support group in the future.
Adverse events during an intervention are major concerns in any form of experimental study. For studies that focus on exercise for older adults, it is important to balance the benefits of physical activity while reducing the risk of cardiovascular disease, which is a risk for older patients [55,56,57,58]. Few studies reported any adverse events resulting from the exercise interventions, and no deaths were attributed to exercise training in any of the included RCTs. The study by Cormie et al., which included prostate cancer patients with bone metastases, showed no adverse effects of skeletal complications or changes in the use of pain medication throughout the intervention [26]. There was one serious adverse event in a person with no history of cardiac disease; an aerobic group participant experienced a MI; however, he subsequently had a full recovery [27]. Participation in moderate- to high-intensity exercise (≧ 6 METs) has been shown to result in a higher risk of MI than low-intensity exercise [59]. Therefore, we suggest that exercise intensity be monitored and exercise programs for prostate cancer patients begin with light to moderate intensity (40 to 50% VO2max), which is recommended for beginners and persons at risk of cardiovascular events [57]. Prostate cancer patients desiring to perform at intensity levels higher than 50% VO2max should be under supervision.
Limitations
The generalization of these results is limited due to the screening process for specific articles and excludes patients with poor physical functional status (e.g., musculoskeletal, cardiovascular, and neurological disorders or cognitive dysfunction). In addition, it may not be possible to directly extrapolate these findings to a global population because all included studies were conducted in developed Western countries.
Conclusions
This meta-analysis provides evidence of the benefits of exercise interventions on social and cognitive functioning among patients with prostate cancer when compared with patients receiving standard oncology care. Although meta-analysis of the individual four studies that assessed cognitive functioning showed no significant improvement, the fixed-effect model demonstrated a significant improvement in cognitive functioning compared to controls. None of the ten included RCTs reported any deaths due to an exercise intervention. Although one study reported an exceptional case of a patient experiencing a MI in the exercise group, the patient subsequently made a full recovery following the adverse event. Although the MI was a rare event, this finding indicates patients must be thoroughly assessed before initiation of an exercise intervention and monitored during participation in the program. Evidence suggests that when prescribing age-appropriate exercise programs for older patients, the preferable exercise intensity should begin at 40–50% VO2max (approximately the range of 60–70% HRmax); exercise intensity of more than 70% HRmax must be performed under supervision.
Implications for future research
Our findings of this meta-analysis have implications for future research. The limited number of studies that assessed cognition suggests that additional research is needed on the benefits of an exercise intervention on cognitive functioning. The subgroup analyses provide evidence for approaches to future research, which could improve social functioning. The improvements that were seen for prostate cancer patients who participated in an exercise program were most significant when the intervention occurred during treatment was group-based and lasted at least 12 to 16 weeks; the significance was greater when the length of the intervention was 24 to 48 weeks. Our findings suggest that these might be important variables for optimizing the benefits of an exercise intervention.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
American Cancer Society [ACS] (2017) Survival rates for prostate cancer Retrieved from https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html
Rhee H, Gunter JH, Heathcote P, Ho K, Stricker P, Corcoran NM, Nelson CC (2015) Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int 115(Suppl 5):3–13. https://doi.org/10.1111/bju.12964
Mundell NL, Daly RM, Macpherson H, Fraser SF (2017) Cognitive decline in prostate cancer patients undergoing ADT: a potential role for exercise training. Endocr Relat Cancer 24:R145–r155. https://doi.org/10.1530/erc-16-0493
O'Higgins CM, Brady B, O'Connor B, Walsh D, Reilly RB (2018) The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer 26:3353–3364. https://doi.org/10.1007/s00520-018-4318-7
Watts S, Leydon G, Birch B, Prescott P, Lai L, Eardley S, Lewith G (2014) Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open 4:e003901. https://doi.org/10.1136/bmjopen-2013-003901
Forsythe LP, Kent EE, Weaver KE, Buchanan N, Hawkins NA, Rodriguez JL, Ryerson AB, Rowland JH (2013) Receipt of psychosocial care among cancer survivors in the United States. J Clin Oncol Off J Am Soc Clin Oncol 31:1961–1969. https://doi.org/10.1200/jco.2012.46.2101
Medina-Perucha L, Yousaf O, Hunter MS, Grunfeld EA (2017) Barriers to medical help-seeking among older men with prostate cancer. J Psychosoc Oncol 35:531–543. https://doi.org/10.1080/07347332.2017.1312661
Cormie P, Turner B, Kaczmarek E, Drake D, Chambers SK (2015) A qualitative exploration of the experience of men with prostate cancer involved in supervised exercise programs. Oncol Nurs Forum 42:24–32. https://doi.org/10.1188/15.Onf.24-32
Trinh L, Arbour-Nicitopoulos KP, Sabiston CM, Alibhai SM, Jones JM, Berry SR, Loblaw A, Faulkner GE (2015) A qualitative study exploring the perceptions of sedentary behavior in prostate cancer survivors receiving androgen-deprivation therapy. Oncol Nurs Forum 42:398–406. https://doi.org/10.1188/15.Onf.398-406
Fox L, Cahill F, Burgess C, Peat N, Rudman S, Kinsella J, Cahill D, George G, Santaolalla A, Van Hemelrijck M (2017) Real world evidence: a quantitative and qualitative glance at participant feedback from a free-response survey investigating experiences of a structured exercise intervention for men with prostate cancer. Biomed Res Int 2017:3507124. https://doi.org/10.1155/2017/3507124
Lee CE, Kilgour A, Lau YK (2012) Efficacy of walking exercise in promoting cognitive-psychosocial functions in men with prostate cancer receiving androgen deprivation therapy. BMC Cancer 12:324. https://doi.org/10.1186/1471-2407-12-324
Zopf EM, Bloch W, Machtens S, Zumbe J, Rubben H, Marschner S, Kleinhorst C, Schulte-Frei B, Herich L, Felsch M, Predel HG, Braun M, Baumann FT (2015) Effects of a 15-month supervised exercise program on physical and psychological outcomes in prostate cancer patients following prostatectomy: the ProRehab study. Integr Cancer Ther 14:409–418. https://doi.org/10.1177/1534735415583552
Schmid D, Leitzmann MF (2014) Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 25:1293–1311. https://doi.org/10.1093/annonc/mdu012
Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM (2012) Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 104:815–840. https://doi.org/10.1093/jnci/djs207
Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, Deng Y, Chen Q, Wei S, Nie S, Liu L (2016) The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med 50:339–345. https://doi.org/10.1136/bjsports-2015-094927
Bourke L, Smith D, Steed L, Hooper R, Carter A, Catto J, Albertsen PC, Tombal B, Payne HA, Rosario DJ (2016) Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol 69:693–703. https://doi.org/10.1016/j.eururo.2015.10.047
Teleni L, Chan RJ, Chan A, Isenring EA, Vela I, Inder WJ, McCarthy AL (2016) Exercise improves quality of life in androgen deprivation therapy-treated prostate cancer: systematic review of randomised controlled trials. Endocr Relat Cancer 23:101–112. https://doi.org/10.1530/erc-15-0456
Gardner JR, Livingston PM, Fraser SF (2014) Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol Off J Am Soc Clin Oncol 32:335–346. https://doi.org/10.1200/jco.2013.49.5523
Menichetti J, Villa S, Magnani T, Avuzzi B, Bosetti D, Marenghi C, Morlino S, Rancati T, Van Poppel H, Salvioni R, Valdagni R, Bellardita L (2016) Lifestyle interventions to improve the quality of life of men with prostate cancer: a systematic review of randomized controlled trials. Crit Rev Oncol Hematol 108:13–22. https://doi.org/10.1016/j.critrevonc.2016.10.007
Baumann FT, Zopf EM, Bloch W (2012) Clinical exercise interventions in prostate cancer patients--a systematic review of randomized controlled trials. Support Care Cancer 20:221–233. https://doi.org/10.1007/s00520-011-1271-0
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343:d5928. https://doi.org/10.1136/bmj.d5928
Cormie P, Galvao DA, Spry N, Joseph D, Chee R, Taaffe DR, Chambers SK, Newton RU (2015) Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int 115:256–266. https://doi.org/10.1111/bju.12646
Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA (2013) Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis 16:328–335. https://doi.org/10.1038/pcan.2013.22
Galvao DA, Spry N, Denham J, Taaffe DR, Cormie P, Joseph D, Lamb DS, Chambers SK, Newton RU (2014) A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol 65:856–864. https://doi.org/10.1016/j.eururo.2013.09.041
Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU (2010) Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol 28:340–347. https://doi.org/10.1200/jco.2009.23.2488
Livingston PM, Craike MJ, Salmon J, Courneya KS, Gaskin CJ, Fraser SF, Mohebbi M, Broadbent S, Botti M, Kent B (2015) Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: a multicenter cluster randomized controlled trial (ENGAGE). Cancer 121:2646–2654. https://doi.org/10.1002/cncr.29385
Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W, Fossa SD, Thorsen L (2015) Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol 54:1805–1813. https://doi.org/10.3109/0284186x.2015.1037008
Jones LW, Hornsby WE, Freedland SJ, Lane A, West MJ, Moul JW, Ferrandino MN, Allen JD, Kenjale AA, Thomas SM, Herndon JE 2nd, Koontz BF, Chan JM, Khouri MG, Douglas PS, Eves ND (2014) Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol 65:852–855
Monga U, Garber SL, Thornby J, Vallbona C, Kerrigan AJ, Monga TN, Zimmermann KP (2007) Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil 88:1416–1422. https://doi.org/10.1016/j.apmr.2007.08.110
Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, Gorecki M, Ozga-Majchrzak O, Milecki T, Milecki P (2016) Physical exercise for functional capacity, blood immune function, fatigue, and quality of life in high-risk prostate cancer patients during radiotherapy: a prospective, randomized clinical study. Eur J Phys Rehabil Med 52:489–501
Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, Milecki P (2017) Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer. A randomized controlled trial of a 12month exercise program. Pol Arch Intern Med 127:25–35. https://doi.org/10.20452/pamw.3888
Borenstein M (2009) Introduction to meta-analysis. John Wiley & Sons, Chichester
Dwan K, Gamble C, Williamson PR, Kirkham JJ (2013) Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PLoS One 8:e66844
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Riley RD, Higgins JP, Deeks JJ (2011) Interpretation of random effects meta-analyses. BMJ 342:d549
Kriston L (2013) Dealing with clinical heterogeneity in meta-analysis. Assumptions, methods, interpretation. Int J Methods Psychiatr Res 22:1–15. https://doi.org/10.1002/mpr.1377
Borenstein M, Higgins JP (2013) Meta-analysis and subgroups. Prev Sci 14:134–143. https://doi.org/10.1007/s11121-013-0377-7
Phan K, Tian DH, Cao C, Black D, Yan TD (2015) Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 4:112–122. https://doi.org/10.3978/j.issn.2225-319X.2015.02.04
Langston B, Armes J, Levy A, Tidey E, Ream E (2013) The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support Care Cancer 21:1761–1771. https://doi.org/10.1007/s00520-013-1751-5
Keogh JW, MacLeod RD (2012) Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manag 43:96–110. https://doi.org/10.1016/j.jpainsymman.2011.03.006
Keilani M, Hasenoehrl T, Baumann L, Ristl R, Schwarz M, Marhold M, Sedghi Komandj T, Crevenna R (2017) Effects of resistance exercise in prostate cancer patients: a meta-analysis. Support Care Cancer 25:2953–2968. https://doi.org/10.1007/s00520-017-3771-z
Gao Y-F, He W-Y, He X-Y, Huang Y-L, Gou X (2017) Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: an update meta-analysis. Medicine 96:e7368. https://doi.org/10.1097/md.0000000000007368
Yang B, Wang J (2017) Effects of exercise on cancer-related fatigue and quality of life in prostate cancer patients undergoing androgen deprivation therapy: a meta-analysis of randomized clinical trials. Chin Med Sci J 32:13–21
Vashistha V, Singh B, Kaur S, Prokop LJ, Kaushik D (2016) The effects of exercise on fatigue, Quality of life, and psychological function for men with prostate cancer: systematic review and meta-analyses. Eur Urol Focus 2:284–295. https://doi.org/10.1016/j.euf.2016.02.011
Dunn J, Casey C, Sandoe D, Hyde MK, Cheron-Sauer MC, Lowe A, Oliffe JL, Chambers SK (2018) Advocacy, support and survivorship in prostate cancer. Eur J Cancer Care 27:e12644. https://doi.org/10.1111/ecc.12644
Cormie P, Oliffe JL, Wootten AC, Galvao DA, Newton RU, Chambers SK (2016) Improving psychosocial health in men with prostate cancer through an intervention that reinforces masculine values - exercise. Psycho-oncology 25:232–235. https://doi.org/10.1002/pon.3867
Hyde MK, Zajdlewicz L, Wootten AC, Nelson CJ, Lowe A, Dunn J, Chambers SK (2016) Medical help-seeking for sexual concerns in prostate cancer survivors. Sex Med 4:e7–e17. https://doi.org/10.1016/j.esxm.2015.12.004
Hyde MK, Newton RU, Galvao DA, Gardiner RA, Occhipinti S, Lowe A, Wittert GA, Chambers SK (2017) Men's help-seeking in the first year after diagnosis of localised prostate cancer Eur J Cancer Care 26 https://doi.org/10.1111/ecc.12497
Yousaf O, Grunfeld EA, Hunter MS (2015) A systematic review of the factors associated with delays in medical and psychological help-seeking among men. Health Psychol Rev 9:264–276. https://doi.org/10.1080/17437199.2013.840954
Wenger LM (2013) 'Living under assault': men making sense of cancer. Eur J Cancer Care 22:389–399. https://doi.org/10.1111/ecc.12042
Karunanithi G, Sagar RP, Joy A, Vedasoundaram P (2018) Assessment of psychological distress and its effect on quality of life and social functioning in cancer patients. Indian J Palliat Care 24:72–77. https://doi.org/10.4103/ijpc.Ijpc_104_17
Cheng S-J, Yu H-K, Chen Y-C, Chen C-Y, Lien W-C, Yang P-Y, Hu G-C (2013) Physical activity and risk of cardiovascular disease among older adults. Int J Gerontol 7:133–136. https://doi.org/10.1016/j.ijge.2013.03.001
Lachman S, Boekholdt SM, Luben RN, Sharp SJ, Brage S, Khaw K-T, Peters RJG, Wareham NJ (2018) Impact of physical activity on the risk of cardiovascular disease in middle-aged and older adults: EPIC Norfolk prospective population study. Eur J Prev Cardiol 25:200–208. https://doi.org/10.1177/2047487317737628
Fleg JL (2016) Salutary effects of high-intensity interval training in persons with elevated cardiovascular risk F1000Research 5 https://doi.org/10.12688/f1000research.8778.1
American College Of Sports Medicine [ACSM] (2014) ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, Philadelphia
Mittleman MA, Mostofsky E (2011) Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation 124:346–354. https://doi.org/10.1161/circulationaha.110.968776
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Fang, YY., Lee, YH., Chan, JC. et al. Effects of exercise interventions on social and cognitive functioning of men with prostate cancer: a meta-analysis. Support Care Cancer 28, 2043–2057 (2020). https://doi.org/10.1007/s00520-019-05278-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05278-y