Abstract
Background
Older adults undergoing cancer surgery are at greater risk for poor postoperative outcomes. Caregivers also endure significant burden. Participation in perioperative physical activity may improve physical functioning and enhance overall well-being for both patients and caregivers. In this study, we assessed the feasibility of a personalized telehealth intervention to enhance physical activity for older (≥ 65 years) gastrointestinal (GI) and lung cancer surgery patients/caregivers.
Methods
Participants completed four telehealth sessions with physical therapy/occupational therapy (PT/OT) before surgery and up to 2 weeks post-discharge. Outcomes included preop geriatric assessment, functional measures, and validated measures for symptoms and psychological distress. Pre/post-intervention trends/trajectories for outcomes were explored.
Results
Thirty-four patient/caregiver dyads (16, GI; 18, lung) were included. Accrual rate was 76% over 8 months; retention rate was 88% over 2 months. Median for postop of a 6-min walk test, timed up and go, and short physical performance battery test scores improved from baseline to postop. Participant satisfaction scores were high.
Conclusion
Our conceptually based, personalized, multimodal, telehealth perioperative physical activity intervention for older patient/caregiver dyads is feasible and acceptable. It offers an opportunity to improve postoperative outcomes by promoting functional recovery through telehealth, behavior change, and self-monitoring approaches.
Trial registration
ClinicalTrials.gov Identifier: NCT03267524
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Major thoracic and abdominal procedures for gastrointestinal (GI) and lung cancers are complex. Patients are at risk for postoperative physical and psychological symptoms, including pain, dyspnea, fatigue, and distress [1, 2]. These symptoms, along with abrupt functional declines during the immediate postoperative period, contribute to a deterioration in overall quality of life (QOL) [3, 4].
Older adults undergoing cancer surgery are at greater risk for poor postoperative outcomes, including morbidity, increased length of hospital stay, and impaired functional status [5, 6]. The number of older adults is growing, with a projected rise to 70 million in 2030. This demographic shift leads to a projected 67% increase in cancer incidence for adults aged 65 years and older [7]. Thus, the population undergoing cancer surgery is increasing in age. The aging population, combined with pressures to promote early postoperative discharge, creates clinical challenges and place increasing burden on patients and their caregivers.
Caregivers, including family and friends, endure significant distress as they witness their care recipient struggle with a cancer diagnosis and the decision to undergo surgery [8, 9]. Supporting a care recipient through surgery not only causes considerable disruptions in the caregiver’s personal life but also leads to deteriorations to their overall well-being [10]. Thus, both older patients and their caregivers struggle with the challenges of surgery.
Interventions that promote postoperative functional recovery are based on the principle that perioperative physical activity may potentially provide patients with a “physiologic buffer” to withstand the stress of surgery [11]. In addition, randomized trials in chronic illnesses and cancer suggest that interventions that promote self-management are effective in improving physical and psychosocial health, health behaviors, and healthcare resource use (ER visits, at home nursing care, readmissions) [12, 13]. Thus, participation in perioperative physical activity that are based on the self-management framework has the potential to reduce postoperative complications, minimize functional decline, and improve well-being for both patients and caregivers [14]. Physical activity interventions are needed to target older surgery patients and their caregivers in the perioperative setting. The objectives of this study were to (1) determine the feasibility and acceptability of a personalized telehealth perioperative physical activity intervention for older lung and GI cancer surgery patients and their caregivers and (2) describe the trends, trajectories, and patterns of functional recovery and self-reported outcomes before and after surgery and intervention.
Methods
Study and intervention design
The intervention is based on the chronic care self-management model (CCM), which aims to empower patients, build self-efficacy, and improve outcomes through proactive planning and skills building [15]. It provided one-on-one coaching to optimize physical and psychological functioning before and after surgery. Classic behavioral change strategies were integrated, which included SMART (specific, measurable, attainable, relevant, timely) goal setting, identifying challenges/barriers to physical activity, problem-solving to overcome the challenges/barriers, and skills building related to functional recovery.
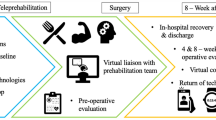
Intervention content was administered by trained physical therapist/occupational therapist (PT/OT) in five sessions through videoconferencing (Zoom) during the perioperative period. (Table 1). Beyond the five telehealth sessions, no additional contacts (either by telephone or in-person) were initiated for intervention delivery purposes. Before session #1, participants completed comprehensive geriatric assessment and objective functional measures, including the 6-min walking test (6MWT), timed up and go (TUG), and short physical performance battery (SPPB). The interventionists used the assessment data to develop a personalized walking program for the patients and caregivers. The program included a target goal for daily steps and recommendations on use of lower extremity exercises that are tailored to the patient’s functional status before surgery.
In session 1 (at least 7 days before surgery), PT/OT focused on (1) the importance of staying physically active, (2) goal setting, (3) identifying and overcoming challenges through problem-solving, and (4) development of a personalized walking and lower extremity exercise (sit to stand, step up and down-front, step up and down-sideways, standing wall push away) program. Before session 2, the interventionist repeated objective functional assessments (6MWT, TUG, SPPB), and used the data to re-design the walking program (target daily steps and lower extremity exercises) based on the patient’s postoperative functional status. In session 2 (before hospital discharge), goals, challenges, and walking/exercise program were refined, and strategies to overcome barriers to staying active after discharge were discussed.
In sessions 3, 4, and 5 (day 7, 14, and 2–4 weeks post-discharge), additional coaching and support were provided. Additional tailoring of the walking program and lower extremity exercises were made on target daily steps for post-discharge functional recovery. A print manual with intervention content was provided to participants. Several self-monitoring approaches to enhance adherence were included: (1) physical activity diary, (2) wristband pedometer (Vivofit 3; Garmin Ltd) wearing on the non-dominant hand for monitoring daily steps, and (3) training and encouraging caregivers to serve as “coaches” for patients.
Sample and setting
Patient eligibility criteria included (1) diagnosis of lung or GI (colorectal, gastric, pancreas, liver) cancers, (2) scheduled to undergo surgery, (3) age ≥ 65 years, and (4) ability to read and understand English. Caregiver eligibility criteria included (1) family member/friend identified by the patient as the primary caregiver before and after surgery, (2) age ≥ 21 years, and (3) ability to read and understand English. All eligible participants who met the study inclusion criteria were identified and recruited from one National Cancer Institute–designated comprehensive cancer center in Southern California over a 7-month period.
Outcome measures
Geriatric assessment was completed using a measure developed by Hurria and colleagues [16]. It includes the following domains: physical function, cognition, nutritional status, social support, comorbidity, psychological status, and polypharmacy. Objective measures of patient functional status included (1) pedometer-obtained daily steps, (2) 6MWT [17,18,19], TUG [20], and SPPB [21]. Patient- and caregiver-reported outcomes included psychological distress (distress thermometer) [22,23,24,25,26]. Patients also completed the MD Anderson Symptom Inventory (MDASI) lung [27] or GI [28] module for symptom assessment.
Study procedures
The study protocol was approved by the Institutional Review Board, and all participants provided written informed consent. Following informed consent, patients completed preoperative comprehensive functional assessments (geriatric assessment, 6MWT, TUG, SPPB), and baseline self-reported measures were completed; these were completed at least 7–14 days before surgery. Participants were also given a wristband pedometer for self-monitoring. They were instructed to wear the pedometer on their non-dominant hand 24/7 throughout the duration of the study, with the exception of day of surgery. The Vivofit 3 is water-proof, and has a battery life of 1 year. Participants were also provided with the intervention resource manual, and were instructed to refer to the manual during the intervention sessions. Research staff assisted participants with pedometer set-up, and also worked with them to select and set-up an engagement device for Zoom telehealth sessions.
Session 1 was administered after baseline assessments and at least 7–14 days before surgery via videoconferencing. Session 2 (in-person encounter) began with re-assessment of functional outcomes (6MWT, TUG, SPPB) and self-reported measures, followed by delivery of the session 2 content; these procedures were all completed within 24 h of planned discharge. Sessions 3, 4, and 5 (telehealth) were completed at days 2, 7, and 2–4 weeks post-discharge. All outcomes were also re-assessed at 2–4 weeks post-discharge; a satisfaction survey was also completed at this time to assess intervention acceptability. Pedometer data were continuously collected throughout the study period.
Statistical analysis
All analyses were generated using SAS 9.4®. Data were summarized using mean and standard deviation or median and range for continuous data, and frequency and percentage for categorical data. Established instruments were scored according to standard instructions, and appropriate descriptive statistics were computed. Outcomes included calculating the percentage of patients who demonstrated adherence with wearing the pedometer before and after surgery. All results were stratified by participant type (patient vs. caregiver), as well as by diagnosis (lung vs. GI). Data from instruments that were completed at multiple time points were summarized by individual time point. Baseline patient demographics were compared by diagnosis, and p values were provided to identify any underlying differences, using t test or Wilcoxon rank-sum test for continuous data and chi-square or Fischer’s exact test for categorical data. Exploratory analyses were conducted to examine change in functional and self-reported outcomes, from baseline to each of the follow-up time points. The Wilcoxon signed rank test was used to test whether paired differences were significantly different than 0.
Results
Feasibility and sociodemographic characteristics
Between November 2017 and June 2018, 45 potential patients/caregiver dyads who were eligible for study participation were screened. Of this total, 11 declined participation (24.4%). Reasons for declining included being no time (6), or being overwhelmed (5). Thirty-four dyads (16, GI; 18, lung) consented to participate in the study (average of 4 dyads per month), yielding an accrual rate of 75.6% over 8 months. Eight dyads (26 out of 34) dropped out of the study, yielding an attrition rate of 23.5%. Reasons for drop-out included too busy (3), too sick (2), and no longer interested (3).
Sociodemographic characteristics of the 68 patients and caregivers enrolled in this study are listed in Tables 2 and 3. Lung surgery patients were older than GI surgery patients, with median ages of 74 and 68 respectively (p = 0.03). Lung surgery caregivers were also older than GI surgery caregivers, with median ages of 71 and 67 respectively (p = 0.02). Forty-one percent of patients were female, 82% were of white race, and 94% were married. Fifty-nine percent of the caregivers were female, 73.6% were of white race, and 88% were married.
Acceptability
Accrual rate was 76% over 8 months and retention rate was 88% over 2 months. Intervention acceptability (as measured by self-reported satisfaction mean scores [29,30,31]) were high overall for both patients and caregivers (3.2/4.0 and 3.5/4.0, respectively). The caregivers reported higher satisfaction with the telehealth approach than patients (3.2/4.0 versus 2.9/4.0, respectively). Both patients and caregivers reported high satisfaction with use of a pedometer for self-monitoring daily steps (3.3/4.0). The overwhelming majority of patients (93.3%) thought that the timing of the intervention (starting before surgery) was appropriate.
Functional outcomes
Functional capacity at 2–4 weeks post-discharge, as measured by the 6MWT, exceeded baseline values for GI patients (411 m vs. 396 m, p = 0.7) as well as lung patients (420 m vs. 426 m, p = 0.8) (Tables 4 and 5). Functional mobility, as measured by mean TUG scores, gradually improved from baseline to post-discharge for lung patients (10.0 s to 9.1 s, p = 1.0). However, TUG scores worsened slightly in the GI patients from baseline to post-discharge (8.5 s to 9.9 s, p = 0.1). Neither of the changes observed were statistically significant, based on our exploratory analysis. For lower extremity physical performance status, exploratory analysis revealed significant improvements in mean SPPB score of greater than 1 point from before surgery to 2–4 weeks post-discharge (GI, 10.5–7.5; p = 0.001; lung, 10.2–8.2; p = 0.01).
Daily steps trends and trajectory
Preoperative patient adherence to pedometer use was 79%, and 68% post-discharge. Overall, the median number of preoperative daily steps was 6324; this number decreased to 1050 during hospitalization and gradually increased to 2927 in the first 2 weeks after discharge. Examination of daily steps from discharge to 4 weeks post-discharge revealed that the median daily steps were lowest during hospitalization, regardless of diagnosis (Fig. 1). During hospitalization, lung cancer patients had higher number of daily steps than GI cancer patients (median of 1331 vs. 164).
Patient- and caregiver-reported outcomes
Overall, symptom severity was mild over time (0–10 scale, higher scores represent higher severity). For lung cancer patients, the preoperative median MDASI symptom severity score was mild (0.2/10); this increased to 3.3/10 at hospital discharge (p = 0.01 compared to preop). By 2 to 4 weeks post-discharge, median symptom severity score was 2.3/10 (p = 0.3 compared to preop). For GI cancer patients, similar trends were observed, with preoperative symptom severity of 1.2/10, increasing to 2.2/10 at hospital discharge (p = 0.0006 compared to preop), and 1.9/10 by 2–4 weeks post-discharge (p = 0.003 compared to preop).
The trajectory of patient and caregiver distress scores is depicted in Fig. 2. Similar trajectories were observed for all patients, with mild distress levels reported preoperatively; levels increased to moderate distress prior to discharge, with gradual improvements at 2–4 weeks post-discharge. For caregivers, distress levels were higher than patients at all time points prior to 2 weeks post-discharge, but followed a similar trajectory of gradual improvements after surgery.
Discussion
Our findings suggest that a conceptually based, personalized, telehealth perioperative physical activity intervention in older adults with cancer and their caregivers is feasible and acceptable. Accrual and retention rates were acceptable (greater than 70%), and participants generally reported satisfaction with the intervention as indicated by quantitative data derived from the satisfaction tool. We were successful in accruing and retaining both patients and their caregivers on the study. The intervention offers an opportunity to improve postoperative outcomes by promoting perioperative physical activity through telehealth, behavior change, and self-monitoring approaches. We chose to focus on older adults with cancer, a population at higher risk for postoperative morbidity and functional decline. Currently, only about 7% of published randomized trials target older adults with cancer [32]. Older adults may require a more personalized approach to physical activity [33]. We included several preoperative measures, including comprehensive geriatric assessment, to tailor the intervention based on a patient’s co-morbid conditions, tolerance, and preference.
We observed a pattern of gradual improvements in objective functional measures and subjective, self-reported outcomes postoperatively. However, the improvements were preempted by dramatic declines for all objective functional measures postoperatively; this underscores the abrupt and immediate impact of surgery on the patient’s overall functional status. The analyses were exploratory, and we are unable to statistically determine whether the changes are related to the intervention. However, the outcome patterns are consistent with published trials, where an immediate decline after surgery followed by gradual improvements is observed [14, 34]. In addition, several of the outcome score changes were clinically meaningful, particularly for the objective functional measures. A change of 14.0 to 30.5 m is clinically important for 6MWT [35]; for TUG, the minimum clinically important difference is 3.4 s [36]. Minimally, significant changes for SPPB are 0.3 to 0.8 points [37, 38]. Clinically, these improvements in the functional assessment of patients undergoing both lung and GI cancer surgery may potentially translate into a quicker return to baseline function. This suggests that when this study is expanded, the improved functional measures and quicker return to baseline could ultimately result in potential improvements in surgical outcomes, such as shorter length of stay, increased percentage of patients discharged to home instead of to inpatient rehabilitation, and potentially to decrease 30-day hospital readmissions. The observed higher distress levels in caregivers underscores the need to address caregiver needs in the perioperative setting. We have previously observed similar trends in lung cancer surgery [1].
The concept of perioperative physical activity is not new, and several published trials, including prehabilitation, reported benefits on surgical outcomes [14]. A recent study from Spain reported enhanced aerobic capacity and reduced postoperative complications with a 6-week, in-person program [39]. Although promising, this model of multiple preoperative sessions is challenging to implement in the USA, where the time between initial surgical consult and day of surgery is much shorter. In-person interventions may be prohibitive due to patient/caregiver travel burden [40].
Our telehealth and home-based intervention design aimed to address these implementation challenges and minimize participant burden. First, exercise adoption and adherence are determined by behavioral, physical, psychological, environmental, and social factors [41, 42]. The intervention is based on classic principles of behavior change, as the intent is not only to maximize perioperative outcomes but also to promote long-term physical activity behaviors in older adults with cancer. The benefits of physical activity participation should, in older adults, be long term rather than just focused around one specific trajectory (treatment) of the entire cancer continuum. Second, we included caregivers and supported their role in participating in the prescribed program, because social support is an effective facilitator of physical activity [40]. Including the caregivers is increasingly important as there is increased pressure to decrease postoperative length of stay. Thus, the caregivers are assuming the burden of caregiving after discharge. Finally, self-monitoring is a classic strategy to engage patients in behavior adoption and adherence [43]. Participants in this study used wristband pedometers to monitor their daily step activities. We have previously shown that fewer daily steps were correlated with higher risk for postoperative complications in major abdominal cancer surgery [30]. The present study builds on our experience and is unique in that it uses a combination of a personalized upfront assessment and physical activity plan, involving the caregivers throughout the entire process, integrating wearable devices for self-monitoring, and leveraging telehealth for participant accessibility to the intervention.
Limitations
Several limitations to this study warrant discussion. First, our small sample size was chosen with the intent to determine proof-of-concept; thus, findings are preliminary and limited to feasibility and acceptability. Secondly, the study population was heterogeneous, composed of GI and lung cancer patients undergoing a wide range of surgical procedures with different risk and complication profiles.
Conclusions
Our conceptually based, personalized telehealth perioperative physical activity intervention is feasible and acceptable for older adults undergoing GI or lung cancer surgery and their caregivers. Future directions include a randomized trial using the novel multiphase optimization strategy (MOST) design [44] to identify components of the intervention that contribute meaningfully to postoperative functional capacity improvements in older adults with cancer.
References
Kim JY, Sun V, Raz DJ, Williams AC, Fujinami R, Reckamp K, Koczywas M, Cristea M, Hurria A, Ferrell B (2016) The impact of lung cancer surgery on quality of life trajectories in patients and family caregivers. Lung Cancer 101:35–39. https://doi.org/10.1016/j.lungcan.2016.08.011
Mortensen AR, Thyo A, Emmertsen KJ, Laurberg S (2018) Chronic pain after rectal cancer surgery - development and validation of a scoring system. Color Dis 0(0). https://doi.org/10.1111/codi.14436
Pulvirenti A, Pea A, Rezaee N, Gasparini C, Malleo G, Weiss MJ et al (2019) Perioperative outcomes and long-term quality of life after total pancreatectomy. Br J Surg 0(0). https://doi.org/10.1002/bjs.11185
Poghosyan H, Sheldon LK, Leveille SG, Cooley ME (2013) Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer 81(1):11–26. https://doi.org/10.1016/j.lungcan.2013.03.013
Sun V, Burhenn PS, Lai L, Hurria A (2017) The impact of comorbidity on surgical outcomes in older adults with cancer. Semin Oncol Nurs 33(1):80–86. https://doi.org/10.1016/j.soncn.2016.11.008
Tan HJ, Saliba D, Kwan L, Moore AA, Litwin MS (2016) Burden of geriatric events among older adults undergoing major cancer surgery. J Clin Oncol 34(11):1231–1238. https://doi.org/10.1200/JCO.2015.63.4592
Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA (2009) Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27(17):2758–2765. https://doi.org/10.1200/JCO.2008.20.8983
Frambes D, Given B, Lehto R, Sikorskii A, Wyatt G (2018) Informal caregivers of cancer patients: review of interventions, care activities, and outcomes. West J Nurs Res 40(7):1069–1097. https://doi.org/10.1177/0193945917699364
Ferrell B, Wittenberg E (2017) A review of family caregiving intervention trials in oncology. CA Cancer J Clin 67(4):318–325. https://doi.org/10.3322/caac.21396
Schwartz AJ, Riedel RF, LeBlanc TW, Desai D, Jenkins C, Mahoney E et al (2019) The experiences of older caregivers of cancer patients following hospital discharge. Support Care Cancer 27(2):609–616. https://doi.org/10.1007/s00520-018-4355-2
Fry BT, Hallway A, Englesbe MJ (2018) Moving toward every patient training for surgery. JAMA Surg 153(12):1089. https://doi.org/10.1001/jamasurg.2018.1658
Merluzzi TV, Pustejovsky JE, Philip EJ, Sohl SJ, Berendsen M, Salsman JM (2019) Interventions to enhance self-efficacy in cancer patients: a meta-analysis of randomized controlled trials. Psycho-oncology 0(ja). https://doi.org/10.1002/pon.5148
Howell D, Harth T, Brown J, Bennett C, Boyko S (2017) Self-management education interventions for patients with cancer: a systematic review. Support Care Cancer 25(4):1323–1355. https://doi.org/10.1007/s00520-016-3500-z
Treanor C, Kyaw T, Donnelly M (2018) An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv 12(1):64–73. https://doi.org/10.1007/s11764-017-0645-9
McCorkle R, Ercolano E, Lazenby M, Schulman-Green D, Schilling LS, Lorig K, Wagner EH (2011) Self-management: enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin 61(1):50–62. https://doi.org/10.3322/caac.20093
Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, Rodin M, Panageas KS, Holland JC, Saltz L, Kris MG, Noy A, Gomez J, Jakubowski A, Hudis C, Kornblith AB (2005) Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 104(9):1998–2005. https://doi.org/10.1002/cncr.21422
Enright PL (2003) The six-minute walk test. Respir Care 48(8):783–785
Lord SR, Menz HB (2002) Physiologic, psychologic, and health predictors of 6-minute walk performance in older people. Arch Phys Med Rehabil 83(7):907–911
Harada ND, Chiu V, Stewart AL (1999) Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil 80(7):837–841
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39(2):142–148
Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik JM (2011) Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 66(1):89–96. https://doi.org/10.1093/gerona/glq167
National Comprehensive Cancer N (2003) Distress management. Clinical practice guidelines. J Natl Compr Cancer Netw 1(3):344–374
Zwahlen D, Hagenbuch N, Carley MI, Recklitis CJ, Buchi S (2008) Screening cancer patients’ families with the distress thermometer (DT): a validation study. Psycho-oncology 17(10):959–966. https://doi.org/10.1002/pon.1320
Zwahlen D, Hagenbuch N, Jenewein J, Carley MI, Buchi S (2011) Adopting a family approach to theory and practice: measuring distress in cancer patient-partner dyads with the distress thermometer. Psycho-oncology 20(4):394–403. https://doi.org/10.1002/pon.1744
Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD (2007) Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer 55(2):215–224. https://doi.org/10.1016/j.lungcan.2006.10.001
Tuinman MA, Gazendam-Donofrio SM, Hoekstra-Weebers JE (2008) Screening and referral for psychosocial distress in oncologic practice: use of the Distress Thermometer. Cancer 113(4):870–878. https://doi.org/10.1002/cncr.23622
Mendoza TR, Wang XS, Lu C, Palos GR, Liao Z, Mobley GM, Kapoor S, Cleeland CS (2011) Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the M D Anderson Symptom Inventory. Oncologist 16(2):217–227. https://doi.org/10.1634/theoncologist.2010-0193
Wang XS, Williams LA, Eng C, Mendoza TR, Shah NA, Kirkendoll KJ, Shah PK, Trask PC, Palos GR, Cleeland CS (2010) Validation and application of a module of the M. D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI). Cancer. 116(8):2053–2063. https://doi.org/10.1002/cncr.24920
Sun V, Raz DJ, Ruel N, Chang W, Erhunmwunsee L, Reckamp K, Tiep B, Ferrell B, McCorkle R, Kim JY (2017) A multimedia self-management intervention to prepare cancer patients and family caregivers for lung surgery and postoperative recovery. Clin Lung Cancer 18(3):e151–e1e9. https://doi.org/10.1016/j.cllc.2017.01.010
Sun V, Dumitra S, Ruel N, Lee B, Melstrom L, Melstrom K et al (2017) Wireless monitoring program of patient-centered outcomes and recovery before and after major abdominal cancer surgery. JAMA Surg 152(9):852–859. https://doi.org/10.1001/jamasurg.2017.1519
Reb A, Ruel N, Fakih M, Lai L, Salgia R, Ferrell B, Sampath S, Kim JY, Raz DJ, Sun V (2017) Empowering survivors after colorectal and lung cancer treatment: pilot study of a self-management survivorship care planning intervention. Eur J Oncol Nurs 29:125–134. https://doi.org/10.1016/j.ejon.2017.06.003
Bruns ER, van den Heuvel B, Buskens CJ, van Duijvendijk P, Festen S, Wassenaar EB, van der Zaag E, Bemelman WA, van Munster B (2016) The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Color Dis 18(8):O267–O277. https://doi.org/10.1111/codi.13429
Looijaard S, Slee-Valentijn MS, Otten RHJ, Maier AB (2018) Physical and nutritional prehabilitation in older patients with colorectal carcinoma: a systematic review. J Geriatr Phys Ther 41(4):236–244. https://doi.org/10.1519/JPT.0000000000000125
Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F (2018) Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg 153(12):1081–1089. https://doi.org/10.1001/jamasurg.2018.1645
Bohannon RW, Crouch R (2017) Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 23(2):377–381. https://doi.org/10.1111/jep.12629
Gautschi OP, Stienen MN, Corniola MV, Joswig H, Schaller K, Hildebrandt G, Smoll NR (2017) Assessment of the minimum clinically important difference in the timed up and go test after surgery for lumbar degenerative disc disease. Neurosurgery. 80(3):380–385. https://doi.org/10.1227/NEU.0000000000001320
Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, Vaes B, Legrand D, Verghese J, Wang C, Stenholm S, Ferrucci L, Lai JC, Bartes AA, Espaulella J, Ferrer M, Lim JY, Ensrud KE, Cawthon P, Turusheva A, Frolova E, Rolland Y, Lauwers V, Corsonello A, Kirk GD, Ferrari R, Volpato S, Campo G (2016) Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med 14(1):215. https://doi.org/10.1186/s12916-016-0763-7
Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ et al (2009) What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 13(6):538–544
Barberan-Garcia A, Ubre M, Roca J, Lacy AM, Burgos F, Risco R et al (2018) Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 267(1):50–56. https://doi.org/10.1097/SLA.0000000000002293
Ferreira V, Agnihotram RV, Bergdahl A, van Rooijen SJ, Awasthi R, Carli F et al (2018) Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer 26(8):2717–2723. https://doi.org/10.1007/s00520-018-4109-1
Moorthy K, Wynter-Blyth V (2017) Prehabilitation in perioperative care. Br J Surg 104(7):802–803. https://doi.org/10.1002/bjs.10516
Koll TT, Semin JN, Grieb BM, Dale W (2017) Motivating older adults with cancer to keep moving: the implications of lifestyle interventions on physical activity. Curr Oncol Rep 19(10):68. https://doi.org/10.1007/s11912-017-0623-4
Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME (2018) Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psycho-oncology 27(3):713–724. https://doi.org/10.1002/pon.4612
Collins L (2018) Optimization of behavioral, biobehavioral, and biomedical interventions: the multiphase optimization strategy (MOST). Springer, University Park, Pennsylvania
Funding
Research reported in this publication was supported by the COH Center for Cancer and Aging. It also included work performed in the Biostatistics and Mathematical Modeling Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have not conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official view of the Center for Cancer and Aging or the National Institutes of Health.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lafaro, K.J., Raz, D.J., Kim, J.Y. et al. Pilot study of a telehealth perioperative physical activity intervention for older adults with cancer and their caregivers. Support Care Cancer 28, 3867–3876 (2020). https://doi.org/10.1007/s00520-019-05230-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05230-0