Abstract
Purpose
The primary objective was to investigate the association between the amount of time spent in moderate-to-vigorous intensity physical activity (MVPA) and cancer-related fatigue (CRF) before, during, and 2 years after start of treatment.
Methods
The results of the present study are based on data from the study “Early rehabilitation of cancer patients.” Two hundred and forty patients (109 females) with one of the following cancer types were included: breast, colorectal, prostate and testicular cancer, and lymphoma. Chalder’s fatigue questionnaire (FQ) was used to map CRF at baseline, 4, 8, 12, and 24 months post-inclusion. Baseline was at the time of diagnosis, before treatment start. Physical activity was recorded using SenseWear armband (SWA) at baseline, 4 and 24 months post-inclusion.
Results
One hour increased MVPA daily at baseline was associated with lower fatigue with − 0.8 at 4 months’ follow-up (p < 0.001), − 0.7 at 8 months’ follow-up (p = 0.001), − 0.6 at 12 months’ follow-up (p = 0.008), and − 0.5 at 24 months’ follow-up (p < 0.043). The participants maintained and improved PA level at the two follow-up points.
Conclusion
The results imply that the amount of time spent in moderate to vigorous intensity physical activity at baseline can modify cancer related fatigue during and after cancer treatment. The participants managed to maintain and improve their activity level at the two follow-up points. Future research should map fatigue and measure activity, with objective measurement units, at several measurement points to map activity level over time and to substantiate these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of improved therapy, people with cancer survive longer but they have to deal with long-term consequences [1]. Cancer-related fatigue (CRF) is recognized as one of the most common and distressing side effects of the disease and its treatment [2,3,4]. Cancer-related fatigue has been increasingly recognized as an important symptom both during and after cancer treatment, with a profound impact on the physical, mental, and emotional functioning of the patient [5]. It affects the patients’ normal daily activity, work, social relationships, and mood [2, 3].
Cancer-related fatigue is characterized by lack of energy, decreased physical ability, weakness, diminished attention or concentration, decreased interest or motivation to engage in usual activities, and severe tiredness [2, 5]. Patients describe CRF as an unexpectedly intense and severe tiredness, which is not relieved by rest or sleep [5]. It has been reported that 60–100% of cancer patients will experience CRF, depending on the cancer type and treatment. Onset of CRF may precede start of treatment, and CRF severity usually increases during cancer treatment, including treatment with radiation, chemotherapy, hormonal, and/or biological therapies [2, 6]. Usually, CRF improves during the year after the treatment is completed, but still a significant amount of patients experience fatigue for months or years after successful treatment [1, 2].
During the last decade, results from a number of published studies illustrates that physical activity (PA) may partly prevent and/or reduce CRF. Large meta-analyses conclude that non-pharmacological interventions such as PA and psychological interventions during active cancer treatment can improve CRF [1, 5, 7, 8]. On the other hand, pharmacological interventions have not been proven to significantly improve CRF [9, 10]. In addition to psychosocial interventions, nutritional counseling, cognitive behavioral therapy for sleep, and bright light therapy, the National Comprehensive Cancer Network (NCCN) guidelines for cancer-related fatigue recommend PA for reducing CRF during treatment [11]. This is in contrast to earlier recommendations that cancer patients should rest if they felt fatigued [1].
There is a consistent positive overall effect on fatigue through PA [7]. While the knowledge concerning fatigue is growing, there is still a lack of knowledge about the relationship between fatigue and PA [1, 8, 12], and specifically regarding the optimal amount of PA required to partly prevent and/or reduce CRF. To our knowledge, few studies have performed objective measures of the participants’ PA level at several time points, in order to examine the relationship between the amount of time spent in moderate-to-vigorous intensity physical activity (MVPA) and the level of CRF. The primary objective of the present study was to investigate the relationship between the amount of time spent in MVPA, measured by SenseWear armband (SWA), and self-reported CRF during treatment and 2 years after baseline. The secondary objective was to examine the participants’ level of MVPA before and during treatment and 2 years after treatment completion.
Methods
Design
The results of the present study are based on data from the study: “Early rehabilitation of cancer patients [13].”
In the main study, the participants were randomized to individual stress management intervention in two steps (Ia and Ib) or control (C), which meant treatment as usual. All patients received information regarding the benefits of PA and PA recommendations that were based on the NCCN guidelines [11]. All the patients were also encouraged to participate in a rehabilitation program at the Department of Oncology and Medical Physics. The rehabilitation program offered group based PA with an instructor, individual and group based, structured conversations, and course within relevant theme. The recruitment and the intervention program have been described previously [13, 14].
Study participants
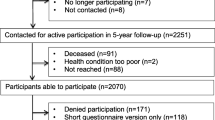
During May 2011 to June 2013, 1987 patients with a recent diagnosis of breast, colorectal, prostate or testicle cancer, or lymphoma were referred the Department of Oncology and Medical Physics, Haukeland University Hospital Bergen. Of these, all individuals over the age of 18, with stage I–III disease and scheduled for neo/adjuvant or curative treatment, i.e., chemotherapy, radiation therapy or hormonal therapy or any combination of these therapies, were considered for inclusion in the study. Exclusion criteria were on-going psychiatric condition (as determined by medical chart review), lack of fluency in Norwegian, or a previous diagnosis of cancer. After receiving information about the neo/adjuvant/curative treatment at the clinic, eligible patients (n = 677) received written information about the study by mail, informing them that they would be contacted by telephone and asked to participate in the study by project staff. Of these, 371 patients rejected participation, and 15 patients (2%) did not return the baseline questionnaires. Thus, 291 patients (43%) accepted participation in the study and returned the signed informed consent form and the baseline questionnaires by mail. Due to technical problems with the parsing, only 240 of patients (38%) wore SenseWear™ Pro3 Armband (Body Media Inc., Pittsburgh, PA, USA) (SWA) at baseline.
Participating patients were included in the study, a mean of 114 days post-diagnosis (as defined by the date on the histopathological report). Demographic and medical data for participating patients are shown in Table 1. The Regional Committee for Medical and Health Research Ethics in Western Norway and the Norwegian Center for Research approved the project, Dnr 2010/1911. We have not performed power calculations for this study.
Data collection
Assessment points for fatigue were baseline and 4, 8, 12, and 24 months post-inclusion. The questionnaires were sent by mail to the participants accompanied by written instructions and a prepaid return envelope. The project staff checked the returned questionnaires for incomplete responses and contacted the participant to complete them. In order to secure the points of assessment, the participants were sent a reminder within 14–21 days if the questionnaires had not been returned. If the questionnaires had still not been returned after three reminders, the participant was excluded from the study. SenseWear armbands were mailed to participants upon receipt of the baseline questionnaires. Assessment points for PA level were at baseline, 4, and 24 months post-inclusion.
Demographic data, e.g., age, residential area, and social status were collected using a brief questionnaire. Medical records supplied medical data, such as diagnosis, other health complaints, and primary treatments.
Primary outcome, CRF, was measured using the Chalder’s fatigue questionnaire (FQ) [15], also known as the Chalder’s fatigue scale. The questionnaire has been used in population studies and has normative data available for comparison with cancer patients [16]. FQ is a brief and easy scale to administer, and still it covers two aspects of fatigue: mental fatigue (four items) and physical fatigue (seven items), as well as total fatigue (all items) [16, 17]. It consists of 11 questions where the patients answer one of the following statements “Better than usual”, “No more than usual”, “Worse than usual”, or “Much worse than usual”, as well as two questions mapping the duration and scope of fatigue. In the present study, the responses were scored on a Likert scale (0, 1, 2, 3). The scores are weighted as follows: better than usual = 0, no more than usual = 1, worse than usual = 2, and much worse than usual = 3. The top score is 33, and a higher score indicates worse fatigue. Total fatigue is calculated as the sum of the Likert scores for the whole scale [16, 18].
Physical activity level was recorded by SWA. According to manufacturer instructions, the armband was carried on the left overarm/triceps for seven consecutive days. Females who had had breast surgery on the left side could place the armband on the right arm. SWA is an activity monitor that is easily used by participants. The armband uses a 2-axis accelerometer, a heat flux sensor, a galvanic skin response sensor, a skin temperature sensor, and a near-body ambient temperature sensor to capture data. It has been found valid during free-living activities compared with the doubly labeled water method [19]. SenseWear armband seems to provide accurate and reliable estimates, also in cancer patients [20]. Physical activity is presented as hours per day. In the present study, all patients who had been wearing SWA > 4 days were included in the analysis. A valid day required 80% wearing time, 19.2 h. Cut-off points for MVPA were set to 3 metabolic equivalents (METs) [21]. Due to technical problems, such as the battery ended, a considerable amount of data from the SenseWear monitor, from baseline to the two SWA follow-up points, were lost.
Statistical analysis
Descriptive data used to characterize the sample are presented as median, with range. A linear regression model was used to investigate the association between PA at baseline and follow-up, adjusted for intervention group or control group. To assess the time dependent association between CRF and PA, we estimated a linear mixed effect model with simple contrast (changes from baseline) for physical fatigue, mental fatigue, and total fatigue depending on PA at baseline, time, and their interaction. All models were estimated without adjustments, as well as adjusted for sex, age, and both sex and age. We used an ANOVA for nested models to select the best model.
Significance level for all statistical tests was set to 0.05. SPSS 23 (IBM, Armonk) and R 3.3 [22] with the package nlme 3.1 [23] were used to compute the statistical analyses. Matlab 9.0 (Mathworks Inc., Natick) was used to generate the graphics.
All analyses were made according to the “intention to treat” principle. For participants who did not complete all items on scales, the missing value was replaced by the mean of the available individual item scores within each subscale, provided that at least half of these had been completed.
Results
Participants
Forty-five percent of the participants were females, and breast cancer was the most common cancer type (41%) among women. For men, the most common cancer type was prostate cancer (43%). Fifty-six percent of the participants had surgery (Table 1).
Amount of time in MVPA
Mean MVPA at baseline was 1.7 h per day. The amount of time spent in MVPA increased to 1.8 and 3.3 h at 4 and 24 months follow-up, respectively (Fig. 1). A linear regression model showed that PA registration at 4 months was positively associated with PA baseline score (p < 0.001). Thirty-seven percent of the participants reported PA within the rehabilitation program at the Department of Oncology and Medical Physics during treatment, and the mean number of physical sessions reported was 15.
Registration of fatigue
The mean values (standard deviations) for the FQ subscales at each point of assessment are presented in Fig. 2. The figure shows a significantly increased level of fatigue (p < 0.001) at 4 months’ follow-up, persisting at 8, 12, and 24 months’ follow-up. A linear regression model showed that fatigue score at 4 months was positively associated with baseline score (p < 0.001).
Associations between FQ and MVPA
Higher MVPA at baseline were associated with less increase in fatigue between baseline and 4, 8, 12, and 24 months (p = 0.01). Example given 1 h increased PA at baseline was associated with reduced total fatigue at the first follow-up with − 0.8 at 4 months’ follow-up (p < 0.001), − 0.7 at 8 months’ follow-up (p = 0.001), − 0.6 at 12 months’ follow-up (p = 0.008), and − 0.5 at 24 months’ follow-up (p < 0.043). Over time, this association got weaker, but stayed significant (Table 2). We could only detect an association between fatigue at several follow-up points and PA at baseline.
Discussion
The main aim of the present study was to investigate the association between the amount of time spent in MVPA and the level of CRF. The results demonstrated that higher PA at baseline was significantly associated with lower CRF at follow-up. In present study, where the primary objective was to investigate the association between the amount of time spent in MVPA and CRF over time, we look at the whole group together. The intervention concerning stress management in the main randomized controlled trial did not focus on improving PA. All patients, who participated in the RCT “Early rehabilitation of cancer patients”, had the opportunity to engage in the organized PA at the department. The only difference was that patients in the intervention group received information that PA was a good way to manage stress reactions at their first stress management session. In addition, the sessions took place at the department, which might have inspired the patients in the intervention group to engage in the organized PA [13] to a larger extent than the control group. Therefore, we adjusted for group affiliation, but could not find that group affiliation which had an impact on the results. The secondary objective was to observe the participants level of MVPA before, during, and 2 years after the treatment. The participants’ maintained time spent in MVPA at 4 months’ follow-up. Time spent in MVPA increased from 4 months’ to 24 months’ follow-up. The results also showed a large increase in fatigue from baseline to the first follow-up (p < 0.001) and that fatigue stayed at the same high level at 8, 12 and 24 months’ follow-up (p < 0.001).
The results in present study support a dose-response relationship between MVPA and CRF and found that less time spent in MVPA is associated with higher amount of CRF. This promotes a vicious cycle of CRF and PA, as increased CRF often has a negative impact on PA [2]. Phillips, Awick [24] found in their study that lifestyle activity was only inversely associated with fatigue duration while sedentary time was positively associated with fatigue duration, which also supports a vicious cycle of CRF and PA. We wanted to prospective examine the relationship by objective measures of MVPA, but we have not focused on sedentary time. To our knowledge, the study by Phillips, Awick [24] is one of few studies examining this relationship prospectively and objectively measured activity in breast cancer survivors prospectively by accelerometer. They found an association between higher MVPA and lower fatigue interference at 6 months’ follow-up. The study by Phillips, Awick [24] differs from the present study with the fact that they only had one objective measure of the activity at baseline. Additionally, the participants had already finished their cancer treatment when they were included in the study. Despite these differences, the findings provide support for a dose-response relationship between MVPA and CRF [24], as well as the findings in the present study. Present study can contribute with information concerning MVPA and CRF during cancer treatment and post treatment. By using three measurement points for SWA; baseline, 4 months’, and 24 months’ follow-up, we got a picture of the patients’ activity over time. The participants managed to maintain and even improve their activity level at the two follow-up points.
To our knowledge, few other studies have used objective methods for measuring PA in this setting. Many studies base their intervention on PA, but the results give little information about the amount of time the participants have been active, and the other studies do not report on PA change over time [4, 25,26,27,28,29,30,31]. In addition, most of these studies use subjective measurement methods, such as various questionnaires and Borg scales. In many studies, participants engage in supervised PA and are encouraged to be physically active in their leisure time. When reporting their activity, recall bias, including variations in the subjective perception of the activity, is a relevant issue.
The use of several measurement points for fatigue provides an indication of fatigue over time. The results showed a large increase in CRF from baseline to the first follow-up. Fatigue may be elevated before treatment onset, but usually it increases during cancer treatment, which includes treatment with radiation, chemotherapy, hormonal, and/or biological therapies. Both cancer diagnosis and type of cancer treatment affect CRF [2]. In that context, the results showing the large increase from baseline to the first follow-up seems logical, especially since all of the patients at that point had started the treatment. The fatigue score at 4 months was significantly associated with the baseline score (p < 0.001). This finding is in line with other findings that show that the strongest and most consistent predictor for increased fatigue is pre-treatment fatigue. Patients who report higher levels of fatigue before treatment also report increased fatigue immediately after treatment and over the following years [2]. In present study, the increased level of fatigue persisted at 8, 12, and 24 months follow-up. This shows that fatigue does not resolve itself once treatment is completed and that treatment for CRF is required. The results showed that 1 h increased activity at baseline was associated with reduced fatigue at follow-up (Table 2). According to the literature, patients who are more fatigued typically report lower levels of PA. Lack of PA may lead to physical deconditioning, which makes everyday tasks more challenging and can potentially contribute to development and persistence of fatigue [2].
Despite lack of knowledge about the relationship between PA and CRF, some possible explanations have been suggested for the beneficial effect of PA on fatigue. A possible mechanism for the effectiveness of resistance exercise in reducing CRF among breast and prostate cancer survivors may be the attenuation of progressive muscle waste and disruption [32]. Segal, Reid [33] found a reduction in fatigue in patients who performed strength (p = 0.010) and aerobic exercise (p = 0.004) while receiving radiation therapy for prostate cancer. Buffart, Galvao [34] and Gardner, Livingston [35] found that resistance and aerobic exercise intervention significantly reduced fatigue in patients undergoing androgen deprivation therapy for prostate cancer. However, they could not explain the intervention effects on fatigue by improved muscle strength, aerobic capacity or walking speed, nor by reduced inflammation.
Fatigue may also be influenced by psychosocial factors, including depression and anxiety, which can be improved by PA [34, 36, 37]. Strong correlations between the incidence of depression and fatigue in patients with cancer have been reported [2, 32]. The association between fatigue and depression is complex. Fatigue can occur as a symptom of depression, as depression is known to be a predisposing factor for the development of chronic fatigue. At the same time, fatigue may precipitate feelings of depression since it affects the person’s mood, work, activity level, and/or sleep [32, 38]. Schmidt, Wiskemann [39] investigated the effect of resistance exercise among breast cancer patients during adjuvant chemotherapy. They found that the patients with baseline depression had substantially higher fatigue, and the levels remained high or decreased over time with no significant between-group differences.
Strengths and limitations
Using objective measurement methods is a strength and has several benefits. However, it is important to be aware that the SWA has certain weaknesses. A validation study of PA monitors showed that SWA, compared with indirect calorimetry, overestimated time in MVPA by 2.9% and underestimated very vigorous intensity PA [40]. It is important to bear in mind the possible overestimation of time spent in MVPA when considering the results. At the same time, it is important to remember the Hawthorne effect in use of objective measurement methods [41].
It may be relevant to consider the data on participants’ activity level during and post treatment in the context of the considerable dropout rate. To our surprise, the participants maintained their activity level during treatment. While this finding is encouraging, it is also unexpected. At 4 months’ follow-up, most of the patients were in the middle of cancer treatment, and we know from earlier studies that treatment can have a negative impact on patients’ physical shape and their ability to be physically active. It is possible that participants who used SWA at the follow-up points were the ones least affected by the cancer treatment or the ones who were the most physically active to start with. At the same time, it is important to remember the possible impact of the Hawthorne effect in use of objective measurement methods, which implies that study participants change their behavior due to an awareness of being observed [41]. It is conceivable that participants may have increased their activity level because of wearing the SWA.
For the purpose of mapping the participants CRF, the FQ was used. Minton and Stone [17] recommend using the FQ in circumstances where a multidimensional fatigue instrument is required. Although the instrument was not originally developed for use in cancer patients, the scale has robust psychometric properties and has been extensively used in other populations. The scale has been used to measure CRF in more than 2000 patients, although its main use has been in the investigation of chronic fatigue syndrome [16]. The dropout from baseline until 12 months is low, possibly because the questionnaire is brief and manageable. When mapping CRF, it is important to bear in mind that the prevalence of CRF can vary widely depending on the chosen measurement tool [17].
Conclusion
The results imply that the amount of time spent in moderate-to-vigorous intensity physical activity at baseline can modify cancer related fatigue during and after cancer treatment. The participants managed to maintain and even improve their activity level at the two follow-up points. Future research should map fatigue and measure activity with objective measurement units, at several measurement points, to map activity level over time and to substantiate these results.
References
Cramp F, Byron-Daniel J (2012) Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 11:97
Bower JE (2014) Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11(10):597–609
Al-Majid S et al (2015) Effects of exercise on biobehavioral outcomes of fatigue during cancer treatment: results of a feasibility study. Biol Res Nurs 17(1):40–48
Travier N et al (2015) Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med 13:121
Tomlinson D et al (2014) Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil 93(8):675–686
Scott K, Posmontier B (2017) Exercise interventions to reduce cancer-related fatigue and improve health-related quality of life in cancer patients. Holist Nurs Pract 31(2):66–79
Minton O, Jo F, Jane M (2015) The role of behavioural modification and exercise in the management of cancer-related fatigue to reduce its impact during and after cancer treatment. Acta Oncol 54(5):581–586
Velthuis MJ et al (2010) The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 22(3):208–221
Hilfiker R et al (2018) Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 52(10):651–658
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, Scarpato J, Smith T, Sprod LK, Miller SM (2017) Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 3(7):961–968
National Comprehensive Cancer Network, NCCN clinical practice guidelines in oncology (NCCN guidelines) cancer-related fatigue. 2018.
Mishra SI et al (2012) Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev 8:379
Arving C et al (2018) Early rehabilitation of cancer patients-an individual randomized stepped-care stress-management intervention. Psychooncology
Arving C et al (2013) Early rehabilitation of cancer patients - a randomized controlled intervention study. BMC Cancer 13:9
Chalder T et al (1993) Development of a fatigue scale. Journal of Psychomatic Research:147–153
Loge JH, Ekeberg Ø, Kaasa S (1998) Fatigue in the general norwegian population: normative data and assoiations. J Psychosom Res 45:53–65
Minton O, Stone P (2009) A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 20(1):17–25
Neuberger GB (2003) Measures of fatigue; the fatigue questionnaire, fatigue severity scale, multidimensional assessment of fatigue scale, and short form-36 vitality (energy/fatigue) subscale of the short form health survey. Arthritis & Rheumatism (Arthritis Care & Research) 49(5S):9
St-Onge M et al (2007) Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr 85(3):742–749
Cereda E, Turrini M, Ciapanna D, Marbello L, Pietrobelli A, Corradi E (2007) Assessing energy expenditure in cancer patients: a pilot validation of a new wearable device. JPEN J Parenter Enteral Nutr 31(6):502–507
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359
R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2016.
Pinheiro, J., et al., nlme: linear and nonlinear mixed effects models. R package version 3.1-127. 2016.
Phillips SM, Awick EA, Conroy DE, Pellegrini CA, Mailey EL, McAuley E (2015) Objectively measured physical activity and sedentary behavior and quality of life indicators in survivors of breast cancer. Cancer 121(22):4044–4052
Truong PT, Gaul CA, McDonald R, Petersen RB, Jones SO, Alexander AS, Lim JT, Ludgate C (2011) Prospective evaluation of a 12-week walking exercise program and its effect on fatigue in prostate cancer patients undergoing radical external beam radiotherapy. Am J Clin Oncol 34(4):350–355
Steindorf K, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, Habermann N, Beckhove P, Owen R, Debus J, Wiskemann J, Potthoff K (2014) Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 25(11):2237–2243
van Waart H et al (2015) Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 33(17):1918–1927
Juvet LK et al (2017) The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast 33:166–177
Lipsett A, Barrett S, Haruna F, Mustian K, O'Donovan A (2017) The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast 32:144–155
Mishra SI et al (2012) Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev 8:460
Brown JC et al (2011) Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomark Prev 20(1):123–133
Ryan JL et al (2007) Mechanisms of cancer-related fatigue. Oncologist 12(Suppl 1):22–34
Segal RJ et al (2009) Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 27(3):344–351
Buffart LM, Galvão DA, Chinapaw MJ, Brug J, Taaffe DR, Spry N, Joseph D, Newton RU (2014) Mediators of the resistance and aerobic exercise intervention effect on physical and general health in men undergoing androgen deprivation therapy for prostate cancer. Cancer 120(2):294–301
Gardner JR, Livingston PM, Fraser SF (2014) Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol 32(4):335–346
Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 4(2):87–100
Bower JE (2007) Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun 21(7):863–871
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR (2000) Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 18(4):743–753
Schmidt ME et al (2015) Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer 137(2):471–480
Berntsen S, Hageberg R, Aandstad A, Mowinckel P, Anderssen SA, Carlsen KH, Andersen LB (2010) Validity of physical activity monitors in adults participating in free-living activities. Br J Sports Med 44(9):657–664
McCambridge J, Witton J, Elbourne DR (2013) Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 67:267–277
Acknowledgments
We thank all participants and professional staff of participating institutes. The study is ethically approved, and founded by charity awards from the Grieg foundation as well as the Norwegian Cancer Society.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nilsson, M., Arving, C., Thormodsen, I. et al. Moderate-to-vigorous intensity physical activity is associated with modified fatigue during and after cancer treatment. Support Care Cancer 28, 3343–3350 (2020). https://doi.org/10.1007/s00520-019-05176-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05176-3