Abstract
Purpose
To assess changes in neutropenia-related hospitalization, myelosuppressive chemotherapy, and primary prophylactic colony-stimulating factor (PP-CSF) use in elderly cancer patients receiving myelosuppressive chemotherapy.
Methods
We identified annual cohorts of patients aged ≥ 66 years with breast cancer, lung cancer, or non-Hodgkin lymphoma (NHL) initiating myelosuppressive chemotherapy during 1995–2015 using Medicare 5% (1994–2008) and 20% (2007–2015) data. We described myelosuppressive chemotherapy changes by febrile neutropenia (FN) risk category (high, intermediate, unclassified), PP-CSF use, and, in the first cycle of myelosuppressive chemotherapy, neutropenia-related hospitalization (ICD-9-CM: 288.0X, first 5 positions). We evaluated hospitalization trends using a logistic regression model with spline curve of calendar year adjusting for baseline characteristics.
Results
Annual cohorts included 1451–2114 eligible patients for 1995–2007 and 5272–7603 for 2008–2015. Myelosuppressive chemotherapy use with high/intermediate FN risk increased from 31% in 1995 to 56% in 1999, stabilized through 2008 (range 56–61%), then decreased to 52% in 2015. PP-CSF use increased from 5.5% in 1995 to 52.7% in 2015, mainly due to pegfilgrastim introduction in 2002. Crude neutropenia-related hospitalization incidence decreased from 5.2% in 1995 to 2.7% in 2015; adjusted incidence decreased, on average, by 4.7% yearly before 2010 (p < 0.0001) and was flat from 2010 onward (p = 0.53).
Conclusions
Among elderly patients with breast cancer, lung cancer, or NHL receiving myelosuppressive chemotherapy, PP-CSF use increased substantially after 2002. Neutropenia-related hospitalization incidence in the first cycle decreased yearly before 2010 and was flat afterward. Further studies are needed to understand overall decreasing neutropenia-related hospitalization trends and effects of changes in myelosuppressive chemotherapy and FN management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy has evolved in the past two decades as myelosuppressive regimens became more common from the mid-1990s to the mid-2000s [1], and the use of taxane-based chemotherapy increased accompanying decreases in anthracycline-based chemotherapy for breast cancer after 2005 [2]. While newer targeted therapies and immunotherapies have been enthusiastically welcomed in recent years, most cancer patients continue to receive myelosuppressive chemotherapy [3]. Febrile neutropenia (FN) is a serious complication of myelosuppressive chemotherapy that occurs more commonly in the first cycle [4, 5] and is associated with significant morbidity, mortality, and healthcare costs [6,7,8]. FN is considered a medical emergency generally requiring hospitalization, which on average lasts from 6 to 11 days [6, 9,10,11]. Neutropenia-related hospitalization (NRH) results in chemotherapy dose reductions, delays, and discontinuations, compromising disease control, quality of life, and overall survival [12,13,14,15,16].
Given the demonstrated efficacy of primary prophylactic colony-stimulating factors (PP-CSF) in reducing FN [17,18,19,20], current clinical guidelines recommend PP-CSF for patients receiving chemotherapy regimens associated with high FN risk (> 20%) [21,22,23,24]. Additionally, for patients receiving chemotherapy regimens associated with intermediate FN risk (10–20%), the National Comprehensive Cancer Network (NCCN®) guidelines [21] recommend considering PP-CSF if at least one patient-level FN risk factor (e.g., age > 65 years) is present. However, this important preventive measure is routinely underutilized in the real-world setting among patients receiving high FN-risk regimens [25, 26]. Literature is lacking regarding changes in chemotherapy regimens by FN risk category, changes in PP-CSF use, and incidence of NRH in elderly cancer patients. Available studies are limited to single cancer types with older data [27] or selected regimens [28].
The objectives of this study were to estimate the proportion of elderly patients receiving myelosuppressive chemotherapy by FN risk category (high, intermediate, or unclassified), PP-CSF use, and in the first cycle of myelosuppressive chemotherapy, NRH incidence using annual cohorts of Medicare patients diagnosed with breast cancer, lung cancer, or non-Hodgkin lymphoma (NHL)—the cancers most commonly treated with myelosuppressive chemotherapy.
Materials and methods
Study design and data source
We conducted an ecological trend study using Medicare 5% (1994–2008) and 20% (2007–2015) sample data from the Centers for Medicare & Medicaid Services (CMS). The CMS-sponsored Medicare program is the primary health insurer for 97% of the US population aged ≥ 65 years and covers hospital, physician, and outpatient medical services provided to beneficiaries [29]. The 5% and 20% Medicare samples are systematic random samples of all Medicare beneficiaries generated by selecting those with 05, 20, 45, 70, or 95 in the last two positions of their health insurance claim numbers, and those with 0 or 5 in the last position, respectively. Data included in the annual denominator file containing demographic information for each patient include date of birth, sex, race, place of residence (state, county, and zip code), Medicare enrollment status, and the annual claims-based standard analytic files including Part A institutional and Part B physician/supplier claims. This study was approved by the Office for Human Subjects Research of Hennepin Healthcare System.
Patients and cohorts
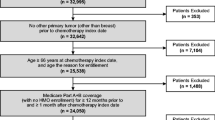
We created annual cohorts of patients initiating myelosuppressive chemotherapy for female breast cancer, lung cancer, or NHL from January 1, 1995, to September 30, 2015 (Fig. 1). We included patients aged ≥ 66 years at chemotherapy initiation who survived the subsequent ≥ 6 days (to ensure the time period needed to identify regimen and day of chemotherapy completion in the first cycle). Patients were also required to be continuously enrolled in Medicare Parts A and B for at least 365 days before and 6 days after myelosuppressive chemotherapy initiation, without enrollment in a health maintenance organization. Chemotherapy initiation was defined as first myelosuppressive chemotherapy treatment in the year, with no claims for myelosuppressive chemotherapy agents in the preceding 365 days; we used Healthcare Common Procedure Coding System (HCPCS) codes to identify the chemotherapy regimens (Online Resource 1). We excluded patients who had received radiotherapy or had evidence of stem cell transplant (Online Resource 2) in the 365 days before and 6 days after myelosuppressive chemotherapy initiation.
Study design for annual cohorts of patients who initiated myelosuppressive chemotherapy. Eligible patients had breast cancer, lung cancer, or NHL and had initiated myelosuppressive chemotherapy in each year from January 1, 1995, to September 30, 2015. The annual cohorts were identified using Medicare 5% (1994–2008) and 20% (2007–2015) sample data. The Medicare 5% and 20% data from 1994 and 2007, respectively, were used for baseline derivation purposes only and the 5% data from 2008 for follow-up purpose only. The 2015 cohort was a partial-year cohort including patients who initiated myelosuppressive chemotherapy from January 1, 2015, to August 31, 2015. September 2015 was a follow-up only month. In this example, completion of chemotherapy occurred on day 3 of the first cycle. All chemotherapies were administered on day 1 of the first cycle. NHL, non-Hodgkin lymphoma; PP-CSF, primary prophylaxis with colony-stimulating factor
We identified chemotherapy cycles and regimens in Medicare Part A outpatient and Part B carrier claims from January 1, 1995, to September 30, 2015, using the algorithm described by Weycker et al. [30]. Briefly, the first myelosuppressive chemotherapy cycle started on the initiation date (day 1) and ended at the next administration, at least 6 days but no more than 35 days after initiation. If no second myelosuppressive chemotherapy cycle started before day 35, the first cycle was considered completed at the earliest of day 35 after initiation, the day before regimen change, death, stem cell transplant, radiation, disenrollment from Part A or B, or September 30, 2015.
We defined chemotherapy regimen based on the HCPCS Level II codes for parenterally administered antineoplastic agents (myelosuppressive and non-myelosuppressive) on claims with service dates from day 1 to day 6 of the first cycle and, on the same claims, an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for female breast cancer, lung cancer, or NHL. Regimens were classified as “high” or “intermediate” with regard to FN risk based on the NCCN® clinical guidelines on the use of myeloid growth factors for 2005–2017 [31, 32] (Online Resource 3). For regimens listed multiple times with different risk classes, the latest risk class was used. Regimens not classified as high or intermediate FN risk anytime during the study period were grouped into an “unclassified” FN risk category. We further excluded regimens in the “unclassified” category with the identified first cycle length of < 11 days, since these are likely weekly regimens that would not usually prompt PP-CSF use. The date of the last administration of myelosuppressive chemotherapy agents in the first 6 days of the cycle was defined as the date of cycle completion.
The three cancers were defined based on ICD-9-CM diagnosis codes reported on the same claims for chemotherapy agents used to define the regimens (see Online Resource 4). To ensure that the identified regimen was used to treat the cancer of interest, patients with evidence of more than one cancer reported on chemotherapy claims in the first 6 days of the cycle were excluded.
Baseline and follow-up periods
We defined the baseline period as 365 days prior to chemotherapy initiation (Fig. 1). Patient-level covariates, including comorbid conditions, length of hospital stay, and neutropenia-related hospitalization (NRH), were defined during the baseline period, and age was assessed at the chemotherapy initiation date (Online Resources 5 and 6). The follow-up period started on the day of chemotherapy initiation and ended on the last day of the first chemotherapy cycle.
Study outcomes
Study outcomes were assessed during the follow-up period and included receiving myelosuppressive chemotherapy regimens by FN risk category (high, intermediate, or unclassified), PP-CSF use (overall and by CSF type), and NRH in the first chemotherapy cycle.
Receiving myelosuppressive chemotherapy regimen by FN risk category is described under “Patients and cohorts” above. PP-CSF use was defined as the administration of pegfilgrastim or any short-acting CSF (filgrastim, tbo-filgrastim, filgrastim-sndz, or sargramostim) on Part A outpatient/Part B claims with the corresponding HCPCS Level II codes (Online Resource 2) at or up to 5 days after completion of the first chemotherapy cycle. NRH was defined as Part A inpatient claims with ICD-9-CM diagnosis code 288.0X [33] (in the first 5 positions) occurring between day 7 and the last day of the first cycle. The first 6 days of the cycle were excluded for NRH assessment, because the neutrophil nadir usually occurs after day 6 and FN risk is reported to be very low in the first 6 days [34, 35].
Data analysis
For patient characteristics, numbers (n), percentages, means, and standard deviations are reported. For the outcomes of interest, proportions and 95% confidence intervals (CIs) are reported for each annual cohort overall and by cancer type. Temporal trends are illustrated using bar plots and line plots of the percentages over years. For NRH rates, we further evaluated trends using a logistic regression model with spline curve of calendar year adjusting for baseline patient characteristics (age, race, sex), FN risk category of myelosuppressive chemotherapy regimens, number of comorbid conditions, and history of hospitalization (hospital length of stay for any cause and NRH admission). Average percentage change over a year was estimated for different periods by replacing the spline curve with a piecewise-linear function. Because the logistic regression models the odds ratio, interpreting the year effect estimated from the model is difficult. Since the sample size was large and the probability of an event was low, the distribution of the number of events was closely approximated by the Poisson distribution. We therefore used a Poisson regression with a log link function to enable the estimation of the rate ratio.
Results
Patients
Cohort selection for select years is shown in Online Resource 7. Annual cohorts of eligible female breast cancer, lung cancer, or NHL patients combined included patients ranging from 1451 to 2114 during 1995–2007 and from 5272 to 7603 during 2008–2015 (Table 1, Online Resource 8). By cancer type, the ranges for the 1995–2007 and 2008–2015 cohorts were 409 to 627 patients and 1612 to 2143 patients for breast cancer (Table 1, Online Resource 9), 504 to 821 patients and 1783 to 2910 patients for lung cancer (Table 1, Online Resource 10), and 538 to 717 patients and 1877 to 2640 patients for NHL (Table 1, Online Resource 11), respectively.
The proportion of patients aged ≥ 80 years for the three cancers combined increased from 32% in 1995 to 36% in 2015 (range 30–37%; Table 1, Online Resource 8). By cancer type, the proportion of breast cancer patients aged ≥ 80 years decreased from 31% in 1995 to 27% in 2015 (range 31–25%; Table 1, Online Resource 9), but the proportion of lung cancer patients increased from 25% in 1995 to 35% in 2015 (range 25–37%; Table 1, Online Resource 10) and that of NHL patients increased from 39% in 1995 to 44% in 2015 (range 34–46%; Table 1, Online Resource 11).
Receiving myelosuppressive chemotherapy regimens by FN risk category
Temporal trends in myelosuppressive chemotherapy regimens with high/intermediate FN risk are shown in Fig. 2. For breast cancer, lung cancer, and NHL combined, the proportion of patients who received regimens with high/intermediate FN risk rapidly increased from 31% in 1995 to 56% in 1999, became fairly stable through 2008 (range 56–61%), then gradually decreased to 52% in 2015 (Fig. 2(a)). The trends differed among the three cancer types (Fig. 2(b)–(d)). For breast cancer, the proportion of patients, who received regimens with high/intermediate FN risk steadily increased from 22% in 1995 to 70% in 2007, was stable through 2010, and then slowly decreased to 65% in 2015 (Fig. 2(b)). For lung cancer, this proportion steadily increased from 50% in 1995 to 70% in 1997, stabilized through 2007 (range 67–74%), and then rapidly decreased to 47% in 2015 (Fig. 2(c)). For NHL, this proportion was < 20% before 1997, increased to 36% in 1997, and became relatively stable through 2015 (range 40–46%) (Fig. 2(d)).
PP-CSF use
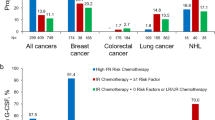
Temporal trends in PP-CSF use for all regimens combined and by FN risk category are shown in Figs. 3 and 4, respectively. For the three cancers combined, PP-CSF use with any agent increased from 5.5% in 1995 to 52.7% in 2015 (range 5.3–54.6%) (Fig. 3(a)). The trends were similar by cancer type; PP-CSF use with any agent increased from 3.2% in 1995 to 63.2% in 2015 for breast cancer (range 1.7–63.3%; Fig. 3(b)), from 4.8% in 1995 to 36.5% in 2015 for lung cancer (range 4.0–40.8%; Fig. 3(c)), and from 8.0% in 1995 to 59.2% in 2015 for NHL (range 8.0–61.2%; Fig. 3(d)). The increase mainly occurred in 2002–2004, attributed to the introduction of pegfilgrastim in 2002 (Fig. 3(a)–(d)). By FN risk category, PP-CSF use was more common in patients receiving regimens with high/intermediate FN risk than in patients receiving other regimens (Fig. 4(a)–(d)).
PP-CSF use by FN risk category (high/intermediate or unclassified) for (a) breast cancer, lung cancer, and NHL combined; (b) breast cancer; (c) lung cancer; and (d) NHL. aRegimens not classified as high or intermediate FN risk anytime during the study period. FN, febrile neutropenia; NHL, non-Hodgkin lymphoma; PP-CSF, primary prophylaxis with colony-stimulating factor
NRH in the first chemotherapy cycle
For the three cancers combined, the crude NRH rate decreased over the total study period from 5.2% in 1995 to 2.7% in 2015 (range 5.2–2.5%; Fig. 5(a)). In general, the crude rate decreased for each cancer type, with some variations. The crude rate decreased from 3.9% in 1995 to 2.6% in 2015 for breast cancer (range 3.9–1.9%; Fig. 5(b)), from 4.4% in 1995 to 1.4% in 2015 for lung cancer (range 4.5–1.4%; Fig. 5(c)), and from 6.9% in 1995 to 4.1% in 2015 for NHL (range 8.2–3.6%; Fig. 5(d)).
Overall unadjusted incidence (%) of neutropenia-related hospitalization in the first chemotherapy cycle for (a) breast cancer, lung cancer, and NHL combined; (b) breast cancer; (c) lung cancer; and (d) NHL. Neutropenia-related hospitalization definition was based on ICD-9-CM diagnosis codes for neutropenia (288.0X) in the first 5 diagnosis positions from Part A inpatient claims. ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NHL, non-Hodgkin lymphoma
After adjustment for changed patient characteristics, the NRH rate for the three cancers combined decreased, on average, by 4.7% (95% CI, − 6.4 to 3.8%) each year before 2010 (p < 0.0001) and was flat from 2010 onward (p = 0.53). For breast cancer, the rate decreased by 16% (95% CI, − 29 to 0%) each year before 1999 (p = 0.05), stabilized between 1999 and 2010 (p = 0.71), and then decreased by 2.6% (95% CI, − 7.8 to 2.9%) each year (not statistically significant, p = 0.35). For lung cancer, the rate decreased by 5.2% (95% CI, − 6.5 to 3.9%; p < 0.0001) each year over the study period. For NHL, the rate decreased by 5.9% (95% CI, − 7.1 to 4.7%; p < 0.0001) per year before 2010, then was flat after 2010 (p = 0.60).
Discussion
To our knowledge, this is the first population-based study describing temporal trends over two decades in chemotherapy regimens by FN risk categories, PP-CSF use, and NRH rate in elderly Medicare patients receiving myelosuppressive chemotherapy for female breast cancer, lung cancer, or NHL. For the three cancers combined, the use of high or intermediate FN risk regimens nearly doubled from 1995 to 1999, became stable through 2008 between 56 and 61%, and then slowly decreased to 52% in 2015. We observed a substantial increase in PP-CSF use from 6% in 1995 to 53% in 2015, with a rapid increase from 2002 to 2004 after pegfilgrastim introduction in 2002. Crude incidence of NRH during the first cycle following myelosuppressive chemotherapy decreased from 5.2% in 1995 to 2.7% in 2015. After adjustment for changing baseline patient characteristics that included FN risk category, the incidence of NRH declined, on average, by 4.7% each year before 2010 and became stable from 2010 onward. Patterns of PP-CSF use in the first chemotherapy cycle over time were similar for the individual cancers, but trends in chemotherapy regimens by FN risk category and NRH incidence differed.
Comparison of our findings regarding trends in chemotherapy regimens by FN risk category with findings from published literature is challenging, primarily because the prior published studies included a specific regimen [27], evaluated high FN risk regimens alone [28] or any chemotherapy [36], or used state-specific data with some differences in classification of regimens’ FN risk category [25]. In their study, Ramsey and colleagues [25] used four health insurance databases (Medicare, Medicaid, and 2 commercial) linked to the Western Washington State Surveillance, Epidemiology, and End Results (SEER) registry to study the patterns of CSF use among breast, colorectal, or non-small cell lung cancer patients who received myelosuppressive chemotherapy from 2002 to 2005. The authors reported that high or intermediate FN risk regimens were administered to 68% and 40% of Medicare patients with breast and non-small cell lung cancer, respectively [25]. In our study, 55–66% of patients with breast cancer treated with chemotherapy between 2002 and 2005 received high or intermediate FN risk regimens, similar to the findings reported by Ramsey et al. [25]. However, we observed much higher use of high or intermediate FN risk regimens for lung cancer, ranging between 67 and 72%. This was due to Ramsey et al. excluding small cell lung cancer or to different classification of the commonly used paclitaxel-carboplatin regimen defined as low risk in their study but intermediate risk in ours.
Our study provides recent data. In the past decade, among patients with lung cancer receiving myelosuppressive chemotherapy, use of high or intermediate FN risk regimens steadily decreased from 2007. This change may reflect the evolving landscape of lung cancer treatment, including newer targeted therapies and immunotherapies [37, 38], and the increasing proportion of elderly patients with lung cancer in recent years, which may have motivated oncologists to use less myelosuppressive chemotherapy.
Despite the different use patterns for high or intermediate FN risk regimens across the three cancer types, trends in PP-CSF use in the first cycle of chemotherapy by calendar year, from 1995 to 2015, were similar (3–63% for breast cancer, 5–37% for lung cancer, and 8–59% for NHL), with the rapid increase mainly occurring from 2002 to 2004. Our findings are consistent with the findings from earlier studies among elderly patients with newly diagnosed breast cancer receiving anthracycline- and/or taxane-based regimens [28] or NHL patients receiving cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-based chemotherapy [27]. The rapid uptake of pegfilgrastim after its introduction was shown by Du and colleagues [36] among elderly patients with breast cancer treated with any chemotherapy using the SEER-Medicare data from 2000 to 2009, and by Kozma and colleagues [39] using annual drug sales data obtained from the IMS Health Drug Distribution Database from 1994 to 2008.
Our study showed that PP-CSF use remained relatively stable from 2013 onward among patients with breast cancer or NHL but declined slightly among patients with lung cancer; this decline may be explained by the continuous declining trend in the use of high or intermediate FN risk regimens as newer targeted therapies and immunotherapies became available. Additionally, patients with lung cancer have more comorbid conditions than patients with the other two cancers (Online resources 9–11), possibly associated with cigarette use [40]. Thus, these patients may not tolerate intense myelosuppressive chemotherapies. We could not evaluate the chemotherapy doses delivered because dosing information is not available in claims data.
Although overall the incidence of first-cycle NRH declined over time, the trends differed across the three cancers. After adjustment for patient characteristics, NRH rates declined by 5% per year over the entire study period for lung cancer, in general mirroring the increasing trend in PP-CSF use and the decreasing trend in chemotherapy regimens at high or intermediate FN risk in recent years. For NHL and breast cancer, however, the decline in NRH rates did not continue throughout the study period; instead, rates became stable from 2010 onward for NHL and between 1999 and 2010 for breast cancer after taking into account changed patient characteristics over time. Using the SEER-Medicare linked data from 2002 to 2012, Goyal and colleagues [28] assessed the trends in FN risk (defined as neutropenia or infection or fever-related hospitalization) among elderly women with newly diagnosed breast cancer receiving anthracycline- and/or taxane-based chemotherapy. The authors reported that the observed FN incidence in the first chemotherapy cycle decreased from 8.6% in 2002 to 7.0% in 2012 (ranging between 4.6% in 2005 and 8.6% in 2002) for breast cancer. Over the same study period, in our study, we observed a slight increase in NRH rates from 2.9% in 2002 to 3.5% in 2012 (ranging between 2.0% in 2010 and 3.8% in 2005). The NRH rate in our study was lower because of the broad definition used by Goyal et al. [28].
Certain limitations of our study should be considered. Firstly, we chose a definition of NRH with high specificity (94%) for identifying FN, but given the low sensitivity (67%) of this definition, we likely underestimated the absolute FN burden for this population [33]. However, the use of a consistent NRH definition over time, even if it sacrifices sensitivity for gains in specificity, should not pose a threat to the validity of the NRH trends. Secondly, the FN risk associated with high-risk regimens varies, with a lower end of 20%. Although we controlled for chemotherapy FN risk category (high/intermediate or unclassified) in multivariable regression models, residual confounding likely exists in the assessment of trends in NRH rates. Thirdly, some regimens may be misclassified, particularly for patients who received combination therapy with a new agent during the study period in which a unique HCPCS Level II code was not yet available. We expect the misclassification was minimal. Additionally, data on chemotherapy dosage and intensity, performance status, lab results for kidney and hepatic function, and cancer stage that could affect PP-CSF use are not available in administrative claims data. Moreover, trends in FN patients receiving only outpatient care were not characterized in this study because most older cancer patients with FN received care in an inpatient setting. Thus, the impact of variations in outpatient treatment of FN over time could not be assessed. Lastly, the study findings may not be applied to patients aged younger than 65 years with breast cancer, lung cancer, or NHL, or older patients with Medicare advantage coverage, or patients residing outside the USA.
In conclusion, data from our study show that among elderly patients diagnosed with breast cancer, lung cancer, or NHL who received myelosuppressive chemotherapy from 1995 to 2015, PP-CSF use increased substantially after 2002. Over the same 21-year period, incidence of NRH in the first cycle decreased, on average, by 5% each year before 2010 and was flat from 2010 onward after controlling for patient characteristics. The decline in NRH can be attributed to a multitude of factors including improved understanding of FN risk following myelosuppressive chemotherapy, patient education and early FN management, considerations of patient-level risk factors, and prophylactic use of CSFs. However, the relative stabilization of NRH rates from 2010 onward indicates an existing clinical burden among patients receiving myelosuppressive chemotherapy. The decision to use PP-CSF during chemotherapy to prevent FN is multifactorial, and it is important to quantify FN risk based on all factors. Further studies regarding understanding additional factors responsible for NRH and developing a prediction model for FN risk based on patient-level risk factors, tumor-related characteristics, and the combination of agents and dosing will be helpful to identify strategies to reduce the persistent health burden of FN.
References
Smith RE (2006) Trends in recommendations for myelosuppressive chemotherapy for the treatment of solid tumors. J Natl Compr Cancer Netw 4:649–658
Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS (2012) Decline in the use of anthracyclines for breast cancer. J Clin Oncol 30:2232–2239
Marquart J, Chen EY, Prasad V (2018) Estimation of the percentage of us patients with cancer who benefit from genome-driven oncology. JAMA Oncol 4:1093–1098
Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, Lyman GH (2008) Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw 6:109–118
Lyman GH, Kuderer NM (2003) Epidemiology of febrile neutropenia. Support Cancer Ther 1:23–35
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116:5555–5563
Schilling MB, Parks C, Deeter RG (2011) Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exp Ther Med 2:859–866
Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916–1924
Tai E, Guy GP Jr, Dunbar A, Richardson LC (2017) Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract 13:e552–e561
Li S, Liu J, Bowers C, Garawin T, Kim C, Bensink ME, Chandler DB (2019) Febrile neutropenia-related care and associated costs in elderly patients with breast cancer, lung cancer, or non-Hodgkin lymphoma. Support Care Cancer. https://doi.org/10.1007/s001090000086
Lyman GH, Dale DC, Crawford J (2003) Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 21:4524–4531
Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI (2004) Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol 22:4302–4311
Lyman GH (2009) Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Cancer Netw 7:99–108
Shayne M, Culakova E, Poniewierski MS, Wolff D, Dale DC, Crawford J, Lyman GH (2007) Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 110:1611–1620
Shayne M, Culakova E, Wolff D, Poniewierski MS, Dale DC, Crawford J, Lyman GH (2009) Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer 115:5319–5328
Mhaskar R, Clark OA, Lyman G, Engel Ayer Botrel T, Morganti Paladini L, Djulbegovic B (2014) Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev CD003039
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, Neumann TA, Schwartzberg LS (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164–170
NCCN® Clinical Practice Guidelines in Oncology. Hematopoietic growth factors. V2.2019. National Comprehensive Cancer Network website. Available at: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed 30 May 2019
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, America IDSo (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:427–431
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO, American Society of Clinical O (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for R, Treatment of C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Ramsey SD, McCune JS, Blough DK, McDermott CL, Clarke L, Malin JL, Sullivan SD (2010) Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care 16:678–686
Sosa R, Li S, Molony JT, Liu J, Stryker S, Collins AJ (2017) Use of prophylactic growth factors and antimicrobials in elderly patients with cancer: a review of the Medicare database. Support Care Cancer 25:3123–3132
Elting LS, Xu Y, Chavez-MacGregor M, Giordano SH (2016) Granulocyte growth factor use in elderly patients with non-Hodgkin’s lymphoma in the United States: adherence to guidelines and comparative effectiveness. Support Care Cancer 24:2695–2706
Goyal RK, Tzivelekis S, Rothman KJ, Candrilli SD, Kaye JA (2018) Time trends in utilization of G-CSF prophylaxis and risk of febrile neutropenia in a Medicare population receiving adjuvant chemotherapy for early-stage breast cancer. Support Care Cancer 26:539–548
Mues KE, Liede A, Liu J, Wetmore JB, Zaha R, Bradbury BD, Collins AJ, Gilbertson DT (2017) Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 9:267–277
Weycker D, Li X, Tzivelekis S, Atwood M, Garcia J, Li Y, Reiner M, Lyman GH (2017) Burden of chemotherapy-induced febrile neutropenia hospitalizations in us clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer 25:439–447
NCCN® Clinical Practice Guidelines in Oncology. Myeloid growth factors. National Comprehensive Cancer Network website. Available at: www.nccn.org. Accessed 30 May 2019
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, Hough S, Kloth DD, Kuter DJ, Lyman GH, Mably M, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Roy V, Rugo HS, Saad AA, Schwartzberg LS, Shayani S, Talbott M, Vadhan-Raj S, Vasu S, Wadleigh M, Westervelt P, Burns JL, Pluchino L (2017) Myeloid growth factors, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15:1520–1541
Weycker D, Sofrygin O, Seefeld K, Deeter RG, Legg J, Edelsberg J (2013) Technical evaluation of methods for identifying chemotherapy-induced febrile neutropenia in healthcare claims databases. BMC Health Serv Res 13:60
Li Y, Klippel Z, Shih X, Reiner M, Wang H, Page JH (2016) Relationship between severity and duration of chemotherapy-induced neutropenia and risk of infection among patients with nonmyeloid malignancies. Support Care Cancer 24:4377–4383
Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10:427–437
Du XL, Zhang Y, Hardy D (2016) Temporal and geographic variations in the receipt of colony-stimulating factors and erythropoiesis-stimulating agents in a large retrospective cohort of older women with breast cancer from 2000 to 2009. Am J Ther 23:e411–e421
Davies J, Patel M, Gridelli C, de Marinis F, Waterkamp D, McCusker ME (2017) Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: a systematic review of recently published studies. PLoS One 12:e0175679
Ho C, Ramsden K, Zhai Y, Murray N, Sun S, Melosky B, Laskin J (2014) Less toxic chemotherapy improves uptake of all lines of chemotherapy in advanced non-small-cell lung cancer: a 10-year retrospective population-based review. J Thorac Oncol 9:1180–1186
Kozma CM, Dickson M, Chia V, Legg J, Barron R (2012) Trends in neutropenia-related inpatient events. J Oncol Pract 8:149–155
Cuyún Carter G, Barrett AM, Kaye JA, Liepa AM, Winfree KB, John WJ (2014) A comprehensive review of nongenetic prognostic and predictive factors influencing the heterogeneity of outcomes in advanced non-small-cell lung cancer. Cancer Manag Res 6:437–449
Acknowledgments
Medical writing support was provided by Martha Mutomba (on behalf of Amgen Inc.). The authors thank Chronic Disease Research Group colleague Nan Booth for manuscript editing.
Funding
This study was financially supported by the Amgen Inc.
Author information
Authors and Affiliations
Contributions
Research idea and study design: SL, JL, PG, CK, MB, DC; data acquisition: SL, JL, HG; data analysis/interpretation: HG, JL, SL, PG, CK, MB, DC; statistical analysis: HG, JL; supervision or mentorship: SL, JL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work.
Corresponding author
Ethics declarations
Conflict of interest
Shuling Li, Jiannong Liu, and Haifeng Guo are employees of Chronic Disease Research Group, Hennepin Healthcare Research Institute, which has received project funding from Amgen Inc. Prasad L. Gawade, Christopher Kim, Mark E. Bensink, and David Chandler are employees of and own stock in Amgen Inc.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Formal consent was not required as the article does not contain any studies involving human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 404 kb)
Rights and permissions
About this article
Cite this article
Li, S., Liu, J., Guo, H. et al. Trends in the use of primary prophylactic colony-stimulating factors and neutropenia-related hospitalization in elderly cancer patients receiving myelosuppressive chemotherapy in the USA: 1995–2015. Support Care Cancer 28, 2637–2649 (2020). https://doi.org/10.1007/s00520-019-05080-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05080-w