Abstract
Purpose
This systematic review aims to identify the risk factors for depression in cancer patients undergoing chemotherapy.
Methods
Eight electronic databases were searched from inception of the databases established until August 2017. References for the included studies were retrieved by manual searching. The quality of the eligible studies was appraised by two persons using the 11-item checklist of the Agency for Healthcare Research and Quality (AHRQ).
Results
Among 5988 potentially relevant articles, 43 studies were eligible, with 17 studies of high quality and 25 studies of moderate quality. A total of 65 factors were extracted, including sociodemographic characteristics (n = 20), physiological condition (n = 20), disease and treatment (n = 12), and psychosocial factors (n = 13). Only social support, anxiety, perceived stress, and self-efficacy were found to be consistently associated with depression in cancer patients. There is not enough evidence to support the link between the other 61 factors and depression in cancer patients undergoing chemotherapy.
Conclusions
This review suggests that the development of depression programs should take social support, anxiety, perceived stress, and self-efficacy into account. More original studies with rigorous design are necessary to further confirm those 61 inconclusive risk factors for depression in cancer patients receiving chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy is a common adjuvant treatment for cancer. However, it is also a stressor that can lead to psychological problems in cancer patients [1]. Compared to patients who have not received chemotherapy, cancer patients undergoing chemotherapy are prone to depression [2]. It has been reported that the incidence of depression is 16.8–45% [1, 3], and the incidence of a major depressive disorder is 12–18% among cancer patients undergoing chemotherapy [4]. Depression not only significantly reduces quality of life [5], but can also increase the rate of cancer recurrence [6] and the risk of death [7]. Thus, it is important to explore the associated risk factors for depression, which could help in the development of programs targeted at promoting psychological well-being.
Numerous studies have identified various associated risk factors for depression in cancer patients undergoing chemotherapy. For example, Polikandrioti et al. indicated that older patients experience higher levels of depression than younger patients [8]. However, other studies have indicated that age does not have an impact on depression [1, 9]. Similarly, a number of studies have found that females are more likely to experience depressive symptoms than males [1, 3, 10]. However, other studies have shown no significant correlation between depression and gender [8, 11]. A number of articles have reported that fatigue and sleep disorders were positively associated with depression [12]. However, fatigue and sleep disorders have not been demonstrated as a predictor of depression in regression analysis [13]. In terms of the chemotherapy period, Farrel et al. found that depression levels in chemotherapy patients would rise during consecutive stages of the therapeutic process [14]. Inversely, a prospective study indicated that patient depression peaked on the first day of chemotherapy, subsided significantly at the midway point, and then remained at similar levels until the last day of chemotherapy [1]. Generally, the evidence for depression and associated factors is unclear. Thus, several authors have reviewed the risk factors for depression in cancer patients in order to provide evidence for related clinical research. For example, Haisfield-Wolfe and colleagues conducted a systematic review of adult patients with head and neck cancer [15]. Based on 52 studies published between 1986 and 2008, they concluded that the main associated factors for depression were patient characteristics, physical symptoms, and treatment characteristics. Another systematic review was related to risk factors and psychological adjustment in patients with breast cancer, with depression as one of the dependent variables [16]. This review identified a number of risk factors for depression, including income level, fatigue, extent of social support, coping strategies, and optimism. Recently, a critical review focused on the prevalence of depression, risk factors, and screening for depression in patients with cancer [17]. Involving 21 reviews/meta-analyses, this critical review found that risk factors for depression were a family history of mood disorders, personal psychiatric history, personality traits, a history of stressful life events, loneliness, social isolation, low income, lack of social support, cancer type, cancer stage, inflammation factors, and medicines. These reviews greatly contributed to an understanding of the factors associated with depression in cancer patients, but none targeted cancer patients undergoing chemotherapy. Our systematic review, therefore, aims to identify the associated factors for depression in cancer patients undergoing chemotherapy, by synthesizing the results of existing papers.
Methods
The review protocol was registered on the PROSPERP (CRD 42016036619) (PROSPERO, 2016) [18]. The PRISMA guidelines were used to guide this systematic review [19].
Selection criteria
Inclusion criteria
-
(1)
Participants: patients were undergoing chemotherapy with any type of cancer, aged 18 or above, and any previous treatments before chemotherapy.
-
(2)
Exposure: the study reported one or more than one risk factor for depression.
-
(3)
Outcome: depression was assessed using standardized instruments, such as the Hospital Anxiety and Depression Scale-Depression subscale (HADS-D), Hamilton Depression Scale (HAMD), Beck Depression Inventory (BDI), and Self-Rating Depression Scale (SDS).
-
(4)
Study designs: cross-sectional survey, longitudinal study, cohort study, and case-control study.
-
(5)
Others: studies reported in English or Chinese.
Exclusion criteria
-
(1)
Participants: chemotherapy cancer patients simultaneously receiving radiotherapy.
-
(2)
Study designs: descriptive paper, reviews, and case reports.
-
(3)
Others: studies with duplicate data, deficient data, or unavailable full text.
Search strategy
An electronic search was performed for eligible articles in eight databases: MEDLINE, EMBASE, PsycINFO, Cochrane CENTRAL, EBSCO CINAHL, China National Knowledge Infrastructure (CNKI), WanFang Data, and Chinese Scientific and Technical Periodicals Database. All searches were limited to include only literature published in English or Chinese from inception to 20 August 2017. The search strategies were tailored to each database. For example, the search strategy for MEDLINE was as follows: exp. neoplasms/or (neoplas* or cancer* or tumor* or tumor* or onco* or malignan* or carcinoma* or adenocarcinoma*or choriocarcinoma* or leukemia* or leukemia* or lymphoma* or metastat* or sarcoma* or teratoma*).ab,ti.] and [exp. depression/or (depression or depressions or depressive symptom or symptom, depressive or symptoms, depressive or emotional depression or depression, emotional or depressions, emotional or emotional depressions).ab, ti.] and (exp chemotherapy/or chemotherapy.ab, ti). In addition, the references for the included studies were retrieved by manual searching.
Study selection
After the duplicates were removed, the articles that were to be considered for inclusion were screened by title and abstract. The full text articles were then retrieved for eligibility consideration. The study selection was independently conducted by two reviewers (WSS and YQ), and a consensus was reached by consulting with a third person (XHM).
Quality assessment
The 11-item checklist recommended by the Agency for Healthcare Research and Quality (AHRQ) was adopted to evaluate the quality of the studies that were included [19]. Each item was scored 0, 0, or 1, corresponding to the answers of “no,” “unclear,” and “yes,” respectively. In the scoring system, a score of 8 to 11 suggests high quality; 4 to 7 indicates moderate quality; and 0 to 3 represents low quality. The quality assessment was independently conducted by WSS and YQ.
Data extraction
The data extraction encompassed study design, published details, participant, disease stage and type, stage of treatment, instruments of depression, associated factors, depression score, or the number of depression. When data were missed or unclear, further details would be obtained by consulting the authors. Any disagreement in the data extraction process was negotiated between the two reviewers (WSS and YYQ), in consultation with a third person (XHM).
Data synthesis
In this review, we conducted a qualitative analysis rather than a statistical pooling of outcomes, because of the heterogeneity in the clinical characteristics of the study designs and outcomes. The risk factors for depression were synthesized by collating the study designs and sample size in a narrative manner. During the qualitative synthesis period, uncertainties were clarified, and consensus was reached through regular research team meetings.
Results
Study selection and quality assessment
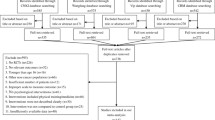
First, the duplicates were discarded and a total of 5988 titles and abstracts remained. Of these, 5807 articles were excluded by screening the titles and abstracts; and 181 articles were further reviewed using the full text. Finally, only 43 articles were included in this systematic review. Study selection details are shown in Fig. 1.
The selected studies included 26 cross-sectional and 17 longitudinal studies. All employed questionnaires or scales to collect data, and described the assessments undertaken for quality assurance. Most studies listed clear inclusion and exclusion criteria (n = 31), and reported the time period used for identifying patients (n = 36). Nearly half of the studies indicated consecutive subjects (n = 17), the rates of patient response and study completion (n = 21), and patient exclusions from analysis (n = 18). Only some studies reported confounding assessment (n = 13), how missing data were handled in the analysis (n = 7), and the percentage of patients with incomplete data or follow-up (n = 11). Overall, most were of high quality (n = 17) or moderate quality (n = 25).
Study characteristics
The 43 studies involved a total of 6151 participants, and had been published during the period from 2004 to 2017. Among these studies, 22 had been published in the past 5 years, while 21 had been published during the period from 2004 to 2012. A total of 31 studies reported the study setting as a hospital (n = 20), clinic (n = 8), or cancer center (n = 3). Most of the studies were conducted in Asia (n = 23) or Europe (n = 12). Depression was assessed by HADS (n = 24), SDS (n = 12), BDI-II (n = 3), Center for Epidemiological Studies-Depression Scale (CES-D) (n = 3), Profile of Mood States-Depression Subscale (POMS-D) (n = 1), or Geriatric Depression Scale—the 15-item version (GDS-15) (n = 1). In total, 64 factors were extracted from the 43 eligible studies, which included sociodemographic characteristics (n = 20), physiological condition (n = 20), disease and treatment (n = 12), and psychosocial factors (n = 12). Table 1 presents the details of eligible study characteristics.
Sociodemographic factors
Among the 43 studies, 15 studies with 2330 participants reported on the relationship between age and depression. Two cross-sectional studies indicated that older adults were more likely to suffer from depression, in comparison with younger groups [8, 20]. However, another cross-sectional study indicated that participants aged 35–50 were the most likely to experience depression [21]. Another 12 studies did not identify any significant association between age and depression [1, 9,10,11, 13, 22,23,24,25,26,27,28].
The correlation between education level and depression was explored by 12 studies with 2170 participants. Only five studies, with 834 participants, found that patients with higher education levels had a higher risk of depressive symptoms. Three studies were of moderate quality [8, 11, 23], and two were of high quality [13, 29]. The remaining seven studies found that education level did not have an impact on depression.
In terms of gender, 11 studies explored the impact of gender on depression in 2177 participants. Four studies with 1149 participants, including three studies of high quality and one study of moderate quality, indicated that female participants had experienced higher depression levels than male participants [1, 3, 10, 30]. However, another study, with 59 participants, found an inverse result [21, 26]. The remaining studies showed no relationship between gender and depression [11, 13, 20, 22, 23].
Five moderate quality studies explored income status as an independent variable for depression in 647 participants. Four studies found no relationship between depression and income [9, 24, 26, 28]. Only one study reported that Chinese cancer patients with less than 1000 yuan in monthly income suffered from severer depressive symptoms than those with more than 1000 yuan in annual income [23].
Four studies evaluated occupation as a risk factor for depression. One study found that pensioners and homemakers experienced higher depression levels than employees and freelancers. However, another three studies did not reach the same conclusion [13, 23, 31].
Additionally, one study showed that patients from nuclear families suffered from higher depression levels, compared to those from blended families [31]. Another study by Park and Yoon revealed that breast cancer patients with more frequent sexual activity had lower depression levels [9]. Indeed, other sociodemographic factors, such as marital status [11, 13, 22,23,24,25,26], race [11, 22], religion [26], ethnicity (Hispanics/non-Hispanics) [22], family history of cancer [28], history of abortion (one or more/none) [25], treatment site (tertiary/public) [11], living with others [13], living alone [3], social status [25], place of residence [25], general status [25], and length of postoperative hospital stay [25] did not identify any significant relationship with depression.
Physiological factors
Four studies explored fatigue as a risk factor for depression in 642 participants. Three of these studies demonstrated that fatigue was negatively related to depression in 431 participants [12, 32, 33]. The other study, with 211 ambulatory cancer patients, showed that fatigue was not significantly associated with depression [13].
Two studies found that patients with inadequate nutritional intake experienced higher levels of depression [3, 8]. Furthermore, participants with no weight change experienced less depression [8], compared to those who had gained or lost weight. Another two studies explored the impact of appetite loss on depression in 291 participants. One study showed that patients with appetite loss were significantly more likely to be depressed [28]. The other indicated a borderline significant relationship between appetite loss and depression. The author suggested loss of appetite could be another indicator of clinical depression [13].
Sleep disturbance was a listed risk factor for depression in three studies that had a total of 577 participants. Two out of the three studies, with 366 participants, provided evidence that patients with a sleep disturbance had higher depression scores, compared to patients without a sleep disturbance [34, 35]. However, when evaluating sleep disturbance as a variable in a multivariate analysis, the third study showed that sleep disturbance was not a significant factor [13].
Three studies with a total of 397 participants explored the relationship between pain and depression. Two studies showed that pain was positively associated with depression in 291 participants [13, 28]. However, another study, with 106 participants, suggested that pain symptom clusters (including shortness of breath, numbness/tingling, dry mouth, and pain) did not impact depression [36].
Two studies explored the relationship between chemotherapy-induced alopecia (CIA) and depression in 379 participants. One study of 168 breast cancer patients indicated that patients with high CIA distress were more likely to be depressed than a group with low CIA distress [37]. The other study did not draw the same conclusion [13].
Two studies indicated that patients with restless leg syndrome (RLS) had severe depression, compared to those without RLS [35, 38].
Two studies investigated serum inflammatory biomarkers. One study found that the trends of tartrate-resistant acid phosphatase 5a (TRACP5a), white blood cells (WBCs), and the number of monocytes, were significantly associated with depression in 62 lung cancer patients [39]. The other study identified cancer antigen 125 levels as an indicator of depression [38].
Three studies respectively reported that low sexual function [9], a low body mass index (BMI) score [22], and menopausal symptoms [9] were significantly associated with depression in cancer patients undergoing chemotherapy.
Additionally, there is no significant association between depression and certain factors, including nausea [13, 14], numbness [13], emetogenic level (moderate/high) [13], Karnofsky Performance Scale Index [10], gastrointestinal symptom clusters (including nausea, vomiting, and poor appetite), and hemoglobin levels [36].
Disease and treatment
Fourteen prospective studies investigated depression in 1561 cancer patients. Three studies [14, 33, 39], with a total of 195 participants, demonstrated that depression levels, or the number of patients with depression, would climb during consecutive stages of the chemotherapy process. Inversely, among 886 participants in four high quality studies and one moderate quality study, depression levels declined during consecutive stages of the therapeutic process [1, 40,41,42,43]. Jiayuan et al. further revealed that the depression scores were highest on the first day of the fourth cycle of chemotherapy [44]. Jane Hipkins followed up on ovarian cancer patients undergoing chemotherapy, and found that patients had higher depression levels at the end of chemotherapy, in comparison with post-treatment patients measured at 3 months after chemotherapy [45]. Chintamani and colleagues indicated that depression levels in a group of chemotherapy cycles > 3 were higher than depression levels in a group of chemotherapy cycles ≤ 3 [31]. However, no relationship was found between chemotherapy cycles and depression in another three studies [25, 29, 46].
Three studies respectively reported that cancer patients who had severe symptoms of cancer [47], received chemotherapy that had little effect [3] or had not response to chemotherapy [31], were more likely to experience depressive symptoms.
Additionally, ten studies reported that some factors were irrelevant to depression, including cancer stage [3, 9, 11, 22, 23, 26], cancer type [13, 26, 28, 48], residual disease after surgery (optimal < 0.5 cm/suboptimal 0.5–2 cm/over 2 cm) [25], intestinal stoma [25], duration of surgical procedure in minutes [25], comorbidities [11], type of treatment [3], and toxicity of chemotherapy [3].
Psychosocial factors
Six studies with a total of 738 participants, including two high quality studies and four moderate quality studies, all showed that having a strong social support network was negatively associated with depression [9, 20, 29, 36, 45, 49]. Four studies with 229 participants, including two high quality studies, one moderate study, and one low quality study, reported there was a significantly positive relationship between anxiety and depression [27, 36, 45, 47]. Two studies consistently showed that perceived stress was positively correlated with depression in 323 participants [34, 50]. Another two studies, with a total of 534 participants, found that self-efficacy was negatively related to depression [51, 52]. One study revealed that negative beliefs [27] and neuropsychological symptom clusters (included drowsiness, difficulty remembering, sadness, distress, sleep disturbances, and fatigue) [36] were positively correlated with depression. Three studies respectively indicated that positive coping strategies [53], hope level [52], and a fighting spirit [36] were negatively related to depression. In addition, the need to control thoughts, cognitive confidence, cognitive self-consciousness, and positive beliefs were all neutral in terms of depression.
Discussion
This is the first systematic review to identify associated factors for depression in cancer patients undergoing chemotherapy. A total of 43 studies met the inclusion criteria, through the database and manual searching. This review indicates that depression in cancer patients undergoing chemotherapy is associated with sociodemographic characteristics, physiological condition, disease and treatment, and psychosocial factors. The main risk factors for depression that were consistently identified were extent of social support, anxiety, perceived stress, and self-efficacy. However, age, educational level, gender, income, occupation, sleep disturbance, fatigue, pain, CIA, appetite loss, and chemotherapy cycles remained controversial.
Sociodemographic risk factors for depression, including age, education level, gender, income, and occupation—were the most widely reported in the studies that were included. However, these are also the most inconclusive factors. Polikandrioti et al. [8] have proposed that in the cases where depression increases with age, it may be due to the fact that elderly patients with cancer, having lost their previous healthy status, often find themselves in life-changing situations, such as functional impairment, loss of spouse, poor social support network, and loss of interest in activities. Conversely, some authors have argued that older adults, who have achieved “emotional regulation” [54], could adopt more passive coping skills than younger patients, leading to a subdued response to cancer and chemotherapy. Seven out of 13 studies in this review have found that education level is negatively associated with depression. Chang-Quan et al. [55] have further explained that patients with less education have less self-efficacy and cognitive function, which may contribute to their higher risk for depression. Three out of five studies included in this review have indicated that females experience higher levels of depression than males. The difference in depression levels between males and females may be attributed to the severity of physical problems, since female patients typically experience greater physical problems when undergoing chemotherapy [1], compared to male patients. It may also be possible that females are more likely to experience depression as a result of the interaction between a cognitive diathesis and a domain-congruent negative life event, such as cancer diagnosis and chemotherapy [56]. Different from previous reviews [16, 17], most studies included in this review have suggested that income was not related to depression in chemotherapy patients. Occupation as a risk factor for depression was only supported by one out of four studies included in this review, which is similar to findings by Brandão et al. [16]. It is worth mentioning that the heterogeneity of grouping in terms of age, educational level, income, and occupation may lead to inconsistent results. Additionally, family type and frequency of sexual activity were reported by only one study, so these factors need to be explored in future studies.
A total of 23 physiological factors were involved in this review, but most were reported by only one or two studies, or the results were controversial. This review has found that nutritional status is significantly associated with depression in chemotherapy patients. It may be due to the fact that patients suffering from malnutrition often experience vitamin deficiency. A longitudinal study with more than 3500 people demonstrated that a higher intake of Vitamins B6 and B12 could decrease the likelihood of depressive symptoms [57]. Moreover, Vitamin B and folic acid have been suggested to play a potential role in a protective mechanism against depressive disorder [58, 59]. This review also revealed that loss of appetite may be another clinical indicator of depression. Hyperinsulinemia may mediate appetite loss and depressive disorders [60], then the sense of satiety deprived people of their appetite. Most studies in our review have indicated that sleep disturbance, fatigue, and pain are risk factors for depression, similar to the review by Haisfield-Wolfe et al. [15]. Inflammation may be one potential mechanism underlying the relationship between depression and sleep disturbance, fatigue, and pain [61, 62]. The inflammatory reaction can occur while a patient is undergoing chemotherapy, or after its completion [63]. Further studies could explore the biological mechanisms underlying the relationship between sleep disturbance, fatigue, pain, and depression, which may contribute to novel therapeutic and preventive interventions for depression. One study investigating breast cancer patients has suggested that CIA was associated with depression. One possible reason may be that patients with CIA distress have lower levels of emotional and social functioning [37]. Additionally, other significant risk factors, such as RLS, sexual function, BMI score, menopausal symptoms, cancer antigen 125, and inflammatory biomarkers (TRACP5a, WBCs, number of monocytes) have respectively been investigated by one or two studies. Further studies with rigorous design are necessary to identify the relationship between these factors and depression.
Thirteen disease and treatment factors have been explored in this review. Among them, severity of cancer, effective chemotherapy, response to chemotherapy, and number of chemotherapy cycles were all found to be related to depression. However, this conclusion requires the support of more original studies. In contrast to a previous review [64], our review found that cancer type is not associated with depression.
Compared to the other risk factors for depression, fewer psychosocial factors were reported. Similar to studies by Brandão et al. [16] and Caruso et al. [17], this review has found that social support or the lack of it was significantly correlated to depression in six studies. Gariépy et al. [65] have revealed that depression can be directly prevented through the benefits of social relationships; or indirectly, social relationships can serve as a buffer against stressful circumstances. Anxiety was also related to depression. This was consistent with the study by Jacobson and Newman, who found that anxiety predicted later depression, and this relationship was partially mediated by avoidance [66]. Perceived stress and self-efficacy also impacted depression in patients undergoing chemotherapy. It is likely that self-efficacy makes a difference in how people feel, think, and act, and influences their appraisal of stressful stimuli (threat, harm, or challenge) [67]. Other significant psychosocial factors, such as positive coping strategies, hope levels, a fighting spirit, and neuropsychological symptom clusters, were respectively reported by one or two studies. Moreover, existing studies have seldom explored the mechanism of depression and psychosocial factors. Future studies could explore how and why these factors affect depression in chemotherapy patients.
The majority of the studies in this review are of high or moderate quality, but there are some limitations. First, all selected studies were descriptive, which may mean they failed to identify the causality between depression and the factors they described. Second, some of the included studies only analyzed the data using univariate analysis, T test, and χ2test. Thus, it is possible that the impact of confounding factors could not be eliminated. Third, most studies had only a small sample size, with the exception of two studies, which both recruited a large sample. Finally, all included studies chose self-report instruments to assess depression levels in cancer patients undergoing chemotherapy. It is worth noting that the prevalence of depression is substantially higher when self-report instruments, instead of diagnostic instruments, are employed [63].
There are some limitations in this review. First, a meta-analysis was not employed to synthesize the data, due to insufficient data contained in the studies, an insufficient number of studies assessing the same factors, and different measurements of depression or heterogeneity of sample grouping. Therefore, we performed a qualitative analysis in this review. Second, we restricted the languages to English and Chinese, which may have resulted in the omission of studies published in other languages. Third, gray literature was not included, so we might have missed some studies. Fourthly, the previous treatments, such as surgery or radiation therapy may influence depressive symptom of cancer patients, but they were not considered in this review. Finally, 55 out of 65 factors were identified by only one or two studies, so the evidence of these factors on depression is not enough.
Conclusion
This review shows that depression is associated with a complex interplay of factors, rather than a single factor, in cancer patients receiving chemotherapy. This review suggests that the development of programs for depression should take social support levels, anxiety, perceived stress, and self-efficacy into account. More studies with rigorous design are necessary to further confirm the inconclusive factors of depression, and explore the mediators and moderators of depression.
References
Bergerot CD, Mitchell HR, Ashing KT, Kim Y (2017) A prospective study of changes in anxiety, depression, and problems in living during chemotherapy treatments: effects of age and gender. Support Care Cancer 25:1897–1904
Piacentine LB, Miller JF, Haberlein S, Bloom AS (2015) Perceived cognitive changes with chemotherapy for breast cancer: a pilot study. Appl Nurs Res 29:9–11
Duc S, Rainfray M, Soubeyran P, Fonck M, Blanc JF, Ceccaldi J, Cany L, Brouste V, Mathoulinpélissier S (2016) Predictive factors of depressive symptoms of elderly patients with cancer receiving first-line chemotherapy. Psycho-Oncology 26:15–21
Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N (2011) Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 12:160–174
Saevarsdottir T, Fridriksdottir N, Gunnarsdottir S (2010) Quality of life and symptoms of anxiety and depression of patients receiving cancer chemotherapy. Cancer Nurs 33:e1–e10
Chen SJ, Chang CH, Chen KC, Liu CY (2016) Association between depressive disorders and risk of breast cancer recurrence after curative surgery. Medicine 95:e4547
Schneider S, Moyer A (2009) Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 115:3304–3305
Polikandrioti M, Evaggelou E, Zerva S, Zerdila M, Koukoularis D, Kyritsi E (2008) Evaluation of depression in patients undergoing chemotherapy. Health Sci J 2:162–172
Park H, Yoon HG (2013) Menopausal symptoms, sexual function, depression, and quality of life in Korean patients with breast cancer receiving chemotherapy. Support Care Cancer 21:2499–2507
Jehn CF, Becker B, Flath B, Nogai H, Vuong L, Schmid P, Lüftner D (2015) Neurocognitive function, brain-derived neurotrophic factor (BDNF) and IL-6 levels in cancer patients with depression. J Neuroimmunol 287:88–92
Fagundes C, Jones D, Vichaya E, Lu C, Cleeland CS (2014) Socioeconomic status is associated with depressive severity among patients with advanced non-small cell lung cancer: treatment setting and minority status do not make a difference. J Thorac Oncol 9:1459–1463
Yan L, Yuan C (2011) Levels of fatigue in Chinese women with breast cancer and its correlates: a cross-sectional questionnaire survey. J Am Assoc Nurse Pract 23:153–160
Akechi T, Okuyama T, Uchida M, Nakaguchi T, Sugano K, Kubota Y, Ito Y, Kizawa Y, Komatsu H (2012) Clinical indicators of depression among ambulatory cancer patients undergoing chemotherapy. Jpn J Clin Oncol 42:1175–1180
Farrell C, Brearley SG, Pilling M, Molassiotis A (2013) The impact of chemotherapy-related nausea on patients’ nutritional status, psychological distress and quality of life. Support Care Cancer 21:59–66
Haisfield-Wolfe ME, Mcguire DB, Soeken K, Geiger-Brown J, De Forge BR (2009) Prevalence and correlates of depression among patients with head and neck cancer: a systematic review of implications for research. Oncol Nurs Forum 36:107–125
Brandão T, Schulz MS, Matos PM (2017) Psychological adjustment after breast cancer: a systematic review of longitudinal studies. Psycho-Oncology 26:917–926
Caruso R, Nanni MG, Riba M, Sabato S, Mitchell AJ, Croce E, Grassi L (2017) Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncol 56:146–155
Shuangshuang W, Yanqing Y, Huimin X, Xiuyan L, Ying C (2016) Systematic review and meta-analysis of the risk factors for depression in cancer chemotherapy patients. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016036619. Accessed 24 March 2016
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Lijun F, Shujing W, Hua W (2011) Prevalence of depression and the relationship between depression and social support in chemotherapy patients. J Qilu Nurs 17:31–32
Mei T (2008) Prevalence of depression in chemotherapy patients. Chin Gen Pract Nurs 6:1601–1603. https://doi.org/10.3969/j.issn.1674-4748.2008.18.002
Faul LA, Jim HS, Minton S, Fishman M, Tanvetyanon T, Jacobsen PB (2011) Relationship of exercise to quality of life in cancer patients beginning chemotherapy. J Pain Symptom Manag 41:859–869
Jianci Z (2015) Influencing factors of negative emotions and well - being in chemotherapy patients and observation of psychological intervention. Chin J Prim Med Pharm 22:765–767. https://doi.org/10.3760/cma.j.issn.1008-6706.2015.05.045
Xu L (2014) The change of quality of life, anxiety and depression and the influencing factors of patients with lung cancer before and after chemotherapy. Dissertation,Tianjin Medical University
Mielcarek P, Nowicka-Sauer K, Kozaka J (2017) Anxiety and depression in patients with advanced ovarian cancer: a prospective study. J Psychosom Obstet Gynecol 37:57–67
Pandey M, Sarita GP, Devi N, Thomas BC, Hussain BM, Krishnan R (2006) Distress, anxiety, and depression in cancer patients undergoing chemotherapy. World J Surg Oncol 4:68
Quattropani MC, Lenzo V, Filastro A (2017) Predictive factors of anxiety and depression symptoms in patients with breast cancer undergoing chemotherapy. An explorative study based on metacognitions 23:67–73
Meihua W, Yanyun W, Xiuying C, Gong Z, Xiaohong C, Xiuyu Y (2004) Investigation on the influence factors of depression in chemotherapy patients and nursing countermeasures. Fujian Med J 26:201–202. https://doi.org/10.3969/j.issn.1002-2600.2004.06.150
Wen Q, Shao Z, Zhang P, Zhu T, Li D, Wang S (2017) Mental distress, quality of life and social support in recurrent ovarian cancer patients during active chemotherapy. Eur J Obstet Gynecol Reprod Biol 216:85–91
Heinze S, Egberts F, Rötzer S, Volkenandt M, Tilgen W, Linse R, Boettjer J, Vogt T, Spieth K, Eigentler T (2010) Depressive mood changes and psychiatric symptoms during 12-month low-dose interferon-[alpha] treatment in patients with malignant melanoma: results from the multicenter DeCOG trial. J Immunother 33:106–114
Chintamani GA, Khandelwal R, Tandon M, Jain S, Kumar Y, Narayan N, Bamal R, Srinivas S, Saxena S (2011) The correlation of anxiety and depression levels with response to neoadjuvant chemotherapy in patients with breast cancer. J Royal Soc Med Cardiovasc Dis 2:15
Dehua L, Hui Z, Xiujing G, Jing F (2016) Associations between stress coping strategies and perceived social support in young patients with gynecologic cancers. J Sichuan Univ (Medical Science Edition) 47:402–405. https://doi.org/10.13464/j.scuxbyxb.2016.03.021
Mills PJ, Parker B, Dimsdale JE, Sadler GR, Ancoli-Israel S (2005) The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 69:85–96
Jianhong L, Xiaoli Z, Liuliu Z (2011) Investigation and analysis of psychological status and its related factors for patients with cancer chemotherapy. Int J Nurs (China) 30:1382–1384. https://doi.org/10.3760/cma.j.issn.1673-4351.2011.09.052
Saini A, Berruti A, Ferini-Strambi L, Castronovo V, Rametti E, Giuliano PL, Ramassotto B, Picci RL, Negro M, Campagna S (2013) Restless legs syndrome as a cause of sleep disturbances in cancer patients receiving chemotherapy. J Pain Symptom Manag 46:56–64
Kim SH, Kim M, Lee HS (2016) Symptom clusters in Korean patients with metastatic cancer undergoing palliative chemotherapy. J Hosp Palliat Nurs 18:292–299
Choi EK, Kim IR, Chang O, Kang D, Nam SJ, Lee JE, Lee SK, Im YH, Park YH, Yang JH (2014) Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psycho-Oncology 23:1103–1110
de Moor JS, de Moor CA, Basenengquist K, Kudelka A, Bevers MW, Cohen L (2006) Optimism, distress, health-related quality of life, and change in cancer antigen 125 among patients with ovarian cancer undergoing chemotherapy. Psychosom Med 68:555–562
Chou HL, Chao TY, Chen TC, Chu CM, Hsieh CH, Yao CT, Janckila AJ (2016) The relationship between inflammatory biomarkers and symptom distress in lung cancer patients undergoing chemotherapy. Cancer Nurs 40:e1–e8
Bergerot CD, Clark KL, Nonino A, Waliany S, Buso MM, Loscalzo M (2015) Course of distress, anxiety, and depression in hematological cancer patients: association between gender and grade of neoplasm. Palliat Support Care 13:115–123
Dai YL, Yang CT, Chen KH, Tang ST (2016) Changes to and determinants of quality of life in patients with advanced non-small-cell lung cancer undergoing initial chemotherapy. J Nurs Res Jnr 25:203–215
Decat CS, de Araujo TC, Stiles J (2011) Distress levels in patients undergoing chemotherapy in Brazil. Psycho-Oncology 20:1130–1133
Meraner V, Gamper EM, Grahmann A, Giesinger JM, Wiesbauer P, Sztankay M, Zeimet AG, Sperner-Unterweger B, Holzner B (2012) Monitoring physical and psychosocial symptom trajectories in ovarian cancer patients receiving chemotherapy. BMC Cancer 12:77
Jiayuan Z, Yuqiu Z, Ziwei F, Yong X, Guangchun Z (2018) Longitudinal trends in anxiety, depression, and quality of life during different intermittent periods of adjuvant breast cancer chemotherapy. Cancer Nurs 41:62–68
Hipkins J, Whitworth M, Tarrier N, Jayson G (2004) Social support, anxiety and depression after chemotherapy for ovarian cancer: a prospective study. Br J Health Psychol 9:569–581
Polat U, Arpacı A, Demir S, Erdal S, Yalcin S (2014) Evaluation of quality of life and anxiety and depression levels in patients receiving chemotherapy for colorectal cancer: impact of patient education before treatment initiation. J Gastrointest Oncol 5:270–275
Gruenigen VEV, Hutchins JR, Reidy AM, Gibbons HE, Daly BJ, Eldermire EM, Fusco NL (2006) Gynecologic oncology patients’ satisfaction and symptom severity during palliative chemotherapy. Health Quality Life Outcomes 4:1–6
Ostacoli L, Saini A, Zuffranieri M, Boglione A, Carletto S, Marco ID, Lombardi I, Picci RL, Berruti A, Comandone A (2014) Quality of life, anxiety and depression in soft tissue sarcomas as compared to more common tumours: an observational study. Appl Res Qual Life 9:123–131
Jihua W, Defang Z, Yingli X (2012) Relationship between depression and social support in chemotherapy patients. Mod J Integr Tradit Chin Western Med 21:4078–4079. https://doi.org/10.3969/j.issn.1008-8849.2012.36.044
Ostacoli L, Saini A, Ferini-Strambi L, Castronovo V, Sguazzotti E, Picci RL, Toje M, Gorzegno G, Capogna S, Dongiovanni V (2010) Restless legs syndrome and its relationship with anxiety, depression, and quality of life in cancer patients undergoing chemotherapy. Qual Life Res Int J Qual Life Aspects Treat Care Rehabil 19:531–537
Xiyuan F, Biru L (2012) Correlative analysis of self-management efficacy and anxiety and depression in patients with gynecological chemotherapy. West China Med J 27:1667–1669 http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFD2012&filename=HXYX201211028&uid=WEEvREcwSlJHSldRa1FhcTdWajFuQ2FYWmErbVJpL3AyUk9wSGtQRWxpZz0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4ggI8Fm4gTkoUKaID8j8gFw!!&v=MjYyNDR1eFlTN0RoMVQzcVRyV00xRnJDVVJMS2ZaT1JzRnluZ1U3dk1MVFhTZHJHNEg5UE5ybzlIYklSOGVYMUw=. Accessed 24 Nov 2012
Xiaohong W, Feng Y (2016) The relationship between self - care ability, hope level and depression in patients with breast cancer during chemotherapy. Matern Child Health Care China 31:3898–3900. https://doi.org/10.7620/zgfybj.j.issn.1001-4411.2016.19.08
Jiangyan S, Liwei W, Huiping L, Yuqing P (2010) Correlation between depression of cancer patients undergoing chemotherapy and coping style and quality of life. J Nurs 17:5–8. https://doi.org/10.3969/j.issn.1008-9969.2010.05.002
Blank TO, Bellizzi KM (2008) A gerontologic perspective on cancer and aging. Cancer 112:2569–2576
Huang CQ, Wang ZR, Li YH, Xie YZ, Liu QX (2010) Education and risk for late life depression: a meta-analysis of published literature. Int J Psychiatry Med 40:109–124
Spangler DL, Simons AD, Monroe SM, Thase ME (1996) Gender differences in cognitive diathesis-stress domain match: implications for differential pathways to depression. J Abnorm Psychol 105:653–657
Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC (2010) Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr 92:330–335
Alpert JE, Fava M (1997) Nutrition and depression: the role of folate. Nutr Rev 55:145–149
Phillips RM (2012) Nutrition and depression in the community-based oldest-old. Home Healthcare Nurse 30:462–471
Licinio-Paixao J (1989) Hyperinsulinemia; a mediator of decreased food intake and weight loss in anorexia nervosa and major depression. Med Hypotheses 28(2):125–130
Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW (2011) Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism. J Clin Oncol 29:3517–3522
Cho HJ, Savitz J, Dantzer R, Teague TK, Drevets WC, Irwin MR (2017) Sleep disturbance and kynurenine metabolism in depression. J Psychosom Res 99:1–7
Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z (2010) Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun 24:968–974
Krebber AMH, Buffart LM, Kleijn G, Riepma IC, Bree RD, Leemans CR, Becker A, Brug J, Straten AV, Cuijpers P (2014) Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psycho-Oncology 23:121–130
Gariépy G, Honkaniemi H, Quesnel-Vallée A (2016) Social support and protection from depression: systematic review of current findings in Western countries. Br J Psychiatry 209:284–293
Jacobson NC, Newman MG (2014) Avoidance mediates the relationship between anxiety and depression over a decade later. J Anxiety Disord 28(5):437–445
Bandura A (1977) Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 84:191–215
Funding
The study was supported by the National Natural Science Foundation of China [8140, 1863].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wen, S., Xiao, H. & Yang, Y. The risk factors for depression in cancer patients undergoing chemotherapy: a systematic review. Support Care Cancer 27, 57–67 (2019). https://doi.org/10.1007/s00520-018-4466-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4466-9