Abstract
Purpose
“Shared decision making” has been proposed as a prerequisite of patient-centered care. However, little is known on factors, which may influence cancer patients’ decision control preferences (DCP) in routine care. This study investigated possible determinants of the patients’ DCP with respect to patient characteristics and patient-reported outcomes (PROs).
Methods
Consecutive patients presenting at a comprehensive cancer center between May 2014 and October 2014 were offered a self-administered electronic questionnaire including standardized PRO measures and patients’ DCP. Results were linked with patient characteristics from the hospital information system and analyzed using cross-sectional methods.
Results
Out of 126 patients participating, 102 (81%; 65% male; mean age 62 years) completed the DCP-item. Overall, 49% (n = 50) preferred shared treatment decision responsibility, 29% (n = 30) preferred to leave the control to his/her physician, whereas 22% (n = 22) preferred to be in control of his/her treatment decision. Higher age (p = 0.035) and elevated distress levels (p = 0.038) were significantly associated with an increased willingness to leave the decision control to the physician. Further sociodemographic and PRO measures were not associated with patients’ DCP.
Conclusion
Our findings demonstrate that DCP assessment in routine cancer care is possible and provides important information to the treating oncologist. Information on DCP combined with PRO may contribute to more individualized decision making in cancer care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patient-centered information and decision making is a requirement both from an ethical and legal perspective [1, 2]. Thus, “shared decision making” has been increasingly advocated in the last two decades to provide information and share control in accordance with patients’ preferences [3, 4]. The appreciation of patients’ preferences regarding participation in decision making is crucial in order to be able to individualize disclosure of information and patient involvement [5, 6]. This importance particularly applies to oncology, not only because cancer care is comprehensive and complex but also because the identification of patients’ preferences for information and control is important to avoid the often occurring conflicts between patients’ expectations and physicians’ decision-making practices [7–9]. Moreover, an individualized process of informing cancer patients and involving them in decisions can have a positive impact on health itself [10]. While patients who wish to be involved in more detail may be harmed by shortcomings of information, evidence exists that patients who are expected by their physicians to take over more control than personally preferred show corresponding higher levels of distress [11]. Therefore, the valid assessment and immediate usability of patient preference seems crucial.
Patients’ Decision control preferences (DCPs) have been explored with a range of instruments including the widely used Control Preference Scale developed by Degner and colleagues [12]. Research on DCP has repeatedly shown that a shared approach to control decision making in healthcare is preferred by the largest group of patients [13]. A considerable body of evidence indicates that sociodemographic factors such as age, gender or education, as well as cultural factors, are associated with patients’ preferences regarding control of decision making in healthcare [14, 15]. However, only few studies have explored the potential relevance of disease-related factors for cancer patients’ DCP. Both, quantitative and qualitative research, indicate that the preferred level of participation of cancer patients can change during the course of the disease [16, 17]. A significant limitation of existing research on DCP in cancer confers to the fact that studies have almost exclusively focused on prostate and breast cancer [8].
More recently, the potential relevance of patient-reported outcomes (PROs) for patients’ DCP has been emphasized in research [18]. In a study with patients with myelodysplastic syndrome by Efficace et al., patients with worse quality of life (QoL) more often preferred a passive role, whereas patients who preferred an active role in decision making experienced better QoL at the time of diagnosis [19]. However, there is a lack of data on DCP and the potential associations with PRO gathered as part of routine cancer care immediately prior to actual decision making between oncologists and their patients.
We investigated DCP and associated health-related and sociodemographic factors that have been elicited in consecutive patients with cancer by means of an electronically displayed and self-administered questionnaire immediately prior to patients’ first consultation at a German Comprehensive Cancer Center. We aimed to assess the distribution of DCP of cancer patients in routine care and to analyze the association of DCP with PRO and sociodemographic factors.
Methods
Study design
We undertook a cross-sectional study embedded in a quality assurance project. Between May 2014 and October 2014, a total of 160 consecutive patients were prospectively approached by nurses as part of the routine admission process prior to their first consultation at the comprehensive cancer center of the University Hospital Dresden, Germany. Reasons for consultation included treatment planning, referral for second opinion at the tertiary cancer center, and discussion of examination results. Patients were already aware of their diagnosis. All patients received standardized information on the study aims and procedures. After declaration of consent for participation, patients answered self-administered, standardized questionnaires on several PRO on a tablet PC. Relevant scores were automatically calculated and transferred to the hospital information system (HIS), where they were linked to sociodemographic and anamnestic data, visualized and accessible for direct use in physician-patient consultation [20].
Measures
Patients answered a self-administered electronic questionnaire, including standardized and validated measures of DCP, QoL, psychological distress, need for psycho-oncological support, nutritional and pain status, and performance status.
Patients’ DCPs of medical decision—the primary outcome of the study—were measured using the standardized and validated Control Preference Scale (CPS) [12]. Patients can pick one statement out of five that best describes their and their physician’s preferred involvement in medical decision making, ranging from active (“I prefer to make the decision about which treatment I will receive”) to passive (“I prefer to leave all decisions regarding my treatment to my doctor”) role. To maintain a sufficient number of cases for statistical analysis per category, adjoining answer options were grouped to three categories towards the overall control preference self-control, shared decision making, and physician control. Global QoL; its dimensions, emotional, physical, social, cognitive, and role functioning; and respective symptom scales, were measured applying the EORTC QLQ-C30 [21], a standardized, well-validated, 30-item questionnaire widely used to assess QoL in cancer patients. Patients indicated their agreement on different questions concerning QoL in the past 7 days (e.g., “Have you felt nauseated”) on a four-point Likert-scale ranging from 1 (“not at all”) to 4 (“very much”) resulting in a summary score ranging from 0 to 100 with a high score representing a higher level in QoL and respective functional dimensions or higher symptom intensity.
Psychologic distress was assessed using the validated German translation of the National Comprehensive Cancer Network Distress Thermometer (DT) [22, 23], a standardized, well-validated, single-item questionnaire, ranging from 0 (“no distress”) to 10 (“extreme distress”).
Need for psycho-oncological support was assessed by the Hornheider Screening Instrument (HSI) [24], a seven-item standardized, validated questionnaire. The final summary score ranges from 0 to 14 with higher scores indicating an increased need for psycho-oncological support.
Nutritional status was assessed applying the Short-Form Mini Nutritional Assessment (MNA) [25], a six-item standardized questionnaire resulting in a summary measure ranging from 0 (“malnourished”) to 14 (“normal nutritional status”). The MNA has been validated in an extensive sample of geriatric patients [26] and is widely used in oncology practice.
Performance status was measured using a patient-reported adaption of the Karnofsky-Index [27] that was transferred [28] to ECOG-performance status, ranging from 0 to 5, with higher scores indicating higher impairment in performance status.
Pain was assessed using the Brief Pain Inventory [29, 30], a standardized, validated questionnaire that assesses several aspects of pain, including its intensity and interference on daily life on a scale ranging from 0 (“no pain”) to 10 (“strongest conceivable pain”).
Compliance with ethical standards
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Patients were informed about the project and asked for their declaration of consent. They were also informed about their right to refuse participation in electronic assessment without any disadvantages and with respect to their treatment and healthcare. The electronic assessment was developed in close collaboration with the data security officer and the responsible IT department of the comprehensive cancer center, and respective approval was obtained. All data management and storage was in accordance with the local data protection guidelines.
Statistical analysis
Hypothesizing that older age, more progressed disease, and higher needs for psycho-oncological support are related to leaving the responsibility of the treatment decision with the physician, we investigated possible determinants of the patients’ DCP using descriptive statistics, Chi-square tests, Fisher’s exact tests, t tests, and Kruskal-Wallis tests, depending on variable type and distribution. All tests were applied using two-sided tests and a significance level of 0.05. Data were analyzed using Stata statistics®, version 12.1.
Possible associations of patients’ DCP with the variables age, psychological distress, global QoL, and its functional dimensions and symptom scales as well as pain severity and pain impairment were analyzed at a continuous level. Treatment intention was analyzed using the categories curative and palliative as noted in the interdisciplinary tumor board, excluding those patients without or unclear intention. Due to low number of patients with higher impairment, ECOG-performance status was re-categorized into three groups (ECOG 1, ECOG 2, ECOG 3–5). Need for psycho-oncologic support (score ≥ 4: need for support, score < 4: no need for support) and nutritional status (0–7 points: malnourished; 8–11 points: at risk of malnutrition; 12–14: normal nutritional status) were grouped according to their respective manuals [24, 25].
Patients with missing data on distress, performance status, need for psycho-oncologic support, nutritional status, pain severity, pain impairment, treatment intention, and sociodemographic information were excluded from the respective group comparison. For the score calculation of functional and symptom scales of the EORTC QLQ-C30, the number of missing items of the respective functional/symptom score were taken into account.
Results
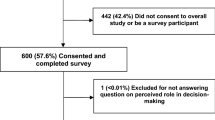
Overall, 126 out of 160 consecutive patients (78%) visiting the outpatient clinic of the German comprehensive cancer center agreed to complete the electronic survey instrument. Participating patients were younger than non-participants (mean age 63.0 years vs. 69.3 years), and treatment intention was more often palliative (22.2 vs. 11.8%). There were no differences in other socio-demographic and clinical parameters between the participating and the non-participating patients. The majority of patients had gastrointestinal tumors. Further information regarding characteristics of participants and non-participants are reported elsewhere [20].
A total of 102 patients (81%) completed the item on the DCP and were included in further analyses. Patients who answered the question on DCP did not differ from non-responders in terms of age, gender, graduation, education, and performance status (Table 1). However, there was a significantly higher proportion of patients treated with curative intent in the group of respondents to the DCP assessment (p = 0.021). Of the 102 responding patients, 67 (66%) were male and 35 (34%) female (Table 1).
The majority of patients (49.0%; n = 50) preferred shared responsibility with regard to treatment decision making. Thirty patients (29.5%) preferred to be in control about treatment decisions, and 21.6% (n = 22) preferred to rather leave the control to the treating physician (Fig. 1).
Decision control preferences of patients (n = 102). Decision control preferences according to Degner et al. [12] of patients (n = 102) including the superordinate categories indicating self-control, shared decision making, and physician control
Table 2 describes patient-reported outcomes for patients responding to the DCP. More than half of these patients (51%) constituted a need for psycho-oncological support. Also, 64% were at risk for malnutrition or already malnourished. Reported outcomes for global health status, functional scales, and symptom scales were varying considerably among all items, with the highest burden reported for fatigue, pain, and insomnia.
As described in Table 2, older age of patients (p = 0.035) and higher levels of distress (p = 0.038) were significantly associated with more willingness to leave the decision with the physician. Patients with better emotional functioning preferred to have self-control when making treatment decisions (p = 0.017). Gender, needs for psycho-oncological support, and treatment intention (curative vs palliative) as a proxy for a more progressed disease status were not significantly associated with DCP. Patients suffering from higher levels of pain severity according to the EORTC QLQ C-30 tended to be more likely to leave control for decision making to the physician compared to patients with lower levels of pain (p = 0.056). Pain impairment was not significantly associated with a specific control preference. Among others, global QoL, functional domains, and symptoms assessed by the QLQ-C30 questionnaire were not significantly associated with DCP. Additionally, performance status, nutritional status, and education and graduation did not reveal significant differences within the three control preference groups. Table 2 provides the details of analysis of the determinants for DCP.
Discussion and conclusion
This study extends previous research by gathering and analyzing data on DCP, a broad set of PRO, and associated sociodemographic factors as part of routine cancer care in a German comprehensive cancer center. Given the previous focus of empirical research on patients’ preferences regarding information and decision making in patients with prostate or breast cancer [8], this study contributes data from a variety of cancer diseases, primarily gastrointestinal tumors.
Compared with two previous studies in patients with colorectal cancer [31, 32], patients included in our study more frequently indicated their wish to participate in decision making or even to take an active role. However, the clinical setting, sociodemographic factors, and cultural difference may limit such a comparison. Furthermore, it seems important to investigate DCP in different malignant diseases, because the type of cancer and symptom burden may be associated with a patient’s preferred or perceived role in decision making [8]. In addition, the matching of involvement in decision making with the preferred role seems important since a mismatch may lead to additional patient burden [11].
In comparison with one of the few studies with cancer patients in Germany [33], our study indicated slightly more patients who preferred to delegate treatment decision to the physician (21.6 versus 17%). This study by Albrecht and colleagues, which included more than 400 patients with melanoma (stage I-III), showed that in 43% of the patients, DCP shifted significantly within the observational period, mostly towards a more active role. Findings from our own qualitative research with pancreatic cancer patients show that factors for such a change in DCP may be explained by an increase of knowledge about the disease, as well as a shift of values and priorities [34].
Furthermore, in this study, we observed a significant association between psychological distress and DCP with higher scores in psychological distress (cut-off ≥5 on the Distress Thermometer [22]), indicating the preference to take a more passive role in decision making. There was also a trend towards a more passive role in patients with higher level of pain severity. On the other hand, patients with better emotional functioning wanted to have more self-control. These findings, which in part resemble the results of others [19], raise the question about how oncologists should systematically explore the current burden of disease in an individual patient. Taking into consideration the potential influence of disease burden and distress in decision making has several implications for patient-centered approach in cancer care: firstly, and particularly bearing in mind the changing phases of psychological stress with high and low distress or changes in the extent of burden of disease, oncologists should explore whether or not the patient is ready for information and decision making prior to a planned consultation. The implementation of a tool that combines the eliciting of PRO and DCP as part of routine care could be used to better identify the appropriate time and situation for treatment decision making. As shown in earlier work, such a standardized, evidence-based approach of collecting PRO can be feasibly implemented into routine clinical care [20, 35]. Such an approach could for example identify patients with a need for a symptom or distress-oriented intervention, before being able to participate in decision making according to his/her preferences.
Secondly and relevant to a more individualized approach to decision making, the eliciting of DCP prior to consultation could be used to guide oncologists in choosing an appropriate approach to decision making. In other words, oncologists could use such information as one component to adapt his/her information style towards a more informative, shared, or paternalistic approach [2, 5], thereby reducing a mismatch with patients preferences.
While the use of such tools could remedy the well-known discrepancy between patients’ preferences and physicians’ misconceptions, it should also be pointed out that relying purely on electronic survey data on DCP elicited prior to the consultation process can also be misleading. One example is that an overall preference for an active role in decision making does not mean that patients in this group are necessarily interested in all details, which from a medical perspective are perceived as relevant. Moreover, it is important to emphasize that adapting decision-making styles in accordance with patient preferences requires knowledge as well as experiential training in ethics and communication [36].
Strengths of this study include the implementation into a realistic clinical setting, so the applicability and generalizability to routine care should be given. The inclusion of consecutive patients decreases the chance for selection bias. However, older ages might be a limiting factor for participation [20]. Study limitations include the relatively low sample size, so further subgroup analyses by tumor entity could not be performed. Statistical power was limited so possible associations between DCP and gender, education, patient symptoms, quality of life, and further clinical characteristics may have been missed. In this context, it should also be explored whether other potentially relevant factors, such as performance state, could be also used as predictors for DCP. The cross-sectional design does not allow drawing causal conclusions and is not suitable to investigate changes of DCP and the observed determinants over time. So far, DCP was only assessed prior to the first patient-physician encounter.
Future studies should capture DCP more often also longitudinally. Moreover, larger samples and possible factors including disease entity and stadium of the disease may be used to further stratify patients with cancer regarding DCP and an individualized approach to decision making. Ultimately, healthcare providers will need to undergo dedicated training modules to incorporate DCP information with regard to tailoring his/her approach to the individual patient.
Conclusion
This study demonstrates that the assessment of DCP in patients with cancer can be incorporated into routine cancer care. DCP in combination with information about psychological distress, QoL, and patient characteristics may contribute to a more individualized approach in informed decision making. Further studies to elicit factors associated with DCP and the impact on patient satisfaction with care are urgently needed.
References
Brody DS (1980) The patient’s role in clinical decision-making. Ann Intern Med 93:718–722. doi:10.7326/0003-4819-93-5-718
Emanuel EJ, Emanuel LL (1992) Four models of the physician-patient relationship. JAMA 267:2221–2226. doi:10.1001/jama.1992.03480160079038
Charles C, Gafni A, Whelan T (1997) Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 44:681–692. doi:10.1016/S0277-9536(96)00221-3
Rockenbauch K, Schildmann J (2011) Shared decision making (SDM): a systematic survey of terminology use and concepts. Gesundheitswesen Bundesverb Ärzte Öffentl Gesundheitsdienstes Ger 73:399–408. doi:10.1055/s-0030-1262870
Elwyn G, Edwards A, Kinnersley P (1999) Shared decision-making in primary care: the neglected second half of the consultation. Br J Gen Pract J R Coll Gen Pract 49:477–482
Towle A, Godolphin W (1999) Framework for teaching and learning informed shared decision making. BMJ 319:766–771
Blinman P, Hughes B, Crombie C et al (2015) Patients’ and doctors’ preferences for adjuvant chemotherapy in resected non-small-cell lung cancer: what makes it worthwhile? Eur J Cancer Oxf Engl 51:1529–1537. doi:10.1016/j.ejca.2015.05.0221990
Tariman JD, Berry DL, Cochrane B et al (2010) Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol 21:1145–1151. doi:10.1093/annonc/mdp534
Caocci G, Voso MT, Angelucci E et al (2015) Accuracy of physician assessment of treatment preferences and health status in elderly patients with higher-risk myelodysplastic syndromes. Leuk Res 39:859–865. doi:10.1016/j.leukres.2015.05.012
Ford S, Fallowfield L, Lewis S (1996) Doctor-patient interactions in oncology. Soc Sci Med 42:1511–1519. doi:10.1016/0277-9536(95)00265-0
Gattellari M, Voigt KJ, Butow PN, Tattersall MHN (2002) When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol Off J Am Soc Clin Oncol 20:503–513
Degner LF, Sloan JA, Venkatesh P (1997) The control preferences scale. Can J Nurs Res Rev Can Rech En Sci Infirm 29:21–43
Frosch DL, Kaplan RM (1999) Shared decision making in clinical medicine: past research and future directions. Am J Prev Med 17:285–294
Florin J, Ehrenberg A, Ehnfors M (2008) Clinical decision-making: predictors of patient participation in nursing care. J Clin Nurs 17:2935–2944
Kaplan RM, Frosch DL (2005) Decision making in medicine and health care. Annu Rev Clin Psychol 1:525–556. doi:10.1146/annurev.clinpsy.1.102803.144118
Brom L, Pasman HRW, Widdershoven GAM et al (2014) Patients’ preferences for participation in treatment decision-making at the end of life: qualitative interviews with advanced cancer patients. PLoS One. doi:10.1371/journal.pone.0100435
Butow PN, Maclean M, Dunn SM et al (1997) The dynamics of change: cancer patients’ preferences for information, involvement and support. Ann Oncol Off J Eur Soc Med Oncol ESMO 8:857–863
Tariman JD, Doorenbos A, Schepp KG et al (2014) Older adults newly diagnosed with symptomatic myeloma and treatment decision making. Oncol Nurs Forum 41:411–419. doi:10.1188/14.ONF.411-419
Efficace F, Gaidano G, Sprangers M et al (2014) Preference for involvement in treatment decisions and request for prognostic information in newly diagnosed patients with higher-risk myelodysplastic syndromes. Ann Oncol Off J Eur Soc Med Oncol ESMO 25:447–454. doi:10.1093/annonc/mdt557
Trautmann F, Hentschel L, Hornemann B et al (2016) Electronic real-time assessment of patient-reported outcomes in routine care-first findings and experiences from the implementation in a comprehensive cancer center. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. doi:10.1007/s00520-016-3127-0
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Mehnert A, Müller D, Lehmann C, Koch U (2006) Die deutsche Version des NCCN Distress-Thermometers: Empirische Prüfung eines Screening-Instruments zur Erfassung psychosozialer Belastung bei Krebspatienten. Z Für Psychiatr Psychol Psychother 54:213–223. doi:10.1024/1661-4747.54.3.213
Roth AJ, Kornblith AB, Batel-Copel L et al (1998) Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer 82:1904–1908
Strittmatter G, Mawick R, Tilkorn M (2000) Entwicklung und klinischer Einsatz von Screening-Instrumenten zur Identifikation betreuungsbedürftiger Tumorpatienten. In: Leb. Aus Med. – Soziol. Perspekt. Hogrefe, Göttingen, Bern, Toronto, Seattle, pp 59–75
Rubenstein LZ, Harker JO, Salvà A et al (2001) Screening for undernutrition in geriatric practice developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 56:M366–M372. doi:10.1093/gerona/56.6.M366
Kaiser MJ, Bauer JM, Ramsch C et al (2009) Validation of the mini nutritional assessment short-form (MNA®-SF): a practical tool for identification of nutritional status. JNHA - J Nutr Health Aging 13:782–788. doi:10.1007/s12603-009-0214-7
Karnofsky D, Burchenal J (1948) The clinical evaluation of chemotherapeutic agents in cancer. In: Eval. Chemother. Agents. Columbia University Press, New York, pp 191–205
Verger E, Salamero M, Conill C (1992) Can Karnofsky performance status be transformed to the eastern cooperative oncology group scoring scale and vice versa? Eur J Cancer Oxf Engl 28A:1328–1330 1990
Cleeland CS (2009) The Brief Pain Inventory User Guide. M.D. Anderson Cancer Center, Houston
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 23:129–138
Beaver K, Bogg J, Luker KA (1999) Decision-making role preferences and information needs: a comparison of colorectal and breast cancer. Health Expect Int J Public Particip Health Care Health Policy 2:266–276
Ramfelt E, Lützen K, Nordström G (2005) Treatment decision - making in a group of patients with colo-rectal cancer before surgery and a one year follow-up. Eur J Cancer Care (Engl) 14:327–335
Albrecht KJ, Nashan D, Meiss F et al (2014) Shared decision making in dermato-oncology: preference for involvement of melanoma patients. Melanoma Res 24:68–74. doi:10.1097/CMR.0000000000000030
Schildmann J, Ritter P, Salloch S et al (2013) ‘One also needs a bit of trust in the doctor ... ’: a qualitative interview study with pancreatic cancer patients about their perceptions and views on information and treatment decision-making. Ann Oncol Off J Eur Soc Med Oncol ESMO 24:2444–2449. doi:10.1093/annonc/mdt193
Schuler MK, Trautmann F, Radloff M et al (2016) Implementation of a mobile inpatient quality of life (QoL) assessment for oncology nursing. Support Care Cancer:1–9. doi:10.1007/s00520-016-3163-9
Politi MC, Studts JL, Hayslip JW (2012) Shared decision making in oncology practice: What do oncologists need to know? Oncologist 17:91–100. doi:10.1634/theoncologist.2011-0261
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The authors have full control of all primary data and would allow the journal to review the data upon request.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Schuler, M., Schildmann, J., Trautmann, F. et al. Cancer patients’ control preferences in decision making and associations with patient-reported outcomes: a prospective study in an outpatient cancer center. Support Care Cancer 25, 2753–2760 (2017). https://doi.org/10.1007/s00520-017-3686-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3686-8