Abstract
Purpose
Evaluation of the quality of care is a key element that healthcare providers now take into consideration to meet patients’ needs, expectations, and values. The FAMCARE scale is one of the most important instruments available to assess the level of satisfaction about care received by patients and families. We describe the validation process used to develop an Italian version (IF) of the original FAMCARE scale for caregivers.
Methods
The IF was prepared according to standard guidelines for translation and transcultural adaptation of self-reported measures. The scale was self-administered to 132 informal caregivers of patients with cancer treated with curative and/or palliative care in a hospice, outpatient, or inpatient setting for at least 1 month. The participant group was composed of spouses (47.73 %), children (31.82 %), siblings (3.03 %), or other relatives (17.42 %). All participants simultaneously completed the EuroQol-5D (EQ-5D) questionnaire to test the construct validity. Twenty-two percent of randomly chosen participants re-completed the test after 1 month to evaluate IF test-retest stability.
Results
The IF showed a strong reliability with internal consistency [α = 0.93, confidence intervals (CI) = 0.91–0.95] and test-retest stability (Pearson r = 0.38; Kendall’s tau-b = 0,25; Spearman’s rho =0.34). Factor analysis identified four factors capable of explaining the 63 % total variance which did not change after the Varimax normalized rotation. Notwithstanding the lack of correlation with the VAS component of the EQ-5D questionnaire, our results highlighted robust psychometric properties of the IF.
Conclusions
IF is a valid translation of the FAMCARE scale and can be used to assess caregiver satisfaction within the Italian context of cancer palliative care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The primary aim of modern healthcare systems is to produce value in health, defined as optimal health outcomes for patients and their families, delivered by high-quality, low-cost services [1]. Satisfaction with care is one of the five major domains measuring patient and caregiver outcomes [2]. In Italy, a national law passed in March 2010 obliged Italian healthcare providers to begin monitoring the quality of services offered. Since then, satisfaction with care has become an integral part of care programs. However, despite the clear need to monitor this aspect of care, the relation between satisfaction and fulfillment of users’ needs and expectations remains to be clarified. In fact, evidence showing a direct relation between real and expected satisfaction [3–6] is in direct contrast to findings of a divergence between the two concepts [7, 8] and is perhaps due to differences in methodological approaches [9].
The construct of satisfaction with the received care is directly associated with the importance of meeting the needs and expectations of patients and their families [10–12]. Nowadays, most oncology teams recognize the impact that informal caregivers have on the effectiveness of cancer management and include them in treatment planning, decision-making, and care implementation [13]. In fact, in the context of life-threatening diseases such as cancer where family members are deeply involved in the care process, caregivers and patients should be considered as a system [14]. As such, both are affected by the received care, e.g., if the need to receive pain relief is not met, both patients and families manifest distress, the former directly and the latter indirectly. Hence, apart from patients themselves, there is no one better equipped than a family member to provide direct feedback on the quality of care received.
The FAMCARE scale is a self-administered questionnaire specifically developed to measure the level of satisfaction perceived by the caregivers of patients with cancer receiving palliative care [15–18]. It is reported to have excellent psychometric properties [8, 19, 20]. Although the scale is available in several languages [21–23], there is still no Italian version. The aim of the present work was to prepare and validate a version of FAMCARE scale for the Italian community.
Materials and methods
Scale translation and adaptation process
The translation and transcultural adaptation guidelines for self-administered outcome measures by Beaton et al. were used as the basis for the procedure [24]. The guidelines include the following six stages:
-
Stage (1) Translation into the target language
Two native Italian translators produced independent forward translations of the FAMCARE scale. The first forward translation was made by a palliative care specialist experienced in care and clinical research, while the second was produced by an expert in communication and in the development of tools for patient and caregiver education.
-
Stage (2) Synthesis of the forward translations
The two forward translations were synthesized into one version by two of the study authors (RC and MM).
-
Stage (3) Backward translations
The synthesized version of the translated scale was then backward translated into English by two independent native English speakers: a bilingual English teacher working in Italy and a bilingual English translator who has long-standing experience in scientific writing and translations. The backward translations were matched to check for semantic and syntactic differences and a synthesized version of the scale was built. The item equivalence of the synthesized backward translation was then performed by comparing the back-translated version of the scale with the original one.
-
Stage (4) Consensus conference
A consensus conference was organized to review the final forward translated Italian version and the backward translated English version of the scale. Conference participants included healthcare professionals with experience in palliative care (palliative care physician, supportive care psycho-oncologist, and oncology nurse), patient and caregiver representatives (i.e., two women and one man for each group of patients and caregivers), and an expert in statistical methodology. A preliminary consensus version of Italian FAMCARE scale was then produced.
-
Stage (5) Pre-test patient survey
The preliminary version of the IF was administered by a research nurse to a random sample of 20 informal caregivers of patients with cancer who were asked to fill in the questionnaire and rate its level of clarity and comprehensiveness, including the title, instructions for use, items, and scale used to respond. They were also asked to specify how long it had taken them to complete the questionnaire, to report any difficulties encountered, and to indicate whether they found any offensive or unacceptable terms within the concrete item phrases (face validity). The problems that emerged from the pre-test study were discussed by the research team to prepare a final Italian version of the scale to use for the subsequent validation study.
-
Stage (6) Approval of original authors
All the documents pertaining to the translation and transcultural adoption process were sent to the original authors for their approval.
Ethical clearance
The study was approved by the Healthcare Authority of the Wide Catchment Area of Romagna (Area Vasta Romagna–AVR) and was carried out in accordance with the Declaration of Helsinki. Ethical clearance was obtained in April 2013, and the study was carried out from May to November of the same year. Informed consent was obtained from the caregivers who decided to take part in the study.
Eligibility criteria
In order to select the study participants in each study center, we approached the informal caregivers of the first, second, or third patients of each week who had been receiving curative cancer treatments or/and palliative care for at least 1 month in the same setting.
The study participants were consenting adult informal caregivers of patients with cancer who had been receiving cancer care curative and/or palliative in one of the study centers for at least 1 month, regardless of healthcare setting (outpatient clinic/inpatient/hospice), disease stage, or ongoing cancer treatments. The caregivers were chosen on the basis of the following definition, “the individual identified by the patient as the person most involved in the care of the patient. The relationship with the patient could be biological, legal, or functional” [8]. All study participants were compliant and were capable of understanding and signing the consensus form and of completing the questionnaires. The participating centers were Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS (Oncology Ward and Outpatient Clinics in Meldola and Forlì) and the community-based hospice in a nearby town (Forlimpopoli).
Sample size calculation
Based on the number of items composing the original scale (20 items), we calculated a sample size of 165 participants, expecting a response rate of 75 %, considering 124 correctly completed questionnaires as a sufficient number for study purposes [25]. We decided to repeat the surveys 4 weeks after the first self-administration in 22 % of randomly chosen caregivers to measure the test-retest stability of the scales.
Procedures
The nursing staff of the Oncology Units involved in the study were trained by the study coordinator of each center on how to screen the caregivers who met the criteria for inclusion in the study. They also provided information about the study to the selected caregivers who agreed to take part in the study and explained how to fill in the questionnaires. Completed questionnaires were forwarded to the study coordinator of each center.
Instruments and measurements
The FAMCARE scale is a self-reporting questionnaire that assesses the level of perceived satisfaction with the care received by both patients and their families [15–18]. It is composed of 20 items rated according to a 5-point Likert scale (1 = very satisfied, 2 = satisfied, 3 = undecided, 4 = dissatisfied, and 5 = very dissatisfied). The items are sorted into four subscales labeled as Physical patient care, Information giving, Availability of care, and Psychosocial care subscale according to the sorting process used by Kristjanson [19].
We used the IF and the Italian version of EuroQol-5 Dimension (EQ-5D) [26] because, considering both patients and caregivers as a system, we expected to find that the quality of life of the caregivers would be influenced by their perceived satisfaction with care [27]. We wanted to test whether there was a correlation between EQ-5D scores and perceived satisfaction and how the satisfaction with care affect family members health status measured by the EQ-5D [28, 29]. The EQ-5D was chosen mainly because of its brevity and ease-of-use.
The EQ-5D is a questionnaire used to evaluate health-related quality of life. In the domain of palliative care, the EQ-5D has proven effective in assessing palliative care patient outcomes [30]. At the time of the IF test, there were no questionnaires, EQ-5D, with Italian translation that was validated to assess quality of life in a general manner (being not disease specific).
The EQ-5D consists of two sections: the first asks participants to give their opinion using a 3-point scale (1 = no problem, 2 = some problems, and 3 = extreme limitation) about health functionality along five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). The second section involves a graphical evaluation of the subject’s perceived level of quality of life (Visual Analog Scale–VAS). This scale ranges from 0 (the worst possible health status) to 100 (the highest attainable standard of health).
Statistical analysis
Descriptive statistics were used for presenting study population characteristics. Mean and standard deviations were used to present scores of individual items of study participants. The total satisfaction score was calculated by summing all the item scores for each subject. Cronbach’s alpha was calculated to assess the internal consistency of the IF and was chosen on the basis of its coefficient sensitivity to measure interrelation levels among items evaluating the same construct. The coefficient was also used to measure internal consistency among items exploring attitudes or opinions.
In order to explore the conceptual structure of the IF, we began with a principal component factor analysis and determined the factor loadings, which are the correlations between the factors and the individual items. Factor analysis and internal consistency were assessed by Barlett’s sphericity test. The measure of sampling adequacy was tested by the Kaiser-Meyer-Olkin (KMO) index as it permitted us to match observed and partial correlations [31, 32]. Moreover, the common factors were extracted using a Varimax rotation with Kaiser normalization to decrease the number of variables involved. The test-retest stability of the scale was evaluated through both parametric (Pearson r) and non-parametric correlations (Spearman’s rho or Kendall’s tau-b).
Finally, the Pearson product-moment correlation coefficient was calculated to assess the relationship between the IF scores and either part of the EQ-5D scores (5D and VAS). As the responses participants provided indicated different levels of problems (e.g., level 1: indicating no problem; level 2: indicating some problems; level 3: indicating extreme problems), participants’ responses were translated into cardinal values by adding the weights recommended by Euroquol Group (www.euroquol.org). Statistical analyses were carried out using R-statistical software (Version 3.1.3).
Results
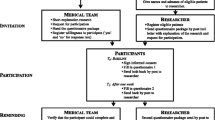
Figure 1 shows details of participant selection in study centers. Of the original 165 participants, 132 completed questionnaires correctly (response rate = 80 %). Study participant characteristics are reported in Table 1. Data on both the original group and the one comprising compliant participants are shown in this table. There were no differences between the demographic characteristics of the two groups. Caregivers not completing all of the questionnaire items was the main cause of missing data.
The total satisfaction score was between 70 and 100, with a mean value of 89.9, a median value of 89.5 and a SD of 7.78. Participants indicated a good level of satisfaction (max score = 2.50, mean score = 1.53, score in the 3rd quartiles = 1.80) in all the items (Table 2). In particular, the mean scores on individual items calculated by sorting the items according to Kristjanson’s four subscales were as follows [19, 20]: 1.54 (SD = 0.45) for Information giving (items 2, 3, 4, 16, and 17), 1.52 (SD = 0.41) for physical care (items 1, 5, 8, 10, 14, 18, and 19), 1.55 (SD = 0.48) for psychosocial care (items 7, 9, 13, and 15), and 1.38 (SD = 0.42) for availability of care (items 6, 11, 12, and 20). The internal consistency of the scale measured by the Cronbach’s α coefficient was high [0.93 % confidence intervals (CI) 0.91–0.94].
Our unrotated factor analysis performed by the extraction method identified four factors (Table 3). The first one yielded a very strong factor with an eigenvalue of 9.08 and three weak factors with eigenvalues of 1.34, 1.15 and 1.05, explaining the 45 %, 7 %, 6 % and 5 % of the proportional variance (63 % of the total variance), respectively. The factors were subsequently confirmed by the Varimax-rotated solution which gave the following eigenvalues: 6.03, 2.47, 2.26 and 1.87, respectively, explaining the 30 %, 12 %, 11 % and 9 % of the proportional variance (i.e. 63 % of the total variance). We then labeled each factor yielded by the factorial analysis in the same way as the original scale, with the exception of the fourth subscale, which was labeled as the item with the highest loading in the respective subscale.
The first factor was labeled as a subscale of Giving information (items 3, 8, 9, 15, 16, 17, 18, 19 and 20), the second as a subscale of Physical care of the patient (items 4, 5,10 and 14), the third as a subscale of Availability of care (items 6, 11, 12 and 13) and the fourth as a subscale of Pain management (items 1, 2, and 7). The KMO index (0.91) and Bartlett’s sphericity test (p value < 0.000; Χ 2 = 1424.7) indicated that factor analysis for the data was appropriate. Table 4 shows the cross-classification of the conceptual structure of Kristjanson’s study and our IF validation population.
With regard to the test-retest analysis, stability over time proved to be low using both parametric (Pearson r = 0.38, n = 37, p = 0.021) and non-parametric correlation tests (Kendall’s tau-b = 0.26, n = 37, p = 0.029; Spearman’s rho =0.36, n = 37, p = 0.026). Despite the correlation that emerged between EQ-5D and EQol VAS (r = 0.72, n = 128, p < 0.001), only a weak correlation was observed between IF scores and EQ-5D, r = 0.17, n = 128, p = 0.047, and no correlation was found with VAS (r = 0.1008, n = 128, p = 0.34).
Discussion
Our analysis of the psychometric properties of the Italian version of the FAMCARE scale revealed a good reliability of the translation in terms of internal consistency. In fact, it emerged that all 20 items of the IF scale contributed to measuring the same construct, i.e., caregivers’ satisfaction with the palliative care received by patients with cancer. “Palliative care” in our clinical context is intended as patient-centered palliative care and represents services that are available for patients with cancer during the course of the disease (early palliative care and palliative care at the end of life). They are offered at the same time as curative treatments and focus on providing patients with relief from symptoms, pain, and stress and on reducing and controlling the side effects of cancer treatments to improve the quality of life for both patients and families. This is why we included caregivers from both palliative care settings in the study [33].
An important aspect of the present study is that it included a retest 1 month after the first questionnaire administration. Four weeks were assumed sufficient to enable us to avoid a potential bias in evaluating test retest stability caused by the effect of learning and recall, as mentioned by Kristjanson, the creator of the original score [19]. However, the results that emerged from both parametric and non-parametric correlations test indicate that other factors may have modulated the responses as a result of changes in the clinical conditions of a patient. In 4 weeks, for example, the retrospective opinions about the care received might have changed. Indeed, as is suggested by the factorial analysis carried out on the data in retest time, item loading differed with respect to test time. Although the retest analysis has a low statistical value given the low number of participants, it suggests that, in the palliative care setting, retrospective opinions about the way in which a loved one was cared for may change over time [19].
A comparison between the construct validity of the IF and that of the EQ-5D revealed a weak correlation with descriptive items of the EQ-5D and no correlation with VAS values. Factor analysis revealed that all 20 items in the FAMCARE scale had high factor loadings and were heavily loaded on one of the four emerging factors. A full comparison between our results and those from other studies is difficult due to differences in the number of factors, combination of items arising within factors, and type of factor analysis used [16, 18, 19, 27, 31], e.g., our fourth subscale differs from that of the original one (Table 4). We hypothesize that the differences between construct structures of the IF and those of the original FAMCARE could be a result of social and disease contexts. Culture, health care systems, and norms may also have influenced factor structure. In our population, the present factor analysis shows that the FAMCARE scale probably does not measure a unitary construct. Given that FAMCARE is a self-reported instrument, the dimensions of satisfaction reflect the significance that patients and families in a specific social context gives to aspects of care (measured by single items) and may also be influenced by a specific phase of the disease. In our population, pain relief appears to be an important dimension of satisfaction with care. The fact that two other items (item 2, information given about prognosis and item 7, conferences held with family about patient’s illness) contribute to this dimension of satisfaction probably indicates that information continuity is essential to fulfill patient and caregiver expectations about this important aspect of care. At the same time, the expectations of patients and families may change during the course of the disease. For example, patient and family expectations about pain relief may be higher in the early stages of cancer than at the end of life, making this dimension an important predictor of satisfaction with care. In the last stages of the disease where patient needs become more complex, the availability of palliative care services becomes more important and expectations about this aspect of care may therefore be higher. This hypothesis is in line with findings from previous FAMCARE validation studies in which the FAMCARE construct structure was defined as setting-dependent. It is also very likely that different settings, i.e., outpatient, long-term care, may have led to differences in factor structure [30]. Although a short version of the FAMCARE exists [23], our values suggest that no pruning process can be performed in the 20 items we translated from the original version of the FAMCARE [19, 20] as each was necessary to measure the level of satisfaction of received care in cancer settings [28, 31].
However, from a theoretical point of view, it can be argued that although some items can be loaded in a specific factor, they might actually fit another factor better. This may be the case for item nos. 8 “Speed with which symptoms are treated”, 9 “Doctor’s attention to patient’s description of symptoms,” 18 “How thoroughly the doctor assesses the patient’s symptoms” and 19 “The way tests and treatment are followed up by the doctor”, which may be more closely related to the factor “Physical care of the patient” than the factor “Given information”.
The study has a number of limitations. The first concerns the fact that we only tested IF in hospital settings. Independent evidence shows that FAMCARE is setting-dependent [30]. Rodriguez et al. reported that in long-term care settings, two items (6 and 12) did not measure care-related satisfaction expressed by family members. In the same vein, Ringdal et al. stated that the FAMCARE scale tested in family caregivers who were close to patients with advanced cancer undergoing palliative care tended to be unidimensional [18]. Although the selection of study participants may have created a bias, we tried to keep it to a minimum by using a selection approach in which we proposed the study to the caregiver of the first, second, or third patient fulfilling selection criteria each week in each study center, i.e., patients receiving cancer care for at least 1 month in the same setting. A further potential limitation concerns the population we tested in that it was mainly composed of female caregivers. However, although at first sight this might appear as a bias, there is evidence to suggest that the majority of caregivers are, in fact, females [34, 35]. Above limitations notwithstanding, the rigorous methods and sample size of the study provide reliable results that will enable the IF to be used in Italian cancer care settings.
Conclusions
The evaluation of the quality of a service has become an important aspect of healthcare. However, quality of care is also a highly complex area requiring the use of indicators that are accurate and easy to use. The level of satisfaction with care is considered a useful indicator to assess the quality of the services provided. Italian Health Authorities, and in particular, local palliative care networks, are required to provide services that fully meet the needs of patients and caregivers. However, this is not an easy task as there are no instruments capable of assessing the quality of palliative care within an Italian context. Given the FAMCARE scale’s intrinsic capacity to measure one of the healthcare quality indicators in the cancer palliative care context, we decided to validate an Italian version of the scale [15, 16]. The IF showed good psychometric properties, indicating its potential suitability for use in settings such as the hospice and hospital.
Today, satisfaction with care is one of the core domains of healthcare value through which to evaluate the performance of the services provided. Although the concept of value in health differs according to the social context considered, satisfaction remains one of the common domains of value [36].
It is thus essential to introduce valid instruments into clinical practice that are capable of providing comparable data to measure the domains of values produced in healthcare settings. The Italian version of the FAMCARE scale validated in this study possesses such characteristics and could be recommended for use in clinical practice. Furthermore, measuring the quality of a service through tools designed to measure satisfaction could help service providers to understand whether the care provided fits in with the needs, requirements, and expectation of patients and caregivers.
References
Porter ME (2009) A strategy for health care reform--toward a value-based system. N Engl J Med 361:109–112
Palos GR, Hare M (2011) Patients, family caregivers, and patient navigators: a partnership approach. Cancer 117:3592–3602. doi:10.1002/cncr.26263
Fox JG, Storms DM (1981) A different approach to sociodemographic predictors of satisfaction with health care. Soc Sci Med A 15:557–564
Linder-Pelz S (1982) Social psychological determinants of patient satisfaction: a test of five hypotheses. Soc Sci Med 16:583–589
Hsieh MO, Kagle JD (1991) Understanding patient satisfaction and dissatisfaction with health care. Health Soc Work 16:281–290
Williams S, Weinman J, Dale J, Newman S (1995) Patient expectations: what do primary care patients want from the GP and how does to meeting expectations Affect patient satisfaction? Fam Pract 12:193–201
McKay A, Goldberg EM, Fruin DJ (1973) Consumers and a social services department. Social Work Today 4:486–491
Medigovich K, Porock D, Kristjanson LJ, Smith M (1999) Predictors of family satisfaction with an Australian palliative home care service: a test of discrepancy theory. J Palliat Care 15:48–56
Staniszewska S, Ahmed L (1999) The concepts of expectations and satisfaction: Do they capture the way patients evaluate Their care? J Adv Nurs 29:364–372
Ajzen I, Fishbein M (1980) Understanding attitudes and predicting social behaviour. Prentice-Hall, Englewood Cliffs, New Jersey
Dawson NJ (1991) Need satisfaction in terminal care settings. Soc Sci Med 32:83–87
Wright S (1998) Patient satisfaction in the context of cancer care. Ir J Psychother 19:274–282
Glajchen M (2004) The emerging role and needs of family caregivers in cancer care. J Support Oncol 2:145–155
Kristjanson LJ, Aoun S (2004) Palliative care for families: remembering the hidden patients. Can J Psychiatr 49:359–365
Kristjanson LJ (1986) Indicators of quality of palliative care from a family perspective. J Palliat Care 1:8–17
Kristjanson LJ (1989) Quality of terminal care: salient indicators identified by families. J Palliat Care 5:21–30
Ringdal GI, Jordhøy MS, Ringdal K, Kaasa S (2001) Factors affecting grief reactions in close family members to individuals who have died of cancer. J Pain Symptom Manag 22:1016–1026
Ringdal GI, Jordhoy MS, Kaasa S (2003) Measuring quality of palliative care: psychometric properties of the scales FAMCARE. Qual Life Res 12:167–176
Kristjanson LJ (1993) Validity and reliability testing of the FAMCARE scale: measuring family satisfaction with advanced cancer care. Soc Sci Med 36:693–701
Kristjanson LJ, Leis A, Koop PM, Carrière KC, Mueller B (1997) Family members’ care expectations, care perceptions, and satisfaction with advanced cancer care: results of a multi-site pilot study. J PalliatCare 13:5–13
Can G, Akin S, Aydiner A, Ozdilli K, Oskay U, Durna Z (2011) A psychometric validation study of the Quality of Life and FAMCARE scales in Turkish cancer family caregivers. Qual Life Res 20:1319–1329. doi:10.1007/s11136-011-9867-x
Alnjadat RM, Adnan WAW, Ismail Z (2014) Construct validity and internal consistency of a Malay version of the FAMCARE scale for measures of informal caregivers satisfaction. Sains Malaysiana 43:1415–1420
Ljungberg AK, Fossum B, Fürst CJ, Hagelin CL (2015) Translation and cultural adaptation of research instruments - guidelines and challenges: an example in FAMCARE-2 for use in Sweden. Inform Health Soc Care 40:67–78
Beaton DE, Bombardier C, Guillemin F, Ferraz MB (2000) Guidelines for the process of cross-cultural adaptation of selfreport measures. Spine (Phila Pa 1976) 25:3186–3191
Kline P (1994) An easy guide to factor analysis. Routledge, London, UK
Savoia E, Fantini MP, Pandolfi PP, Dallolio L, Collina N (2006) Assessing the construct validity of the Italian version of the EQ-5D: preliminary results from a cross-sectional study in North Italy. Health and Quality of Life Outcomes 4:47
Cramm JM, Strating MM, Nieboer AP (2012) Satisfaction with care as a quality-of-life predictor for stroke patients and their caregivers. Qual Life Res 21:1719–1725
Kaasa S, Havard Loge J (2003) Quality of life in palliative care: principles and practices. Palliat Med 17:11–20
Carter GL, Lewin TJ, Gianacas L, Clover K, Adams C (2011) Caregiver satisfaction with out-patient oncology services: utility of the instrument FAMCARE and development of the FAMCARE-6. Support Care Cancer 19:565–572
Xia Q, Hwang SS, Chang VT, Osenenko P, Alejandro Y, Yan H, Srinivas S (2005) Validity, reliability and responsiveness of Euroqol (EQ5D) in patients (Pts) receiving palliative care (PC). JCO ASCO Annual Meeting Proceedings (Vol. 23, No. 16_suppl, p. 8082)
Rodriguez KL, Bayliss NK, Jaffe E, Zickmund S, Sevick MA (2010) Factor analysis and internal consistency evaluation of the FAMCARE scale for use in the long-term care setting. Palliat Support Care 8:169–176
Higginson IJ, Donaldson N (2004) Relationship between three palliative care outcome scales. Health Qual Life Outcomes 2:68
Hui D, Mori M, Parsons HA, Kim SH, Li Z, Damani S, Bruera E (2012) The lack of standard definitions in the supportive and palliative oncology literature. J Pain Symptom Manag 43:582–592
Lipszyc B, Sail E, Xavier A (2012) Long-term care: need, use and expenditure in the EU-27. European Commission & Directorate-General for Economic and Financial Affairs. Brussels: European Commission. Retrieved from http://ec.europa.eu/economy_finance/publications/economic_paper/2012/pdf/ecp469_en.pdf
Triantafillou J, Naiditch M, Repkova K, Stiehr K, Carretero S, Emilsson T et al (2010) Informal care in the long-term care system: European Overview Paper for INTERLINKS project (FP7). Athens/Vienna: EuropeanCentre. http://www.euro.centre.org/interlinks
Dziuban CD, Shirkey EC (1974) When is a correlation matrix appropriate for factor analysis? Some decision rules. Psychol Bull 8:358–361
Acknowledgments
The authors are grateful to the original author of the FAMCARE Scale, Prof. Samar Aoun, for authorizing us to use of the questionnaire, thus making our study possible. We also thank the caregivers who accepted to take part in the pre-test study for their invaluable contribution. Special acknowledgements go to the oncology nurses of IRST IRCCS and of the community-based hospice in a nearby town (Forlimpopoli) for their contribution to participant recruitment. Thanks go to Ursula Elbling for editorial assistance.
Statement of authorship
RC and GO designed the study and performed the translation and adaptation process. AZ, MAB and MM were involved in nurses training, participant recruitment and study coordination in the centers, VC was responsible for data registration and analysis. All authors contributed to the drafting of the manuscript and approved the final version for submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to declare for this manuscript. The authors have full control of primary data and agree to allow the journal to review their data if requested.
Rights and permissions
About this article
Cite this article
Chattat, R., Ottoboni, G., Zeneli, A. et al. The Italian version of the FAMCARE scale: a validation study. Support Care Cancer 24, 3821–3830 (2016). https://doi.org/10.1007/s00520-016-3187-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3187-1