Abstract
Background
Despite newer agents, chemotherapy-induced nausea and vomiting (CINV) continues to remain a distressing side effect to a proportion of patients undergoing systemic anti-cancer therapy.
Methods
We recently performed an unplanned secondary analysis on a previously reported negative phase III trial (N08C3) looking at the efficacy of gabapentin/placebo in combination with dexamethasone and a 5HT3 receptor antagonist in the prevention of CINV for 413 patients undergoing regimens with highly emetogenic chemotherapy (HEC). In the current study, we attempted to better understand the higher than expected rate of overall patient satisfaction, despite a low complete response rate in both arms. Additionally, we looked at patient variables and their relationship to rates of CINV.
Results
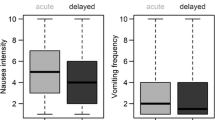
Approximately one third of patients experienced more than mild nausea and reported scores on the Functional Living Index–Emesis that indicated interference with activities. Thirty-five percent reported nausea greater than 2.5 on a scale of 0 to 10 (0 being none), 19 % reported at least one emetic episode, and 49 % reported taking rescue medication. Nausea and vomiting on day 1, cisplatin therapy, and history of motion sickness significantly predicted delayed CINV. Age, combination chemotherapy (HEC with moderately emetogenic), and getting treatment for breast cancer predicted CINV on day 1.
Discussion
These data confirm previous reports that subgroups of patients may be more prone to acute and delayed CINV. Future CINV study design may benefit from a more individualized approach to CINV management, targeting those patients who are truly at risk for CINV despite continued drug development efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) remains a significant source of distress and dissatisfaction among patients receiving cancer treatment. There are two distinct forms of CINV: acute (≤24 h after chemotherapy) and delayed (>24 h after chemotherapy). Even with the emergence of 5HT3 and NK1 receptor antagonists, some patients continue to struggle with delayed CINV, particularly those who are undergoing regimens containing highly emetogenic chemotherapy (HEC) [1]. Prior to the introduction of 5HT3 receptor antagonists, the incidence rate of acute nausea and vomiting was approximately 40 % after cisplatin regimens and delayed nausea and vomiting was experienced by 93 % of the patients [2]. Sixty-eight percent of patients reported both nausea and vomiting; 19 % reported only nausea; 6 % reported only vomiting [2]. In the era of treatment with dexamethasone and 5HT3 antagonists, as well as NK-1 receptor antagonists, the rates of delayed nausea dropped to around 50 %, with delayed emesis in around 30–50 % of patients [3–5].
To address the identified gap in complete control of CINV for all patients, the North Central Cancer Treatment Group (NCCTG, now part of the Alliance for Clinical Trials in Oncology) recently conducted N08C3, a phase III randomized, double blind placebo-controlled trial utilizing gabapentin plus dexamethasone versus placebo with dexamethasone for the prevention of delayed CINV after standard treatment with a 5HT3 receptor antagonist and dexamethasone on the day of chemotherapy. This trial was negative, showing no difference in complete response rates (defined as no emesis and no rescue medications on days 2 through 6) between the two therapy arms [6]. N08C3 demonstrated complete response rates for delayed nausea and vomiting of 47 and 41 % (P = 0.23) in the gabapentin and placebo arms, respectively. Despite the lower than expected complete response rates (less than 50 %), participants rated their overall satisfaction for the control of nausea and vomiting (days 2–6) at a mean of 8.7 (range 0–10 with 10 being completely satisfied) in the group receiving gabapentin and 8.5 in the placebo group [6]. Due to the high rate of satisfaction but lower than expected complete response rates, we re-examined the N08C3 data with the following objectives in mind. The first objective (aim 1) was to identify the prevalence of patients who reported more severe CINV symptoms that interfered with activity. We sought to determine the prevalence of patients reporting, in the delayed phase (days 2–6), more than mild nausea (>2.5), any emesis, or use of rescue medications (as captured on a daily diary). As part of aim 1, we also sought to determine the prevalence of patients reporting scores on the Functional Living Index–Emesis (FLIE) that indicate interference with activity due to CINV (total scores under 108 and subscale scores under 54). The second objective (aim 2) was to determine which characteristics predicted patients that reported a nausea score >2.5 nausea, characteristics of patients with any vomiting, or those who took rescue medications in the delayed phase. The third objective (aim 3) was to calculate the number of patients who reported taking rescue medications without any evidence of emesis or >2.5 nausea during the delayed phase.
Patients and methods
Eligibility criteria
Patients in the parent study were eligible if they had not previously received highly or moderately emetogenic chemotherapy (HEC and MEC) and were scheduled to receive HEC (regimens were required to include at least 7 days between cycles of HEC administration), had an ECOG performance status of 0–2, were able to swallow pills, and were able to provide written informed consent. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Patients were considered not eligible for the parent study if they had current or prior use of (or sensitivity to) gabapentin or other anticonvulsants, lorazepam, diphenhydramine, eszopiclone, and/or dronabinol, or had any planned prophylactic use of these drugs outside of the study regimen during the 6 days of the study, except for the treatment of breakthrough nausea and vomiting. Additionally, patients were excluded for any prior or planned use of aprepitant or any other NK-1 receptor antagonist during the study, since at the time the protocol was written; NK-1 receptor antagonists were not yet the standard of care throughout all cancer treatment facilities. Finally, patients were not allowed to participate in the study if they had received pelvic or abdominal radiotherapy within 1 week prior to study enrollment, or had prior gastrointestinal obstruction, active peptic ulcer disease, uncontrolled heartburn, or a history of nausea and vomiting with any previous chemotherapy.
Treatment
Patients were randomly assigned to receive either gabapentin (300 mg) or placebo pill beginning on the evening of day 1 of their first chemotherapy cycle. The dose was increased on days 2 and 3 to one gabapentin/placebo pill twice daily. On days 4 and 5, patients could be titrated up to one gabapentin/placebo pill three times daily if they were not satisfied with their control. All patients received prophylactic treatment on day 1 for acute CINV with a 5HT3 receptor antagonist (provider/patient choice) and dexamethasone 20 mg. Patients received prophylaxis for delayed CINV on days 2–4 with decreasing doses of dexamethasone (8 mg orally (PO) twice daily (BID), days 2 and 3, and 4 mg PO BID on day 4) with or without a 5HT3 receptor antagonist (clinician preference).
Secondary outcomes analyses
Since there were no significant differences in nausea and vomiting outcomes based on study arm, secondary analyses were conducted utilizing data from the combined sample. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were based on the study database frozen on August 7, 2011.
Aim 1
The first part of aim 1 was to determine the prevalence of patients meeting specified criteria for three outcomes of interest in the patient diary. For each outcome, the number of participants who fit the criterion was divided by those who did not, to determine the prevalence. The first outcome was delayed nausea. In the diary, participants were asked to rate their daily average severity of nausea on a 0 to 10 scale (10 being the worst) over the past 24 h. Patients who met the criterion for delayed nausea reported more than mild nausea, defined as a score of greater than 2.5 on the nausea and vomiting diary, on any of the days 2–6. The maximum reported nausea rating on days 2 through 6 was used for prevalence determination. The second variable was emesis. Participants responded daily to a question asking them how many times they vomited in the past 24 h. Any participant who marked at least 1 on any of the days 2 to 6 were counted as having delayed emesis. For the third variable, use of non-protocol antiemetics, participants responded daily to a question asking if they took any medications for nausea or vomiting in the past 24 h in addition to the study medication. Anyone marking yes on any of the days 2 through 6 was counted as taking rescue medications.
The FLIE total scale and subscales were calculated. Scores of less than 108 have been shown to be associated with a negative impact on activity on the total scale. For the subscales, scores lower than 54 are indicative of a negative impact. The percentage of patients scoring under these cut points was calculated [7].
Aim 2
Separate univariate and multivariate analyses were performed with the following dependent variables: nausea >2.5 days 2–6, any emesis days 2–6, and use of rescue medications days 2–6. In univariate analyses, variables with a p value <0.10 were selected for inclusion in multivariate analyses. Variables controlled for in each model included use of gabapentin, emesis on day 1, nausea severity on day 1, age, gender, disease (breast versus other), use of palonosetron (versus a first-generation 5HT3 receptor antagonist) day 1, type of chemotherapy (cisplatin vs other), use of 5HT3 receptor antagonist after day 1, history of alcoholism, history of motion sickness, and use of moderately emetogenic agents along with the highly emetogenic agents (versus HEC only). Additional independent variables for model 3, where rescue medication use was the dependent variable, included nausea severity on days 2 through 6 and any emesis on days 2 through 6.
Aim 3
For this aim, the sample was split into two groups: patients who took rescue medication (from aim 1) and those who did not. For those who took rescue medication, frequencies were determined for those who reported nausea <2.5 on days 2 through 6 AND did not report any emesis.
Results
Between May 1, 2009 and February 4, 2011, 430 patients were enrolled to study N083C. Of those, 17 never received treatment and are not included in this analysis. Patient characteristics are shown in Table 1. Regarding aim 1, at some time during the delayed phase (day 2–6), 143/413 (35 %) patients reported nausea severity greater than 2.5, 80/413 (19 %) patients reported at least one emetic episode, and 202/413 (49 %) patients took rescue medication. Thirty-three percent of patients scored less than 108 on the FLIE total score, indicating a negative impact on activities. When considering the nausea and vomiting subscales individually, 38 % of patients scored less than 54 on the nausea subscale and 20 % of patients scored less than 54 on the vomiting subscale.
Regarding aim 2, significant predictors of more than mild nausea (>2.5) on days 2–6 in the multivariate model were significant nausea on day 1 and history of motion sickness. Table 2 shows the p values and odds ratios for each of the significant variables in the univariate model and then the p values and odds ratios for the multivariate model. Significant predictors for emesis on days 2 through 6 in the multivariate model included receiving cisplatin-based chemotherapy, experiencing emesis on day 1, and experiencing nausea greater than 2.5 on days 1. Table 3 shows the univariate and multivariate p values and odds ratios. With respect to the use of rescue medication during days 2 through 6, the multivariate model indicates emesis on day 1 or on days 2–6, nausea greater than 2.5 on days 2 through 6, and history of motion sickness were predictive. The use of palonosetron was not a significant factor associated with delayed or acute nausea and vomiting in this sample. Table 4 shows the p values and odds ratios for the univariate and multivariate models.
Since day 1, emesis and nausea were prognostic for similar events on days 2 through 6; baseline demographics were explored to identify variables associated with these day 1 events. Based on univariate and multivariate logistic models, age was the strongest predictor of having emesis on day 1 (p < 0.0001). The odds ratio (95 % confidence interval) for age was 0.937 (0.912 to 0.963), indicating that younger patients were at a greater risk for emesis on day 1. After adjusting for age, no other variables were significant for day 1 emesis.
Variables related to having significant nausea (nausea score >2.5) on day 1 were age (p < 0.0001, odds ratio of 0.943), having breast cancer (p = 0.0054, odds ratio of 2.335), and the addition of any moderate emetogenic agents (p = 0.0149, odds ratio of 2.04).
We also evaluated what factors were predictive of having no emesis and no nausea >2.5 on days 2 through 6. Being of an older age (p = 0.001), not receiving moderately emetogenic agents in addition to highly emetogenic chemotherapy (p < 0.02), and having no emesis or nausea >2.5 on the day of chemotherapy, day 1 (p < 0.0001), were significant predictors in the univariate analysis. However, when all variables were controlled for in the multivariate analysis, only one factor, having no nausea >2.5 on day 1, remained significant (p < 0.0001; odds ratio 6.47 (3.71–11.29)).
Finally, we looked at the characteristics with regard to nausea severity and emesis in patients who took rescue medications during days 2–6 of the study. Table 5 lists these characteristics. Over a third (38 %) of the participants who reported taking rescue medications did not report nausea greater than or equal to 2.5 in severity or any emetic episodes.
Discussion
These data are consistent with many reports already in the literature. First, nausea and vomiting early on (i.e., cycle 1) is associated with CINV in later cycles [8] and there is a very high correlation between nausea and vomiting in the acute and delayed phases [9]. Our data support the idea that controlling CINV on the day of chemotherapy may go a long way in averting CINV on the later days of the cycle (days 2–6). According to this study, characteristics of patients at risk for CINV on day 1 are those who are younger, receiving moderate emetogenic chemotherapy in addition to highly emetogenic chemotherapy, and those receiving treatment for breast cancer. Second, these data confirm that a history of motion sickness is also a significant predictor of nausea and vomiting, as is cisplatin-based therapy [10, 11]. It is interesting that, though age was not predictive in the multivariate analysis, it was predictive for both nausea and vomiting on day 1 and was the only significant variable for emesis on day 1.
It was curious to us that participants rated their satisfaction highly, though by standard definitions of response, less than half had a complete response to their prophylactic medication. Indeed, the prevalence of nausea >2.5 and emesis was 35 % in this study during the delayed phase, yet almost half of the population took rescue medication. Despite the fact that our models demonstrated that both emesis and nausea >2.5 on days 2–6 were significant predictors of those who took rescue medication, our data also reveal that 38 % of people who took rescue medication had mild or no nausea and no emesis. The multivariate analysis provides data to support that a history of motion sickness might have had a role in this. Perhaps patients used rescue medication more preventatively at low levels of nausea if they have had a history of motion sickness in order to avert any unpleasant experiences.
Overall, 19 % of the patients in this study who were receiving HEC had some vomiting throughout days 2 to 6 and just over a third (35 %) had nausea that was rated over 2.5. Earlier studies describing incidence in patients who did not receive aprepitant are a bit higher, with 32 % of patients reporting delayed emesis and 54 % reporting delayed nausea [7]. Rates of breakthrough vomiting in the delayed phase, in studies where patients received an NK-1 antagonist with HEC, range from 10 to 28% [12] and rates of more than mild nausea range from 26 to 30 % [13, 14]. So, while our study did not have complete response rates that were as good as the early studies with NK1 antagonists, the rates of emesis were equivalent and the prevalence of greater than mild nausea is only slightly higher.
The mean score on the total score of the FLIE indicated that the population in this study, on average, did not have CINV that negatively impacted their lives (mean score 108, median 120). The vomiting subscale score mean was 57, median 63, also indicating a lack of negative impact. The nausea subscale mean was 51, median 57, indicating that some patients did experience a negative impact from nausea. The percentage of patients who experienced a negative impact per the FLIE scores was 33 %. This rate is very similar to other studies reporting 23 % [7], 37 % [15], and 34% [16] of patients receiving either HEC or MEC had FLIE scores under 108, indicating negative impacts on their functioning.
In the present pursuit of precision medicine, it would be efficient to design future studies focusing on nausea and vomiting in the 20–40 % who actually are likely to experience these symptoms despite current standard treatment. By targeting this population that needs better treatment, sample sizes could likely be cut in half, making studies more cost efficient. Eligibility criteria that would apply based on our models would include those of a younger age (under 50), those getting a combination of HEC and MEC, and receiving treatment for breast cancer. According to other studies, being female may also be an important eligibility criterion to include [16]. However, perhaps additional variables should be collected to better characterize this group of patients who do not have optimal results from standard antiemetics. Data related to pharmacogenomics [11, 17] and important psychosocial predictors (i.e., expectations, anxiety, coping) may also be useful [10, 18].
Due to the fact that the pathophysiology of chemotherapy-induced nausea and vomiting is known, drug development continues with new agents targeting important receptors being evaluated regularly. Novel designs to target those patients who are currently not benefiting from standard treatment would be a step toward efficient science and perhaps reduce the burden of clinical trials for hundreds of patients who are already experiencing good control of their symptoms.
References
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100(10):2261–2268. doi:10.1002/cncr.20230
Kris MG, Gralla RJ, Clark RA, Tyson LB, O’Connell JP, Wertheim MS, Kelsen DP (1985) Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol Off J Am Soc Clin Oncol 3(10):1379–1384
Campos D, Pereira JR, Reinhardt RR, Carracedo C, Poli S, Vogel C, Martinez-Cedillo J, Erazo A, Wittreich J, Eriksson LO, Carides AD, Gertz BJ (2001) Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol Off J Am Soc Clin Oncol 19(6):1759–1767
Hesketh PJ, Gralla RJ, Webb RT, Ueno W, DelPrete S, Bachinsky ME, Dirlam NL, Stack CB, Silberman SL (1999) Randomized phase II study of the neurokinin 1 receptor antagonist CJ-11, 974 in the control of cisplatin-induced emesis. J Clin Oncol Off J Am Soc Clin Oncol 17(1):338–343
Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A, Kindler H, Grote TH, Pendergrass K, Grunberg SM, Carides AD, Gertz BJ (1999) Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754, 030 Antiemetic Trials Group. N Engl J Med 340(3):190–195. doi:10.1056/nejm199901213400304
Barton DL, Thanarajasingam G, Sloan JA, Diekmann B, Fuloria J, Kottschade LA, Lyss AP, Jaslowski AJ, Mazurczak MA, Blair SC, Terstriep S, Loprinzi CL (2014) Phase III double-blind, placebo-controlled study of gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance). Cancer 120(22):3575–3583. doi:10.1002/cncr.28892
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients′ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol Off J Am Soc Clin Oncol 24(27):4472–4478. doi:10.1200/jco.2006.05.6382
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 15(5):497–503. doi:10.1007/s00520-006-0173-z
dos Santos LV, Souza FH, Brunetto AT, Sasse AD, da Silveira Nogueira Lima JP (2012) Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: a systematic review. J Natl Cancer Inst 104(17):1280–1292. doi:10.1093/jnci/djs335
Roscoe JA, Morrow GR, Hickok JT, Mustian KM, Shelke AR (2004) Biobehavioral factors in chemotherapy-induced nausea and vomiting. J Natl Compr Cancer Netw JNCCN 2(5):501–508
Schwartzberg L (2014) Addressing the value of novel therapies in chemotherapy-induced nausea and vomiting. Expert Rev Pharmacoecon Outcomes Res 14(6):825–834. doi:10.1586/14737167.2014.957683
Aapro M, Carides A, Rapoport BL, Schmoll HJ, Zhang L, Warr D (2015) Aprepitant and fosaprepitant: a 10-year review of efficacy and safety. Oncologist 20(4):450–458. doi:10.1634/theoncologist.2014-0229
Warr DG, Grunberg SM, Gralla RJ, Hesketh PJ, Roila F, Wit R, Carides AD, Taylor A, Evans JK, Horgan KJ (2005) The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from 2 randomised, double-blind, placebo controlled trials. European J Cancer (Oxford, England: 1990) 41(9):1278–1285. doi:10.1016/j.ejca.2005.01.024
Grunberg S, Chua D, Maru A, Dinis J, DeVandry S, Boice JA, Hardwick JS, Beckford E, Taylor A, Carides A, Roila F, Herrstedt J (2011) Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol–EASE. J Clin Oncol Off J Am Soc Clin Oncol 29(11):1495–1501. doi:10.1200/jco.2010.31.7859
Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J (2011) Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 19(6):843–851. doi:10.1007/s00520-010-0915-9
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK (2012) Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 20(1):107–117. doi:10.1007/s00520-010-1073-9
Trammel M, Roederer M, Patel J, McLeod H (2013) Does pharmacogenomics account for variability in control of acute chemotherapy-induced nausea and vomiting with 5-hydroxytryptamine type 3 receptor antagonists? Curr Oncol Rep 15(3):276–285. doi:10.1007/s11912-013-0312-x
Bouganim N, Dranitsaris G, Hopkins S, Vandermeer L, Godbout L, Dent S, Wheatley-Price P, Milano C, Clemons M (2012) Prospective validation of risk prediction indexes for acute and delayed chemotherapy-induced nausea and vomiting. Curr Oncol(Toronto, Ont) 19(6):e414–e421. doi:10.3747/co.19.1074
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Support
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health to the Alliance NCORP Research Base (Jan C. Buckner, M.D., UG1CA189823) and the legacy North Central Cancer Treatment Group Community Clinical Oncology Program (CA37404). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kottschade, L., Novotny, P., Lyss, A. et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3 (Alliance). Support Care Cancer 24, 2661–2667 (2016). https://doi.org/10.1007/s00520-016-3080-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3080-y