Abstract
Purpose
Anemia affects most patients treated for cancer by chemotherapy. It is a known major contributor to fatigue and loss of quality of life and is likely to have a negative effect on prognosis and mortality from cancer. The main purpose of this study was to characterize the management of anemia and iron deficiency in a French oncology day-care center.
Methods
A retrospective study was conducted between May and November 2012 in the oncology day unit of the Jean Godinot Cancer Center (France). The 133 patients included were all over the age of 18 and being treated by chemotherapy and had mild, moderate, or severe anemia.
Results
Over half (58 %) the patients were shown to be receiving no specific treatment for anemia. Iron balance was assessed in 71 patients and iron deficiency diagnosed in 37. Stepwise logistic regression showed that patients with severe to moderate anemia were nearly four times more likely to have an iron balance assessment than those with mild anemia (OR, 3.78; 95 % CI, 1.84–7.76; P = 0.0003). Classical logistic regression shows that older patients (≥70) are three times less likely to have an iron balance assessment than patients <70 years (OR, 0.32; 95 % CI, 0.12–0.86; P = 0.06).
Conclusion
An ideal medical setting for the management of anemia and iron deficiency, and the associated quality-of-life concerns, has yet to be defined for patients with cancer. Screening and treatment of mild to moderate anemia are inadequate, despite the advent of erythropoiesis-stimulating agents. Large scale, multicenter studies are required to define a clear medical framework for the management of anemia and iron deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quality-of-life issues and fatigue associated with cancer and cancer treatments have become the most frequently reported concerns for these patients [1, 2]. Anemia is recognized as a major contributor to fatigue [3], and its incidence in cancer patients has been studied by several authors [4–7]. More recently, chemotherapy-induced iron deficiency has been estimated to affect 32 to 63 % of patients with different tumor types; most of these patients were also anemic [8]. Anemia has a multifactorial etiology [9] and can strongly affect cancer outcomes and survival [10–12], not only in cases of severe anemia but also in mild anemia situations [6, 13].
In 2001, the European Cancer Anemia Study (ECAS) showed that over 60 % of anemic patients did not have any specific treatment for their anemia [14]. The advent of erythropoiesis-stimulating agents (ESA) has improved treatment outcomes and quality of life for cancer patients. The most recent guidelines have evolved towards recommending treating anemia [15–17], with the objectives of improving quality of life in patients with cancer and limiting the use of blood transfusion to the most critical cases.
At the same time, the new FACT-An and FACT-F scales have been shown to be useful measures of patient quality of life [18] and a number of risk factors for chemotherapy-induced anemia have been identified [19, 20]. These developments are paving the way for better management and potential prevention of anemia and iron deficiency in cancer patients.
Nevertheless, the most recent large-scale studies still maintain that anemia and iron deficiency are underdiagnosed [21, 22] and often inadequately treated [14, 23–25].
The aim of the present analysis was to describe the management of anemia and iron deficiency in chemotherapy-treated patients in a French day-care center.
Materials and methods
The present study is a single-center, retrospective analysis of 133 patients with anemia, treated for different types of cancer in the day hospital of the Jean-Godinot Institute (Reims, France). Between May and November 2012, data were collected from 625 consecutive patients regardless of the type of cancer. The patients included were all over the age of 18. Both the purpose and the methodology of the study were explained to each patient before their data were considered for inclusion in the present analysis.
Tumor localization and metastatic status, date of diagnosis, type of chemotherapy, and number of lines of treatment were documented for all patients, as were gender and anthropometric measurements. None of the patients included was treated by radiotherapy. Data on anemia management included hemoglobin (Hb) level, iron status parameters (serum iron and ferritin levels, transferrin saturation [TSAT] [26]), and current treatments (oral or intravenous iron substitution, ESA, blood transfusion). The present analysis is based on the latest values of the clinical parameters registered.
Anemia was rated as severe, moderate, or mild according to the Hb cutoff levels, Hb < 8 g/dL, 8 ≤ Hb ≤ 9.9 g/dL, and 10 ≤ Hb ≤ 11.9 g/dL, respectively [16, 26]. Iron balance assessment was based on at least one of the three iron status parameters. In this study, iron deficiency was defined at the threshold of TSAT < 20 % in line with guidelines prevailing at that time [16, 26, 27]. This criterion has previously been adopted in a number of studies on the management of anemia and iron deficiency [8, 10, 28]. TSAT has also been shown to be a more reliable parameter than serum ferritin levels in an inflammatory context [27, 28]. The threshold level of serum ferritin at 100 ng/mL indicates the likelihood of absolute iron deficiency (<100 ng/mL) or functional iron deficiency (≥100 ng/mL) [8].
Logistic regression was used to identify factors associated with the likelihood of undergoing iron balance assessment. It focused on a number of parameters including age, gender, body mass index (BMI), type of disease, grade of anemia, line of treatment, and metastatic stage. Stepwise regression was used to identify the most relevant model (using the AIC criterion). All analyses were performed using the R software, version 12.5.1 (stats and MASS packages) [29, 30].
Results
The study population comprised 133 patients diagnosed with anemia according to guideline hemoglobin cutoff levels [16, 26]. Median age was 59 years (range 28–91 years); 32 patients were <70 years; there were 30 men. Breast cancer (50 %) and gynecologic cancers (16 %) were the most common diseases of the study population (Fig. 1). More than half the patients (52 %) were at metastatic stage.
Median Hb level was 9.9 g/dL (range 6.5–12.9 g/dL). Patients were stratified according to Hb levels: 65 patients had mild anemia (Hb = 10–11.9 g/dL) and 68 had moderate to severe anemia (Hb = 5.9–9.9 g/dL) (Fig. 2).
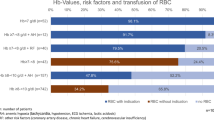
Over half the anemic patients (77/133, 58 %) in our study population were shown to be receiving no treatment for anemia. The median Hb level in this group of patients was 10.4 g/dL, ranging from 8.1 to 12 g/dL. The less severely affected patients were more likely to remain without medical treatment for their anemia, i.e., 50 of the untreated patients had mild anemia vs. 27 who had severe to moderate anemia (Fig. 3).
Iron balance was assessed in 71 anemic patients (53 %). Full assessment, i.e., serum iron and ferritin levels and TSAT, was done in 62 patients and for the nine other patients; the assessment was partial. TSAT data were not available for three patients. Iron balance was more likely to be assessed in the most severely affected patients (45/68) than in those with mild anemia (26/65) (Fig. 3).
These observations were confirmed by the results of a stepwise logistic regression showing that the severity of anemia was the only significant predictor of iron balance assessment being performed. Patients with moderate to severe anemia were nearly four times more likely to have an iron balance assessment than those with mild anemia (OR, 3.78; 95 % CI, 1.84–7.76; P = 0.0003).
Classic logistic regression shows that gynecologic cancer patients were nearly four times more likely to have an iron balance assessment than breast cancer patients (OR, 4.37; 95 % CI, 1.19–15.96; P = 0.03). Likewise, lung cancer patients were also more likely to undergo iron balance assessment, although this difference is not statistically significant (OR, 7.07; 90 % CI, 1.20–41.63; P = 0.07).
The rate of iron balance assessment was also affected by age, though not significantly: older patients (≥70 years) were three times less likely to have iron balance assessment than patients <70 years (OR, 0.32; 95 % CI, 0.12–0.86; P = 0.06).
Other factors such as gender, BMI, and treatment line combined with metastatic stage were not found to be significant predictors of the prescription of iron balance assessment in stepwise classic logistic regression.
Following iron balance assessment, TSAT values below the threshold of 20 % were reported for 37 patients (Fig. 3). The majority (N = 33) underwent a full iron balance assessment, including serum ferritin levels (sFerritin); five patients had sFerritin < 100 ng/mL and 28 had sFerritin ≥ 100 ng/mL. Serum ferritin data was missing for four patients. Iron deficiency was diagnosed in a little over half the patients in the lower Hb level group (24/45) and in half (13/26) those in the higher Hb level group.
Treated patients (56/133, 42 %) received ESA, transfusion, or iron either alone or in any combination of the three (Table 1). For all anemic patients, management with iron supplementation was related to severity of anemia (Fig. 3) and was thus more frequently prescribed to patients with severe to moderate anemia (11/24) than to those with mild anemia (3/13).
The proportion of patients on supportive care and with iron status parameters indicating iron deficiency are presented in Table 1; these patients were treated with at least ESA or transfusion or iron supplementation. A combination of ESA and iron supplementation was administered to six patients who had both TSAT < 20 % and sFerritin ≥ 100 ng/mL.
Discussion
The present analysis of 133 anemic patients shows that in the context of a cancer day-care center, less than half the patients (42 %) were receiving supportive care for anemia. Indeed, little over half (53 %) had undergone any iron balance assessment. The severity of anemia was the only significant predictive factor for the prescription of iron balance assessment, as revealed by stepwise logistic regression.
Our data are consistent with those collected in 2001 in the ECAS study [14] and its French arm published by Schneider et al. [23], underscoring the fact that 60 % of anemic patients were not treated for anemia. The present data are however very different from those collected in France in 2006 and published by Guardiola et al. [24]. Their analysis reported that 17 % of anemic patients were not treated for anemia, while a German analysis from a Web-based prospective survey conducted in 2009–2010 [25] showed that two thirds of patients with Hb ≤12 g/dL were not treated for anemia.
With regard to iron balance assessment, Spielmann et al. found that iron status parameters (TSAT and/or serum ferritin) were evaluated in only one out of five patients (21 %) in a French survey conducted in 2009–2010 [21], leaving four out of five anemic patients not undergoing any iron balance assessment. Ludwig et al. estimated that at least one third of European patients had not undergone any iron status assessment at diagnosis of anemia [22]. Our own observations of the extent of iron status assessment come approximately midway between the findings of these earlier publications.
A number of reasons may explain the differences observed among published data. Firstly, assessments of anemia and iron status vary substantially from one country to another [22]. Secondly, medical practice has wavered over the past decade due to several changes in guidelines [15, 16, 31–33]. Yet another reason may be related to the structure of the population studied in terms of cancer types (solid tumors vs. hematological malignancies), treatment regimens (chemotherapy vs. radiotherapy), or stage and progression of the cancer. However, with regard to findings that anemia is insufficiently treated and iron status insufficiently assessed, our observations clearly tie in with the conclusions of the works cited.
The present analysis shows that elderly patients (≥70 years) are less likely to be referred for iron balance assessment than younger patients (<70 years), even though the difference lacks statistical significance. This was an unexpected finding. It is however known that patients may experience symptoms of anemia at different Hb levels [4, 5, 34] and elderly cancer patients in particular often manifest anemia at higher Hb levels than do anemic patients without cancer [35–37]. This shows the limits of instigating anemia treatment based on a single Hb measurement and also the importance of systematically screening for the symptoms of anemia.
The statistical analysis also showed that patients treated for gynecologic cancer and, less meaningfully, for lung cancer were more likely to undergo iron balance assessment than breast cancer patients. Although not statistically significant, this trend is consistent with the literature reports of a higher prevalence of anemia in lung, gynecologic, and breast cancers [4, 7, 14, 24] and the most severe cases of anemia, i.e., those requiring transfusion, encountered in gynecologic and lung cancers [4]. It is worth recalling that colorectal pathologies are included in the 3 % “other” category of cancer localization and are thus poorly represented in the present study population.
Most of the patients presenting an iron deficiency (TSAT < 20 %) also had normal to elevated sFerritin levels (≥100 ng/mL), which is the combination of iron status parameters that characterizes functional iron deficiency. Data from several controlled clinical trials have shown that intravenous iron supplementation enhances the response to ESA treatment and also reduces the number of ESA doses required in cancer patients [38–42]. In routine clinical practice, serum ferritin levels <100 ng/mL are very likely indicative of insufficient iron stores for ESA therapy to be effective in patients with cancer [8].
Our study presents a number of limitations. First, it was conducted in a single cancer center on a small cohort. In addition, the sample population was not well balanced in terms of tumor localization: half the patients were being treated for breast cancer and only a small number for colorectal cancer, whereas the latter patients are particularly affected by anemia issues. And lastly, this study describes the situation of the patients at a given time-point. It was not designed to include any information regarding follow-up and, in particular, any further examinations that may be required to identify the origins of anemia and iron deficiency.
Conclusion
The present analysis highlights the fact that an ideal medical setting for the management of anemia and iron deficiency, and the associated quality-of-life concerns, has yet to be defined for patients with cancer. Treatment patterns could be improved by systematic iron status assessment in all cancer patients, both before and during chemotherapy cycles. Our results, based on a small cohort from a single cancer center, suggest that current screening and treatment, particularly for mild to moderate forms of anemia, are inadequate, despite the introduction of treatment with erythropoiesis-stimulating agents. Large-scale, multicenter studies focusing on daily practice are required to clearly define a clear medical structure for the management of anemia and iron deficiency according to its specific origin.
References
Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N (2000) Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum Ann Oncol 11(8):971–975
Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D (2002) Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 95(1):155–163
Sabbatini P (2000) Contribution of anemia to fatigue in the cancer patient. Oncology (Williston Park) 14(11a):69–71
Groopman JE, Itri LM (1999) Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 91(19):1616–1634
Demetri GD (2001) Anaemia and its functional consequences in cancer patients: current challenges in management and prospects for improving therapy. Br J Cancer 84(Suppl):131–137
Gordon MS (2002) Managing anemia in the cancer patient: old problems, future solutions. Oncologist 7(4):331–341
Barrett-Lee PJ, Ludwig H, Birgegard G, Bokemeyer C, Gascon P, Kosmidis PA, et al. (2006) Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology 70(1):34–48
Aapro M, Osterborg A, Gascon P, Ludwig H, Beguin Y (2012) Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol 23(8):1954–1962
Bron D, Meuleman N, Mascaux C (2001) Biological basis of anemia. Semin Oncol 28(2 Suppl 8):1–6
Ludwig H, Muldur E, Endler G, Hubl W (2013) Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol 24(7):1886–1892
Caro JJ, Salas M, Ward A, Goss G (2001) Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 91(12):2214–2221
Harper P, Littlewood T (2005) Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology 69(Suppl):22–27
Sabbatini P (2000) The relationship between anemia and quality of life in cancer patients. Oncologist 5(Suppl):219–223
Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. (2004) The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40(15):2293–2306
Aapro MS, Link H (2008) September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist 13(Suppl):333–336
Schrijvers D, De Samblanx H, Roila F, ESMO Guidelines Working Group (2010) Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol 21(Suppl 5):v244–v27.
Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, et al. (2007) EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 43(2):258–270
Cella D (1997) The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol 34(3 Suppl 2):13–19
Coiffier B, Guastalla JP, Pujade-Lauraine E, Bastit P, Anemia Study Group (2001) Predicting cancer-associated anaemia in patients receiving non-platinum chemotherapy: results of a retrospective survey. Eur J Cancer 37(13):1617–1623
Ray-Coquard I, Le Cesne A, Rubio MT, Mermet J, Maugard C, Ravaud A, et al. (1999) Risk model for severe anemia requiring red blood cell transfusion after cytotoxic conventional chemotherapy regimens. The Elypse 1 Study Group. J Clin Oncol 17(9):2840–2846
Spielmann M, Luporsi E, Ray-Coquard I, de Botton S, Azria D, Lasocki S, et al. (2012) Diagnosis and management of anaemia and iron deficiency in patients with haematological malignancies or solid tumours in France in 2009–2010: the AnemOnHe study. Eur J Cancer 48(1):101–107
Ludwig H, Aapro M, Bokemeyer C, Glaspy J, Hedenus M, Littlewood TJ, et al. (2014) A European patient record study on diagnosis and treatment of chemotherapy-induced anaemia. Support Care Cancer 22(8):2197–2206
Schneider M (2005) Fréquence de l’anémie chez les patients français atteints de tumeurs solides ou d’hémopathies malignes: résultats de l’«European Cancer Anaemia Survey (ECAS)». Oncologie 7(5):397–402
Guardiola E, Morschhauser F, Zambrowski J-J, Antoine E-C (2007) Prise en charge de l’anémie chez les patients présentant une pathologie maligne : résultats de l’étude. F-ACT (Fr Anaemia Cancer Treat) Bull Cancer 94(10):907–914
Link H, Schmitz S (2013) Treatment of cancer-associated anaemia: results from a two-day cross-sectional survey in Germany. Onkologie 36(5):266–272
Association Francophone pour les Soins Oncologique de Support (AFSOS) (2012) Anémie et cancer. Available from: http://ftp.comm-sante.com/SB/anemieetcancer.pdf
Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA (2011) Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 58(12):1241–1251
Cook JD (2005) Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol 18(2):319–332
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, Fourth Edition
R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012.
Lichtin AE (2011) Clinical practice guidelines for the use of erythroid-stimulating agents: ASCO, EORTC. NCCN Cancer Treat Res 157239-48
Gafter-Gvili A, Steensma DP, Auerbach M (2014) Should the ASCO/ASH guidelines for the use of intravenous iron in cancer- and chemotherapy-induced anemia be updated? J Natl Compr Cancer Netw 12(5):657–664
Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, et al. (2010) American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 116(20):4045–4059
Ludwig H, Fritz E (1998) Anemia of cancer patients: patient selection and patient stratification for epoetin treatment. Semin Oncol 25(3 Suppl 7):35–38
Busti F, Campostrini N, Martinelli N, Girelli D (2014) Iron deficiency in the elderly population, revisited in the hepcidin era. Front Pharmacol 583
Eisenstaedt R, Penninx BW, Woodman RC (2006) Anemia in the elderly: current understanding and emerging concepts. Blood Rev 20(4):213–226
Chaves PH, Ashar B, Guralnik JM, Fried LP (2002) Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc 50(7):1257–1264
Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. (2004) Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 22(7):1301–1307
Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS, et al. (2008) Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 26(10):1611–1618
Hedenus M, Birgegard G, Nasman P, Ahlberg L, Karlsson T, Lauri B, et al. (2007) Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 21(4):627–632
Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR (2007) Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 12(2):231–242
Pedrazzoli P, Farris A, Del Prete S, Del Gaizo F, Ferrari D, Bianchessi C, et al. (2008) Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha. J Clin Oncol 26(10):1619–1625
Acknowledgments
Medical writing assistance was provided by Potentiel d’Action (France) and funded by Pierre Fabre Oncology.
Conflicts of interest
Florence Laï-Tiong, Cloé Brami and Olivier Dubroeucq have no conflicts of interest to declare.
Hervé Curé has received accommodation fees from the Pierre Fabre Foundation in 2013 and 2014.
Nicolas Jovenin has received honoraria from Pierre Fabre Oncologie, msd, Sanofi and Amgen. He has received consultancy fees from Eisai and Hospira.
Florian Scotté has received honoraria from Pierre Fabre Oncologie, Roche, Janssen, Sandoz, Hospira, Teva, and Vifor. He has received consultancy fees from AMGEN for his role as an advisory board member and for symposia presentations.
The authors declare that they had full control of all primary data and that they agree to the journal reviewing their data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laï-Tiong, F., Brami, C., Dubroeucq, O. et al. Management of anemia and iron deficiency in a cancer center in France. Support Care Cancer 24, 1091–1096 (2016). https://doi.org/10.1007/s00520-015-2877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2877-4