Abstract
Background
Palliative chemotherapy in patients with nonresectable advanced colorectal carcinoma is performed to prolong survival, alleviate tumor-associated symptoms, and maintain or improve health-related quality of life (HRQOL). In this prospective single-center observational study, we assessed HRQOL across the various lines of palliative chemotherapy.
Methods
HRQOL data were acquired using the EORTC Quality of Life Questionnaire-C30 (QLQ-C30) questionnaire. The first assessment was performed at the beginning of each chemotherapy line, the second after three cycles, and the third at the end of chemotherapy. Further assessments were conducted during checkups every 3 months in our outpatient unit.
Results
In total, 100 consecutive patients with colorectal carcinoma (mean age 66.4 years; 60 % men) treated with palliative chemotherapy were recruited. Generally, QOL deteriorated constantly across time. Physical functioning, fatigue, pain, dyspnea, and appetite worsened steadily from first-line chemotherapy to the later treatment phase. Global QOL, emotional functioning, and role functioning improved slightly after the end of first-line chemotherapy, deteriorated during second-line chemotherapy to the level of first-line chemotherapy, and further deteriorated in the later treatment phases. In additional analyses, we found the largest differences between patients with and without a treatment response for pain (19.0 vs. 37.2 points) and appetite loss (17.4 vs. 32.7 points).
Conclusion
The individual QOL domains deteriorated constantly across time. Our data indicate that patients undergoing first- and second-line palliative chemotherapy experience stabilization of global QOL and psychosocial symptoms. We also found that unselected patients who achieved a treatment response had a lower symptom burden and better QOL than did patients with progressive disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer (CRC) is the third most common cause of cancer death in both men and women in Europe and the USA. The aims of chemotherapy in patients with nonresectable advanced CRC are to prolong survival, control symptoms, and maintain or improve quality of life (QOL) [1].
The median overall survival for patients with unresectable advanced CRC who receive best supportive care alone is approximately 5 to 6 months. Systemic chemotherapy produces meaningful improvements in median survival and progression-free survival [2].
For many decades, 5-fluorouracil was the main active agent in the treatment of CRC, providing a median survival of about 1 year [3]. This has changed markedly since the year 2000. With the approval of irinotecan and oxaliplatin in combination with 5-fluorouracil, the median overall survival has increased to 19 months [4, 5]. The number of therapeutic options for CRC further increased with the addition of several humanized monoclonal antibodies to vascular endothelial growth factor and epidermal growth factor, and the median survival for patients with metastatic disease is now approximately 24 months [6–8].
In addition to delaying disease progression, maintenance of health-related QOL (HRQOL) is a particularly important aim of treatment in patients with metastatic disease [1]. HRQOL can be formally defined as “the extent to which one’s usual or expected physical, emotional, and social well-being are affected by a medical condition and its treatment” [9]. This definition incorporates the two widely accepted aspects of QOL: subjectivity and multidimensionality [10].
In previous randomized trials, HRQOL measurement was performed at baseline and at determined intervals during administration of a specific palliative chemotherapy line [11, 12]. Knowledge of the longitudinal course of QOL across different chemotherapy lines is almost completely lacking. Thus, the objective of our study was to analyze and compare patient-reported HRQOL measured by repeated computer-assisted completion of validated questionnaires in patients with nonresectable advanced CRC while they underwent treatment with several palliative chemotherapy lines.
Patients and methods
Sample
Patients were consecutively included in the study upon starting a palliative chemotherapy line according to standard guidelines [1, 13]. The inclusion criteria were a diagnosis of metastatic CRC, starting palliative chemotherapy, no overt cognitive impairment, age of >18 years, and written informed consent. The first assessment was performed at the beginning of chemotherapy, the second after three cycles, and the third at the end of chemotherapy (after six cycles). Response was assessed using interval radiographic evaluation (every 3 months). Radiographic tumor response was quantified using Response Evaluation Criteria in Solid Tumors (RECIST) [14]. Further interviews were conducted during checkups every 3 months in our clinic and when another chemotherapy line was started for treatment of progressive disease. QOL assessments continued until the patient died or was unable to complete the questionnaire or at a maximum of 3 years after inclusion in the study.
QOL data collection
QOL was assessed with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (QLQ-C30), an internationally validated and widely used cancer-specific QOL questionnaire [15]. This questionnaire is the most frequently used instrument for assessment of HRQOL in patients with CRC. It comprises five functioning scales (physical, role, cognitive, emotional, and social functioning), three symptom scales (fatigue, pain, and nausea and vomiting), a global QOL scale, and six single items that assess additional problems commonly reported by patients with cancer (dyspnea, appetite loss, sleep disturbance, constipation, diarrhea, and financial difficulties). We administered only the QLQ-C30 and not the additional CRC module, the QLQ-CR29, to limit patient burden introduced by questionnaire length and repeated assessments.
Differences in QOL scores of >20, 10 to 20, and 5 to 10 points were considered large, moderate, and small, respectively [16]. To facilitate both data collection and analysis, QOL data capture was performed electronically using tablet PCs running Computer-based Health Evaluation System software [17]. A study nurse gave these tablet PCs to patients and asked them to complete the QLQ-C30. The study nurse provided further information and assistance to patients with questions or concerns. Assessments took place in the patients’ rooms during their inpatient or day clinic stay for computed tomography evaluation.
Clinical and sociodemographic data were collected from the hospital records and entered in the Computer-based Health Evaluation System database to match patient-reported outcome data. This study was approved by the ethics committee of the state medical board of Upper Austria.
Statistical analysis
Analysis of differences in QOL among different chemotherapy lines was performed with linear mixed models. The models comprised the chemotherapy line as the fixed effect and the QOL scores as dependent variables. All chemotherapy lines beyond the second line were collapsed to a category labeled “third+ line.” The variables were divided into separate categories for the period of chemotherapy administration and the period during which patients received no active anticancer treatment (intervals between chemotherapy administrations). Additionally, the model employed a first-order autoregressive covariance structure and a random intercept at the patient level. We conducted an additional analysis of the association between QOL and response to treatment. Because the chemotherapy line was strongly associated with treatment response, we did not include both variables in the same model; instead, we performed another analysis using the same model described above, but with treatment response instead of chemotherapy as the fixed effect.
Results
Patient characteristics
In total, 100 consecutive patients with nonresectable advanced CRC were recruited at the Department of Internal Medicine, Hospital Wels-Grieskirchen, Austria from February 2007 to September 2011 and assessed for a maximum of 3 years. Sixty percent of the patients were men, and the mean patient age was 66.4 years (standard deviation 10.6). At the time of recruitment, 73 patients were starting first-line palliative chemotherapy and 27 patients were at the start of the second-line chemotherapy. Further details are provided in Table 1.
Twenty-five percent of the patients died during the first year after study inclusion, 29 % died during the second year, and 26 % died during the third year. The median survival time after study inclusion was 21.8 months (95 % confidence interval 15.6–28.0).
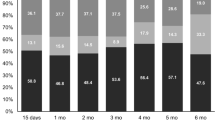
The questionnaire completion rates ranged from 65 to 100 % for assessment time points during the first year after study inclusion. For time points during the second year, the completion rate was between 61 and 70 %, and during the third year, between 45 and 71 %. These percentages refer to the total number of patients alive in that period.
Comparison of QOL across chemotherapy lines
The main focus of our analysis was determination of the trajectories of the individual QOL domains covered by the QLQ-C30 across the various lines of palliative chemotherapy. In general, QOL deteriorated constantly across time. Constipation, financial impact, and taste alterations were the only domains that did not significantly change over time.
Physical functioning, fatigue, pain, dyspnea, and appetite loss worsened more or less steadily from first-line chemotherapy to the later treatment phases; however, physical functioning was similar in the off-treatment period following first-line chemotherapy (70.5 points) and second-line chemotherapy (71.1 points). Additionally, fatigue and pain had nearly the same levels during and after first-line chemotherapy.
Global QOL, emotional functioning, and role functioning improved by about 5 points after the end of first-line chemotherapy, deteriorated during second-line chemotherapy to the level of first-line chemotherapy, and further deteriorated in the later treatment phases. Role functioning deteriorated from 56.7 to 47.8 points immediately after second-line chemotherapy, whereas global QOL and emotional functioning did not decrease until third-line chemotherapy.
Social functioning was similar during and after first-line chemotherapy at 74.8 and 75.1 points, respectively; it stabilized at 64.9 to 68.6 points between second-line chemotherapy and later chemotherapy lines, and showed the most severe impairment at 57.9 points during the off-treatment phases of the third+ chemotherapy lines.
Sleep disturbances remained rather stable at 26.9 to 30.0 points during and after the first two chemotherapy lines and worsened to 35.4 to 44.2 points during and after the third+ chemotherapy lines.
Diarrhea was generally worse during treatment and less severe in the periods between two chemotherapy lines. The average diarrhea score was 29.2 points during third+ chemotherapy; this substantially exceeded the scores during first-line (21.7 points) and second-line (18.8 points) chemotherapy. Further details are shown in Tables 2 and 3 and Figs. 1 and 2.
In a further analysis, we investigated the impact of response to treatment according to RECIST (progressive disease vs. [partial] remission or stable disease) on the various QOL domains. We found that response (partial remission or stable disease) to treatment became significantly less frequent as the number of chemotherapy lines increased (p < 0.001). During first-line chemotherapy, only 18.7 % of the staging examinations indicated progressive disease, whereas this proportion substantially increased during second-line (44.4 %) and third+ chemotherapy lines (62.5 %).
As expected, all differences were in favor of a response to treatment. The largest differences between patients with and without a treatment response were found for pain (19.0 vs. 37.2 points; difference 18.2 points) and appetite loss (17.4 vs. 32.7 points; difference 15.3 points). The other statistically significant differences were <10 points: global QOL 9.2 points, fatigue 8.1 points, social functioning 7.9 points, physical functioning 7.7 points, dyspnea 6.4 points, role functioning 6.2 points, sleep disturbances 5.8 points, emotional functioning 5.4 points, nausea/vomiting 5.0 points, and cognitive functioning 3.7 points. Constipation, diarrhea, financial impact, and taste alterations were not found to be significantly associated with treatment response. None of the QOL scales exhibited a statistically significant interaction between treatment response and chemotherapy, which would have indicated a different association between treatment response and QOL depending on the chemotherapy line. Further details are given in Table 4.
Conclusion
This is the first study to evaluate QOL across chemotherapy lines in unselected patients with nonresectable advanced CRC in clinical practice. Our data indicate that patients undergoing first- and second-line palliative chemotherapy experience stabilization of the global QOL and psychosocial symptoms. Patients undergoing first- and second-line palliative chemotherapy, but not those undergoing third+ chemotherapy, showed stable QOL trajectories. The latter patients reported a substantially higher symptom burden. Similar results have been shown in a few other studies of patients with lung, pancreas, and biliary tract cancers [18–20]. Traditionally, objective end points such as response and survival rates have been used to evaluate the efficacy of chemotherapy in patients with advanced CRC. In recent years, increasingly more trials have incorporated HRQOL as a key end point. The American Society of Clinical Oncology claims that patient outcomes (toxicity, survival, and HRQOL) are more important than cancer outcomes (response rate and duration) [21].
We also investigated the association between our QOL data and the chemotherapy response, which was defined as disease stabilization or better on computed tomography scans every 3 months after starting chemotherapy. An improvement in nearly all QOL scales was shown in patients who achieved disease stabilization. The largest differences between patients with and without a treatment response were found for pain and appetite loss. Our data indicate that unselected patients who undergo treatment with several lines of chemotherapy and achieve a response to treatment benefit not only with regard to survival, as suggested by previous studies [22, 23], but also with regard to QOL.
Whereas the decrease in the number of patients across treatment lines reflected the survival rate in each patient group, we also found a decrease in the proportion of patients who survived and completed the questionnaires. This should to be noted as a limitation of our study affecting in particular our analysis of patients receiving three or more chemotherapy lines.
HRQOL is an important factor to consider when treating patients with cancer, especially those with metastatic disease. At the metastasis stage, it may be wiser to allocate resources to improving patients’ HRQOL rather than investing in expensive and burdensome oncological treatments [24].
One of the major problems associated with assessing QOL during chemotherapy is the timing and frequency of the assessment. The optimal frequency of assessment remains unclear; however, when too much time elapses between two surveys, we cannot detect rapid and clinically important changes in QOL. Approximately 70 % of patients in clinical trials typically complete the baseline QOL measurements, but compliance with follow-up assessments is lower and missing data is a problem [25]. Compliance with QOL assessment in clinical trials that involve regular support from research staff may differ from that in clinical trials that involve routine QOL data collection in practice (e.g., in a busy oncology clinic). Collection of QOL data should become robust, inexpensive, easy, and readily interpretable. The present study showed that electronic questionnaire administration is a feasible way to collect QOL data in daily clinical practice.
Based on our findings, we recommend to assess QOL across the whole treatment trajectories and not just focus on single chemotherapy lines. Routine evaluation of HRQOL during administration of all chemotherapy lines would be optimal to obtain data for comparison of treatment options.
References
Van Cutsem E, Nordlinger B, Cervantes A, Group EGW (2010) Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 21(Suppl 5):v93–v97
Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D (1993) Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 306(6880):752–755
de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, et al. (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15(2):808–815
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355(9209):1041–1047
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22(1):23–30
Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25(12):1539–1544
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29(15):2011–2019
Cella DF (1995) Measuring quality of life in palliative care. Semin Oncol 22(2 Suppl 3):73–81
Aaronson NK (1988) Quality of life: what is it? How should it be measured? Oncology 2(5):69–76 64
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Bennett L, Zhao Z, Barber B, Zhou X, Peeters M, Zhang J, et al. (2011) Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br J Cancer 105(10):1495–1502
ESMO Guidelines Working Group, Van Cutsem EJ (2007) Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 18(Suppl 2):ii25–ii26
Gehring K, Aaronson NK, Gundy CM, Taphoorn MJ, Sitskoorn MM (2011) Predictors of neuropsychological improvement following cognitive rehabilitation in patients with gliomas. J Int Neuropsychol Soc 17(2):256–266
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1):139–144
Holzner B, Giesinger JM, Pinggera J, Zugal S, Schopf F, Oberguggenberger AS, et al. (2012) The computer-based health evaluation system (CHES): a software for electronic patient-reported outcome monitoring. BMC Med Inform Decis Mak 12(1):126
Koeberle D, Saletti P, Borner M, Gerber D, Dietrich D, Caspar CB, et al. (2008) Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol 26(22):3702–3708
Wintner LM, Giesinger JM, Zabernigg A, Sztankay M, Meraner V, Pall G, et al. (2013) Quality of life during chemotherapy in lung cancer patients: results across different treatment lines. Br J Cancer 109(9):2301–2308
Zabernigg A, Giesinger JM, Pall G, Gamper EM, Gattringer K, Wintner LM, et al. (2012) Quality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tract. BMC Cancer 12:390
Outcomes of cancer treatment for technology assessment and cancer treatment guidelines (1996) American Society of Clinical Oncology. J Clin Oncol 14(2):671–679
Glimelius B, Hoffman K, Graf W, Pahlman L, Sjoden PO (1994) Quality of life during chemotherapy in patients with symptomatic advanced colorectal cancer. The Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Cancer 73(3):556–562
Au HJ, Karapetis CS, O'Callaghan CJ, Tu D, Moore MJ, Zalcberg JR, et al. (2009) Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 trial. J Clin Oncol 27(11):1822–1828
Farkkila N, Sintonen H, Saarto T, Jarvinen H, Hanninen J, Taari K, et al. (2013) Health-related quality of life in colorectal cancer. Color Dis Off J Assoc Coloproctol G B Irel 15(5):e215–e222
Ganz PA, Gotay CC (2007) Use of patient-reported outcomes in phase III cancer treatment trials: lessons learned and future directions. J Clin Oncol 25(32):5063–5069
Acknowledgments
We would like to thank Bettina Buchbauer Mag, MSc. and Ina Pühringer Mag, MSc for their contribution to the data collection.
Funding
The study was partly funded by the Forschungsförderungsverein Oberösterreichische Krebshilfe, an independent and non-profit association with the aim of cancer research, cancer prevention, and counseling. The funding source had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mayrbäurl, B., Giesinger, J.M., Burgstaller, S. et al. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Support Care Cancer 24, 667–674 (2016). https://doi.org/10.1007/s00520-015-2828-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2828-0