Abstract
Purpose
Severe chemotherapy-induced gastrointestinal toxicity (CIGT) is common and results in treatment delays, dose reductions, and potential premature treatment discontinuation. Currently, there is no diagnostic marker to predict CIGT. Proinflammatory cytokines, produced via toll-like receptor signaling, are key mediators of this toxicity. Hence, this pilot study investigated the association between immune genetic variability and severe CIGT risk.
Methods
Genomic DNA from 34 patients (10 with severe CIGT) who had received 5-fluoruracil-based chemotherapy regimens was analyzed for variants of IL-1B, IL-2, IL-6, IL-6R, IL-10, TNF-a, TGF-b, TLR2, TLR4, MD2, MYD88, BDNF, CRP, ICE, and OPRM1. Multivariate logistic regression created a prediction model of severe CIGT risk.
Results
There were no significant differences between patients with and without severe CIGT with regards to age, sex, type of cancer, or chemotherapy treatment regimens. The prediction model of severe CIGT risk included TLR2 and TNF-a genetic variability and cancer type (colorectal and gastric). This prediction model was both specific and sensitive, with a receiver operator characteristic area under the curve of 87.3 %.
Conclusions
This is the first report of immune genetic variability, together with cancer type, being predictive of severe CIGT risk. These outcomes are being validated in a larger patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced gastrointestinal toxicity (CIGT), particularly following 5-fluorouracil (5-FU)-based therapies, occurs frequently in cancer patients [1]. Patients with CIGT experience severe consequences such as oral mucositis, increased infection rates, and diarrhea [2, 3]. Consequently, CIGT is a significant burden on patients’ quality of life that causes substantial additional health costs [4] and often results in treatment delays and/or chemotherapy dose reduction. Currently, the risk of CIGT cannot be accurately predicted.
Substantial research has shown that many of the key mediators of CIGT belong to the inflammatory signaling pathway [2]. In particular, activation of the transcription factor nuclear factor kappa B (NF-κB) and up-regulation of its proinflammatory cytokine target genes including tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6 are implicated in modulating injury [5]. These cytokines have been correlated with CIGT severity in clinical studies [6]. A key upstream initiating event leading to this increased proinflammatory cytokine expression is the Toll-Interleukin-1 Receptor (TIR) pathway. This innate immune system pathway includes the pattern recognition toll-like receptors (TLRs) and interleukin-1 (IL-1) family and plays a pivotal role in CIGT [7].
The risk of developing CIGT depends on several variables related to drug treatment and patient characteristics [1], including genetic factors. Genetic polymorphisms in a number of drug targets and genes have been associated with toxicity to 5-FU, including dihydropyrimidine dehydrogenase (DPYD), thymidylate synthase (TYMS), and methylene-tetrahydrofolate reductase (MTHFR) [8]. However, current pharmacogenetic markers lack clinical utility due to poor sensitivity and specificity and provide only a limited role in patient selection. With the emerging role of patient innate immunity in CIGT, the variability of genes encoding mediators of the TLR/IL-1β pathway may be additional important markers of risk as indicated by studies associating TNFA genetic variability with toxicity [9, 10]. This pilot retrospective study aimed to investigate the association between a number of immune genetic loci and severe CIGT following 5-FU-based chemotherapy.
Materials and methods
Study participants
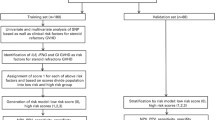
Thirty-four patients who had received 5-FU-based chemotherapy; 5-FU and leucovorin (5-FU/LV), other (5-FU in combination protocols, including FOLFOX 4/6, FEC-D, FEC100, ECF) and capecitabine (in protocols with oxaliplatin and Avastin); from June 2009 to December 2012 at the Flinders Medical Centre, South Australia were recruited. This study was approved by the Southern Adelaide Clinical Human Research Ethics Committee, and all patients provided written informed consent prior to participating in accordance with the Declaration of Helsinki. Mail-out kits were used to collect saliva samples from participants according to manufacturer’s instructions (Oragene DNA collection kit, DNA Genotek Inc., Kanata, Canada). Blinded clinical case note review was used to obtain patient demographic and CIGT data, with patients characterized as having severe CIGT if grade ≥3 toxicity (National Cancer Institutes Common Terminology Criteria for Adverse Events (CTCAE) version 4) was documented on at least one occasion for any individual or combination of the following: nausea, vomiting, diarrhea, and oral mucositis. In addition, if patients had received a dose reduction due to CIGT regardless of symptom grade, they were also classified as having severe CIGT. Patients were excluded if they had received concurrent radiotherapy, had pre-existing conditions associated with mucosal damage (including Crohn’s disease and ulcerative colitis), were unable to give informed consent, were unwilling to participate, were genetically related to other participants in the study, or had a significant (grade 3+) non-gastrointestinal chemotherapy-induced toxicity.
Genetic analysis
Genomic DNA was isolated from a saliva sample. Concentration and purity were assessed by spectrophotometry, and samples were then analyzed for 21 single nucleotide polymorphisms (SNPs) in the following genes using a customized Sequenom MassArray (iPLEX GOLD) assay at the Australian Genome Research Facility (Brisbane, Australia): IL-1B (rs16944, rs1143627, rs1143634), IL-2 (rs2069762), IL-6 (rs10499563), IL-10 (rs1800871, rs1800896), IL-6R (rs8192284/rs2228145), TNFA (rs1800629), TGFB (rs11466314, rs1800469), TLR2 (rs3804100), TLR4 (rs4986790, rs4986791), MD2 (LY96, rs11466004), MYD88 (rs6853), BDNF (rs6265), CRP (rs2794521), ICE (CASP1, rs554344, rs580253), and OPRM1 (rs1799971).
Statistical analysis
Hardy-Weinberg equilibrium was not significant for any SNP (P > 0.05). Chi-square or Fisher’s exact tests were used to examine differences in patient demographics between patients with severe versus without severe CIGT. A general linear model of multivariate logistic regression with step-wise addition of factors (genetic, type of cancer, and patient demographics) was used to build the predictive risk model using a Chi-square test to test for each additional factor. The final model provided individual P values for each of the included factors which, although may not have been <0.05, were important contributors to the overall model. The receiver operator characteristic (ROC) curve percentage area under the curve (AUC) was used to assess the ability of the model to predict severe CIGT. Analysis was conducted using R [11].
Results
Patient demographics, types of cancer, and treatment regimens were not different between patients with severe CIGT (n = 10) compared to those without CIGT (n = 24, Table 1). A range of symptoms contributed to severe CIGT: nausea/vomiting (n = 2), diarrhea (n = 5), and mucositis (n = 3). The multivariate logistic regression created a predictive severe CIGT model that included genetic variability in TLR2 (rs3804100, P = 0.033) and TNFA (rs1800629, P = 0.049) and colorectal (P = 0.028) and gastric (P = 0.069) cancers. With regard to the individual SNPs, patients with either colorectal or gastric cancer who carried at least one variant had a higher risk of severe CIGT compared to patients with wild-type loci (predicted probability >0.95). The inclusion of age, sex, or treatment regimen did not improve the model.
This model was both specific and sensitive for predicting severe CIGT as indicated by the ROC curve AUC of 87 % (Fig. 1a). When only the SNPs were included in the model, specificity and sensitivity were slightly reduced, with the ROC curve AUC of 77 % (Fig. 1b).
Discussion
Our pilot retrospective study is the first to model the combined importance of patient immune genetic variability and type of cancer in determining risk of severe CIGT. This prediction was both specific and sensitive and remained so with inclusion of only the immune genetic variability, as evidenced by comparable ROC curve % AUCs. Hence, we regard this model to be a step towards being able to predict severe CIGT risk in patients with a particular type of cancer.
With regard to the genetic variants included in the model, the impact of both TLR2 (rs3804100, 1350 T > C) and TNF-a (rs1800629, promotor −308 G > A) SNPs on protein expression and function remains unclear. Nonetheless, although there has been no prior investigation of TLR2 SNPs and toxicity to chemotherapy in patients, given that this innate immune receptor recognizes numerous pathogens, including bacteria, to activate immune responses, it is not surprising that it was an important genetic contributor to severe CIGT risk. Indeed, this is supported by prior association of this TLR2 SNP with immune responses to a measles vaccine [12]. In contrast to TLR2, TNFA loci have been previously investigated with regard to toxicity. The TNFA −308 G > A SNP has been related to toxicity severity in patients receiving high-dose chemotherapy prior to stem cell transplantation, while another TNFA promotor SNP (−1031 T > C) was associated with oral mucositis following 5-FU-based therapy [9, 10]. Collectively, these data highlight the importance of TNF-α in mediating CIGT.
In conclusion, this is the first severe CIGT risk model to implicate immune genetic variability of the TLR2 gene and is supportive of prior studies of the TNFA gene, being markers of risk that is both specific and sensitive. These preliminary outcomes have the potential to impact significantly on a patient’s cancer journey through personalizing treatment based on genetics. However, these preliminary findings must be considered within the context of the limitations of this study, notably the small sample size, the retrospective nature of the study, and the requirement for future validation studies in larger populations.
References
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB, Mucositis Study Section of the MASCC and the ISOO (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025
Sonis ST (2007) Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 5(9 Suppl 4):3–11
Stringer AM, Al-Dasooqi N, Bowen JM, Tan TH, Radazum M, Logan RM, Mayo B, Keefe DMK, Gibson RJ (2013) Potential biomarkers of chemotherapy-induced diarrhea: a clinical study of intestinal microbiome alterations; intestinal inflammation and circulating matrix metalloproteinases. Support Care Cancer 21:1843–1852
Carlotto A, Hogsett VL, Mairorini EM, Razulis JG, Sonis ST (2013) The economic burden of toxicities associated with cancer treatment: review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics 31:753–766
Logan RM, Stringer AM (2008) Serum levels of NF-κB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther 7:1139–1145
Morales-Rojas T, Viera N, Moron-Medina A, Alvarez CJ, Alvarez A (2012) Proinflammatory cytokines during the initial phase of oral mucositis in patients with acute lymphoblastic leukaemia. Int J Paediatr Dent 22:191–196
van der Welden W (2011) Mucosal barrier injury, innate immunity, and stem cell transplantation. Radboud University, Nijmegen Medical Centre, Nijmegen
Cortejoso L, Lopez-Fernandez LA (2012) Pharmacogenetic markers of toxicity for chemotherapy in colorectal cancer patients. Pharmacogenomics 13:1173–1191
Sakamoto K, Oka M, Yoshino S, Hazama S, Abe T, Okayama N, Hinoda Y (2006) Relation between cytokine promoter gene polymorphism and toxicity of 5-fluorouracil plus cisplatin chemotherapy. Oncol Rep 16:381–387
Bogunia-Kubik K, Polak M, Lange A (2003) TNF polymorphisms are associated with toxic but not with aGVHD complications in the recipients of allogenic sibling haematopoietic stem cell transplantation. Bone Marrow Transplant 32:617–622
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ovsyannikova IG, Haralambieva IH, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA (2011) The role of polymorphisms in toll-like receptors and their associated intracellular signaling genes in measles vaccine immunity. Hum Genet 130:547–561
Acknowledgments
JM Bowen was a recipient of a National Health and Medical Research Council (Australia) Post-doctoral Training Fellowship. This research was supported by an Australian Dental Research Foundation Inc. Project grant.
Conflict of interest
The authors have no financial relationship with the Australian Dental Research Foundation Inc, have full control of the primary data of this research study, and agree to allow to data review if requested by the journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coller, J.K., White, I.A., Logan, R.M. et al. Predictive model for risk of severe gastrointestinal toxicity following chemotherapy using patient immune genetics and type of cancer: a pilot study. Support Care Cancer 23, 1233–1236 (2015). https://doi.org/10.1007/s00520-014-2481-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2481-z