Summary
Background
This study was undertaken to determine the effect of wood dust on the respiratory system and oxidative stress in furniture workers and to determine whether any associations exist between respiratory parameters and oxidative stress.
Methods
This cross-sectional study was performed on 45 furniture workers and 45 office workers as a reference group in Iran.
The NIOSH method 0600 was used to determine the concentration of particulates. The prevalence of respiratory symptoms was estimated via the European Community Respiratory Health Survey (ECRHS) questionnaire. Oxidative stress biomarkers and respiratory parameters were also measured.
Results
The mean concentrations of respirable and non-respirable dust were found to be 1.51 mg/m3 and 1.23 mg/m3, respectively. Pulmonary function parameters, including forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC, and antioxidant capacity biomarkers such as total antioxidant capacity (TAC) and superoxide dismutase (SOD) were significantly lower, while the prevalence of respiratory symptoms, and malondialdehyde (MDA) levels, were significantly higher in the furniture workers than in the reference group. There were significant positive associations between FVC and FEV1 with SOD and TAC.
Conclusion
The present study results indicated that exposure to wood dust significantly increased respiratory disorders and confirmed the association between lung function parameters and oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that 3.6 million workers in the European Union are exposed to wood dust [1,2,3].
Furniture makers, cabinet makers, carpenters, and veneer/plywood production workers are exposed to wood dust [4, 5].
Wood dust is classified into three categories: total, inhalable and respirable dust [6]. The particles with a mass median aerodynamic diameter of 100 μ are inhalable, and those with a mass median aerodynamic of 4 μ which deposit in the lower respiratory tract and interact with alveolar macrophages, are respirable [7]. It is worth noting that respirable wood dust ranges from 6% to 75% of the total wood aerosol [6, 8].
The American Conference of Governmental Industrial Hygienists (ACGIH) recommends an 8‑h threshold limit values-time weighted average (TLV-TWA) of 0.5 mg/m3 (inhalable fraction) for western red cedar and a TLV-TWA of 1 mg/m3 (inhalable fraction) for all other types of wood [9].
The Occupational Safety and Health Administration (OSHA) proposed a permissible exposure limit-timeweighted average (PEL-TWA) of 5 mg/m3 for respirable dust.
Exposure to wood dust can cause inflammation and irritation of the respiratory system (coughing, wheezing, chronic bronchitis, tightness of the chest, and asthma) [6], dermatitis, urticaria, alveolitis, and decline in lung function, especially FEV1 [10].
Although most of these symptoms occur at concentrations higher than 5 mg/m3 [10], some studies have shown that exposure to wood dust at concentrations below 5 mg/m3 can cause sinusitis and other pulmonary diseases [11]. Elavarasi et al. [12] observed a significant increase in genotoxicity among the furniture workers exposed to wood dust at concentrations lower than 0.3 mg/m3 compared to the reference group. They suggested that lower TLV is required to protect woodworkers.
The first study indicating an increased risk of nasal cancer in woodworkers was published in 1968. This finding was confirmed by a number of subsequent studies [13], and in 1995, wood dust was classified as carcinogenic to humans (Group I) by the International Agency for Research on Cancer (IARC) [14].
While in some studies, exposure to wood dust was associated with impaired lung function [10, 15,16,17], other studies have failed to show such an association [18,19,20,21,22].
For instance, Cormier et al. [20], Arbak et al. [18], and Borm et al. [19] showed normal respiratory parameters in woodworkers. Also, the studies conducted by Jacobsen et al. [21, 22] in 2008 and 2013 revealed no significant difference in FEV1, FVC, FEV1/FVC values between the workers exposed to wood dust and the reference group.
Oxidative stress is considered as one of the mechanisms by which wood dust exerts its toxicity on the lungs [14, 23, 24].
Oxidative stress impairs skeletal muscle contractions leading to the dysfunction of respiratory muscles, particularly of the diaphragm, in patients with severe chronic obstructive pulmonary disease (COPD) [25]. Some studies, such as those conducted by Noertjojo et al. [26] and Fante et al. [27] found obstructive spirometric patterns in the groups exposed to wood dust.
Oxidative stress is an imbalance between the production of free radicals and antioxidant defense. Free radicals have one or more unpaired electrons, and the radicals derived from oxygen and/or nitrogen are the most important free radicals produced in living systems. Direct measurement of oxidative stress biomarkers, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) is not easy [25]. Therefore, stable molecular products formed via the reaction of ROS and RNS with certain biomolecules such as lipids are measured. The main primary products of lipid peroxidation include isoprostanes, malondialdehyde (MDA), thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides (LOOHs), and conjugated dienes (CDs) [25]. MDA is used in many disorders, such as COPD, as a biomarker of oxidative stress [28]. In 2021, Bhat et al. [29] suggested that serum MDA can be used as an indicator of reduced pulmonary function in cases of difficulty in performing spirometry.
The present study aimed to measure exposure to wood dust among furniture and office workers, to compare lung function parameters, the prevalence of respiratory symptoms, and levels of total antioxidant capacity (TAC) and superoxide dismutase (SOD) as biomarkers of antioxidant status, as well as the mean level of MDA as an indicator of oxidative stress between groups and determine whether any associations exist between lung function parameters and oxidative stress.
Methods
Population and sample of the study
This cross-sectional study was performed on 45 furniture workers exposed to wood dust in Yazd Province, Iran. The reference group included 45 office workers with no history of occupational or nonoccupational exposure to dust or other chemicals which can lead to pulmonary diseases. The participants with a working history of less than 6 months, body mass index greater than 30 kg/m2, and those with a personal or family history of acute respiratory infections, chronic inflammatory diseases [30], asthma, heart disease, and those who had used vitamins E and C in the last month were excluded from the study.
Data collection
Estimation of the prevalence of respiratory symptoms
The European Community Respiratory Health Survey (ECRHS) questionnaire was used to estimate the prevalence of respiratory symptoms in the exposed and reference groups [31].
Measurement of lung function parameters
Spirometric data were collected before the coronavirus disease 2019 (COVID-19) outbreak in October 2019. Lung function parameters, including forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), forced expiratory flow at 25% and 75% of the pulmonary volume (FEF25–75), peak expiratory flow (PEF), and FEV1/FVC ratio, were measured at the end of the working shift using a COSMED Pony Fx desktop spirometer (Metabolic, Rome, Italy).
Exposure assessment
The NIOSH method 0600 [32] was used to determine the concentration of respirable dust with a 50% cut-off point of 4.0 μm at the breathing zone of the studied groups. The sampling was performed at a flow rate of 2.20 l/min in the morning shift. To determine the respirable dust content, the 10-mm Higgins Dewell cyclone with a 37-mm PVC filter (pore size 5.0 µm) loaded onto a three-piece filter cassette was used. The rapid circulation of air in the cyclone separates particles according to their equivalent aerodynamic diameter. Thus, the respirable dust particles collect on a filter for analysis, while the larger dust particles fall into the grit pot at the bottom of the cyclone. Before and after sampling, the PVC filter and grit pot were weighed with a Sartorius balance (Goettingen, Germany) to measure respirable and non-respirable dust. The same procedures were used to measure wood dust in the reference group.
Relative humidity, air pressure, and temperature were measured at the sampling site. Dust concentration (mg/m3) was determined after correcting the sampling air volume.
Determination of oxidative stress biomarkers
A 5 ml blood sample was collected from each participant using a syringe and transferred to plain tubes. Serum was separated by centrifugation at 1372 × g for 10 min. The TAC and MDA levels, and SOD activity were measured using a commercially available Zellbio kit (Zellbio Lab, Ulm, Germany) according to the manufacturer’s protocol.
Ethics
The aim of the study was explained to all participants, and they signed informed consent. The study protocol was approved by Shahid Sadoughi University of Medical Sciences Research Ethics Committee (IR.SSU.SPH.REC.1399.125).
Statistical analysis
Data analysis was performed using SPSS Statistics 21. A χ2-test was used to assess respiratory symptoms. Student’s t‑test was used to compare quantitative indices between the exposed and reference groups. A logistic and multiple linear regression analysis was used to model and assess the association between wood dust exposure and prevalence of respiratory symptoms, lung function parameters, and oxidative stress biomarkers after controlling for confounding variables (age, work history, weight, height, smoking status). Pearson’s correlation was used to correlate oxidative stress biomarkers with lung function parameters.
Results
Table 1 shows the mean age, work history, height, weight, smoking status, marital status, and level of education in both exposed and reference groups. The results showed no significant differences in age, work history, height, weight, and smoking status between the two groups; however, differences in marital status and level of education were observed between the two groups. 10 exposed group and 4 reference sroups were smokers, and the difference was not statistically significant.
The mean concentrations of respirable and non-respirable particles in furniture workers were found to be 1.51 mg/m3 and 1.23 mg/m3, respectively. Particles were not detectable in the work environments of the reference group.
The prevalence of respiratory symptoms in the studied groups is presented in Table 2.
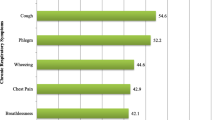
As can be seen, the prevalence rates of all symptoms (except for chronic cough and asthma) were significantly higher in the exposed group than in the reference group (p < 0.05).
As shown in Table 3, some pulmonary function parameters such as FEV1/FVC, FVC, and FEV1were significantly lower in the exposed group than in the reference group.
Table 3 also shows the mean levels of TAC, SOD, and MDA.
The mean values of TAC and SOD were significantly lower in the exposed group than in the reference group (TAC: 1.16 ± 0.13 mM vs. 1.48 ± 0.29 mM; SOD: 9.22 ± 2.42 U/ml vs. 10.59 ± 2.20 U/ml).
Conversely, the MDA level was significantly higher in the exposed group (3.85 ± 2.11 µM) compared with the reference group (2.65 ± 0.68 µM).
Of the exposed subjects, 34 had a normal spirogram, while 13 and 2 exposed subjects had obstructive and restrictive lung disease, respectively. The corresponding values in the reference group were 39, 5, and 2, respectively. There was a significant difference in the prevalence of obstructive pattern between the two groups (p = 0.035). No mixed lung pattern was observed.
To control the effects of confounders such as age, weight, height, work history, and cigarette smoking on the prevalence of respiratory symptoms, binary logistic regression analysis was used.
As shown in Table 4, before and after controlling for confounding variables, there was a significant association between exposure to wood dust and the prevalence of respiratory symptoms (except for chronic cough and asthma) (p < 0.05).
After controlling for confounding variables, the risk of wheezing, chest tightness, coughs, sputum, and shortness of breath increased by 5.89, 4.38, 9.76, 5.83 and 3.47 times, respectively, as a result of exposure to wood dust.
Table 5 shows the association between exposure to wood dust and changes in lung function parameters and oxidative stress biomarkers before and after controlling for confounding variables. As the results show in both before and after controlling for confounding variables, a significant association was observed between wood dust exposure and reduction of FVC, FEV1, FEV1/FVC, SOD, and TAC values, so that these parameters would be reduced by 4.35, 5.44, 5.80, 1.43, and 0.32 units, respectively, after controlling for confounding variables. Exposure to wood dust significantly increased MDA levels.
Table 6 shows the correlation between respiratory parameters and oxidative stress in the studied groups. As shown in Table 6, a significant positive correlation was observed between FVC with SOD (r = 0.24) and TAC (r = 0.21). Also, FEV1 was positively correlated with TAC (r = 0.30) and SOD (r = 0.43).
Discussion
There was no statistically significant difference between the two groups in terms of age, height, weight, work history, and smoking status.
In the present study, the mean concentration of respirable dust (1.51 mg/m3) was higher than non-respirable dust (1.23 mg/m3). This could be explained by the fact that the rotational speed of machines, ambient temperature and humidity, and type of wood (hardwood or softwood) affect the size of particles. More heat generated by the increased speed of the tool during machining, in turn leads to a greater number of respirable dust compared to non-respirable dust [33].
Although mean concentration of respirable dust in this study was below occupational exposure limit set by OSHA, both upper and lower respiratory tract infections and genotoxicity as a result of exposure to wood dust at concentrations less than 0.5 mg/m3 and 0.3 mg/m3, respectively, have also been reported [11, 12].
Thepaksorn et al. [34] reported the mean concentration of respirable dust of 1.11 mg/m3 in 704 wooden toy workers in Thailand. In Mandryk’s study [35] the mean concentrations of inhalable dust in the green mills and dry mills were 1.52 mg/m3 and 1.371 mg/m3, respectively. About 70% of the exposures at the dry mills and 50% of exposures at the green mills were higher than the threshold limit value of 1 mg/m3. The authors suggested that the use of old equipment, poor maintenance of the local ventilation system, leaking ventilation ducts, and the shortage of respirators have caused high dust exposure levels among the workers.
A study in 25 EU countries on 3.6 million workers exposed to wood dust, including carpenters, sawmill workers, furniture workers, forestry workers, and other wood industry workers, demonstrated that 16% of the workers were exposed to respirable dust above 5 mg/m3 [36]. In 2009, Osman et al. [10], in a study on 328 workers exposed to wood dust and 328 control group reported that the mean concentration of respirable wood dust was 2.04 mg/m3, which is lower than the ACGIH TLV (5 mg/m3). Of the workers 9.5% were exposed to wood dust at concentrations above 5 mg/m3, and none of the workplaces had ventilation systems.
Moreover, the workers’ exposure to respirable dust exceeded 5 mg/m3 in the studies conducted by Magagnotti et al. [37] on 60 chipper operators in Italy, Abbas et al. [30] on 67 carpenters in Egypt, and Mohammadian et al. [38] on 10 wood workers in Iran.
Neghab et al. [39] in the sawmill workers in Iran, reported the mean concentrations of inhalable and respirable dust of 2.44 mg/m3 and 6.76 mg/m3, respectively, which is higher than ACGIH TLV-TWA. The authors suggested that the use of air jets and dry sweeping, lack of awareness of health effects of wood dust among the workers, and lack of a proper ventilation system might explain the reasons of these results.
The FVC, FEV1, and FEV1/FVC values were significantly lower in the exposed group than in the reference group.
The results are consistent with the findings of other studies on workers exposed to wood dust [10, 15,16,17, 40]. For example, Shamssain et al. [41] in a study of furniture makers, reported significant decreases in all spirometric indices, including FVC, FEV1, FEV1/FVC, FMF, FEF, and PEF in the wood exposed group in comparison with the non-exposed group. In Osman’s study, the FVC values in the exposed group were significantly lower than that of the control group [10].
Beydon et al. [36] also showed that the respiratory parameters, including FVC, FEV1, and FEV1/FVC were significantly lower in sawmill workers exposed to wood dust in comparison to the non-exposed group.
Additionally, in the study conducted by Hosseini et al. [42] on 276 carpentry workers in Iran, the FEV1 and FEV1/FVC in the wood exposed group were significantly lower than those of the reference group. Similar findings have been reported by others [39, 43, 44]. After controlling for the important confounders, significant associations remained between exposure to wood dust and a decrease in FVC, FEV1, FEV1/ FVC values.
Similarly, the findings of Hessel et al. [45], Noertjojo et al. [26], and Neghab et al. [39] in the sawmill workers showed a significant association between exposure to wood dust and decreased lung function parameters after controlling the confounding variables.
In contrast, some studies have failed to find such an effect on wood dust. For example, surveys such as those conducted by Cormier et al. [20], Arbak et al. [18], and Borm et al. [19] showed that the pulmonary function of woodworkers was normal.
In both studies conducted by Jacobsen et al. [21, 22] in Denmark in 2008 and 2013, no significant difference in the FEV1, FVC, and FEV1/FVC values was observed between the wood exposed workers and the reference group.
The difference between the results of different studies may be due to differences in the workers’ age, exposure duration [18], wood dust concentrations [22], the type of wood and processing method, used technology, the geographical area, the mill size or climatic conditions, and ventilation system [19, 20]. Wood dust exposure can be reduced by 3–10 times via good housekeeping and ventilation [6].
In the present study, the group exposed to wood dust had a significantly higher prevalence of respiratory symptoms, including coughs (90%), sputum (81.2%), chest tightness (78.6%), wheezing (80%), and shortness of breath (72.7%) when compared with reference group (coughs: 10%, sputum: 18.8%, chest tightness: 21.4%, wheezing: 20%, and shortness of breath: 27.3%). The risk of wheezing, chest tightness, cough, sputum and shortness of breath were 5.65, 4.53, 11, 5.68, and 3.58 times higher, respectively in the exposed group in comparison to the reference group.
In accordance with the present results, in Schlunssen’s study, in Denmark, a significant increase in the cough and wheezing frequency in 54 furniture factories (2033 carpenters and 474 control members) in comparison to a control group was observed [16].
In the study conducted by Osman et al. [10] 53.7%, 43%, 41.2%, and 23.8% of the woodworkers had nasal congestion, red eyes, itchy eyes, and runny nose, respectively.
Similarly, Bislimovska et al. [43] in a study on 37 parquet manufacturing workers and 37 reference group, reported the prevalence of cough (29.7%) and sputum (16.2%) in the exposed group was significantly higher than that of the reference group.
Neghab et al. [39] reported the risk of developing wheezing, chest tightness, sputum, chronic sputum, coughs, chronic coughs, and shortness of breath in sawmill workers to be 4.75, 2.47, 12. 57, 2.76, 4.94, 2.02, and 4.64 times higher than a reference group, respectively.
Similar findings have been reported by others [18, 35, 42, 44]. Additionally, after controlling for confounders, significant associations remained between exposure to wood dust and increased prevalence of respiratory symptoms.
Similarly, Hessel’s study showed that the risk of shortness of breath and wheezing increased by 2.83 and 2.58 times, respectively, as a result of exposure to wood dust [45].
In the present study, 13 exposed workers and 5 reference group subjects had obstructive spirometric patterns, and the observed difference was statistically significant. Similarly, Fante et al. [27], Neghab et al. [39], and Noertjojo et al. [26] reported an increased risk for obstructive pulmonary diseases in woodworkers.
In the present study, the exposed group showed significantly higher levels of MDA and lower levels of SOD and TAC in comparison with the reference group. Additionally, after controlling for confounders, significant associations remained between exposure to wood dust and reduced antioxidant capacity and increased oxidative stress biomarker.
Exposure to wood dust resulted in 0.32, and 1.43 unit decreases in TAC and SOD levels and 1.23 unit increases in MDA in furniture workers in comparison to the reference group.
It is difficult to explain this result because antioxidant capacities may fluctuate due to many changes, such as inflammation, stress, diet, and genetics [46].
Studies in the workers exposed to other agents known to induce oxidative stress revealed inhibition of the antioxidant enzyme, i.e., SOD and glutathione peroxidase (GPx) or depletion of substrate molecules, i.e., glutathione (GSH) in exposed workers in comparison to controls [47, 48].
For example, Tope et al. [49] showed that organophosphates can inhibit the activity of SOD and the concentration of GSH in the farmworkers.
Farahat et al. [6] reported a significant reduction in GPx levels among the wood-exposed carpenters (15.52 ± 5.97 U/mg protein) in comparison with the reference group (28.88 ± 5.39 U/mg protein) and the lowest serum GPX concentration was observed where the highest concentration of respirable wood dust was reported.
Abbas et al. [30] found a significantly higher MDA and lower GSH and GPx in woodworkers in comparison with the control group. The authors suggested that a decrease in antioxidant capacity could lead to oxidative stress and inflammation, in other words, oxidative stress may be the mechanism by which air pollutants cause airway inflammation and lead to acute cardiorespiratory complications.
In Wultsch’s study, woodworkers had a significantly greater mean of MDA when compared with the control group [5]. Gaballah et al. [50] showed that exposure to wood dust was associated with a significant reduction in SOD and GPx levels. These authors suggested that occupational exposure to wood dust is associated with oxidative stress.
There was a significant positive correlation between FVC and FEV1 with SOD and TAC.
Similarly, in 2021, Bhat et al. [29] showed significantly decreased FEV1, FVC, and FEV1/FVC, as well as significantly increased MDA levels in the sawmill workers as compared to a control group. The authors suggested that serum MDA can be used as an indicator of decreased pulmonary function, especially in patients in whom spirometry performing is difficult.
A limitation of this study is that the possible role of marital status and level of education in antioxidant capacities outcomes have not been investigated. Therefore, further studies regarding the role of marital status and level of education would be necessary.
Conclusion
In the present study, there was no statistically significant difference between the two groups in terms of demographic data and the number of cigarette smokers, and smoking intensity. Therefore, these cannot be the cause of the differences in results between the two groups; hence, the changes in pulmonary function parameters and oxidative stress biomarkers can be attributed to exposure to wood dust.
The observed correlation between FVC and FEV1 with SOD and TAC supports the theory that oxidative stress is more likely a mechanism of impaired lung function. According to the results of this study, a diet high in antioxidants (such as fruits and vegetables), as well as appropriate personal protective equipment and workplace safety and training are recommended for the prevention and control of acute and chronic lung diseases in wood workers.
References
Kacha Y, Nayak Y, Varu M, Mehta H, Shah C. Effects of wood dust on respiratory functions in saw mill workers. Int J Basic Appl Physiol. 2014;3(1):122–8.
Danilova M, Stoleski S, Mijakoski D. Respiratory symptoms and ventilatory function in never-smoking males working in dusty occupations. Open Access Maced J Med Sci. 2014;7(4):645–649. https://doi.org/10.3889/oamjms.2014.116.
Cellai F, Capacci F, Sgarrella C, Poli C, Arena L, Tofani L, et al. A cross-sectional study on 3‑(2-Deoxy-β-D-Erythro-Pentafuranosyl) pyrimido [1, 2‑α] Purin-10 (3H)-one deoxyguanosine adducts among woodworkers in Tuscany, Italy. Int J Mol Sci. 2019;20(11):1–10.
Thetkathuek A, Yingratanasuk T, Demers PA, Thepaksorn P, Saowakhontha S, Keifer MC. Rubberwood dust and lung function among Thai furniture factory workers. Int J Occup Environ Health. 2010;16(1):69–74.
Wultsch G, Nersesyan A, Kundi M, Wagner K‑H, Ferk F, Jakse R, et al. Impact of exposure to wood dust on genotoxicity and cytotoxicity in exfoliated buccal and nasal cells. Mutagenesis. 2015;30(5):701–9.
Farahat S, Ibrahim Y, Abdel-Latif M. Genotoxicity and oxidative stress due to exposure to wood dust among carpenters. Egypt J Occup Med. 2010;34(1):83–95.
Batsungnoen K, Riediker M, Suárez G, Hopf NB. From nano to micrometer size particles—a characterization of airborne cement particles during construction activities. J Hazard Mater. 2020;398:1–16.
Long H, Shi T, Borm PJ, Määttä J, Husgafvel-Pursiainen K, Savolainen K, et al. ROS-mediated TNF‑α and MIP‑2 gene expression in alveolar macrophages exposed to pine dust. Part Fibre Toxicol. 2004;1(1):1–8.
American Conference of Governmental Industrial Hygienists (ACGIH). TLvs and BELs. Threshold limits values for chemical substances and physical agents and biological exposure indices. Cincinnati. 2022.
Osman E, Pala K. Occupational exposure to wood dust and health effects on the respiratory system in a minor industrial estate in Bursa/Turkey. Int J Occup Med Environ Health. 2009;22(1):43–50.
Husgafvel-Pursiainen K, editor. Wood dust-related health effects and occupational limit values. Wood dust symposium 15th. Copenhagen. 2004.
Elavarasi D, Ramakrishnan V, Subramoniam T, Ramesh A, Cherian K, Emmanuel C. Genotoxicity study in lymphocytes of workers in wooden furniture industry. Curr Sci. 2002;82:869–73.
Demers PA, Boffetta P, Kogevinas M, Blair A, Miller BA, Robinson CF, et al. Pooled reanalysis of cancer mortality among five cohorts of workers in wood-related industries. Scand J Work Environ Health. 1995;21:179–90.
Pylkkänen L, Stockmann-Juvala H, Alenius H, Husgafvel-Pursiainen K, Savolainen K. Wood dusts induce the production of reactive oxygen species and caspase‑3 activity in human bronchial epithelial cells. Toxicology. 2009;262(3):265–70.
Thepaksorn P, Fadrilan-Camacho VFF, Siriwong W. Respiratory symptoms and ventilatory function defects among para rubber wood sawmill workers in the south of Thailand. Hum Ecol Risk Assess. 2017;23(4):788–97.
Schlünssen V, Schaumburg I, Taudorf E, Mikkelsen AB, Sigsgaard T. Respiratory symptoms and lung function among Danish woodworkers. J Occup Environ Med. 2002;44(1):82–98.
Bolund AC, Miller MR, Jacobsen GH, Sigsgaard T, Schlünssen V. New-onset COPD and decline in lung function among wood dust-exposed workers: re-analysis of a 6-year follow-up study. Ann Work Expo Health. 2018;62(9):1064–76.
Arbak P, Bilgin C, Balbay O, Yesildal N, Annakkaya AN, Ulger F. Respiratory symptoms and peak expiratory flow rates among furniture-decoration students. Ann Agric Environ Med. 2004;11(1):13–7.
Borm P, Jetten M, Hidayat S, van de Burgh N, Leunissen P, Kant I, et al. Respiratory symptoms, lung function, and nasal cellularity in Indonesian wood workers: a dose-response analysis. Occup Environ Med. 2002;59(5):338–44.
Cormier Y, Mérlaux A, Duchaine C. Respiratory health impact of working in sawmills in eastern Canada. Arch Environ Health. 2000;55(6):424–30.
Jacobsen G, Schlünssen V, Schaumburg I, Taudorf E, Sigsgaard T. Longitudinal lung function decline and wood dust exposure in the furniture industry. Eur Respir J. 2008;31(2):334–342.
Jacobsen GH, Schlünssen V, Schaumburg I, Sigsgaard T. Cross-shift and longitudinal changes in FEV1 among wood dust exposed workers. Occup Environ Med. 2013;70(1):22–8.
Gromadzinska J, Wasowicz W. Oxidative stress-inducing workplace agents. Comments Toxicol. 2003;9(1):23–37.
Valavanidis A. Oxidative stress and pulmonary carcinogenesis through mechanisms of reactive oxygen species. How respirable particulate matter, fibrous dusts, and ozone cause pulmonary inflammation and initiate lung carcinogenesis. Springer; 2019. p. 247–65.
Shadab M, Agrawal DK, Aslam M, Islam N, Ahmad Z. Occupational health hazards among sewage workers: oxidative stress and deranged lung functions. J Clin Diagn Res. 2014;8(4):11–13.
Noertjojo HK, Dimich-Ward H, Peelen S, Dittrick M, Kennedy SM, Chan-Yeung M. Western red cedar dust exposure and lung function: a dose-response relationship. Am J Respir Crit Care Med. 1996;154(4):968–73.
Fante D, Mariam T, Mulat E, Demissie W. Prevalence of re-spiratory disorders among woodworkers in Jimma town, southwest Ethiopia. J Pulm Med Respir Res. 2019;5:22.
Khoubnasabjafari M, Ansarin K, Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts. 2015;5(3):123–7.
Bhat BM, D’Souza V. Effect of chronic exposure of wood dust on serum malondialdehyde, C‑reactive protein in sawmill workers and their correlation with pulmonary function parameters. Eur J Mol Clin Med. 2021;8(2):1708–17.
Abbas RA, Roshdy HS, Sharaf SM. Occupational exposure to airborne wood dust during carpentry work and risk of ischemic heart disease: a comparative cross-sectional study. J Am Sci. 2013;9:660–8.
No Authors listed. Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European community respiratory health survey (ECRHS). Eur Respir J. 1996;9:687–95.
National Institute of Occupational Safety and Health (NIOSH). Manual of analytical methods, method 0600: particulates not otherwise regulated, respirable. Atlanta: Centers for Disease Control and Prevention; 1998.
Pędzik M, Przybylska-Balcerek A, Szwajkowska-Michałek L, Szablewski T, Rogoziński T, Buśko M, et al. The dynamics of mycobiota development in various types of wood dust depending on the dust storage conditions. Forests. 2021;12(12):1786.
Thepaksorn P, Thongjerm S, Siriwong W, Ponprasit P. Occupational hazard exposures and health risks at wooden toys industry in southern Thailand. Hum Ecol Risk Assess. 2020;26(8):2162–72.
Mandryk J, Alwis KU, Hocking AD. Effects of personal exposures on pulmonary function and work-related symptoms among sawmill workers. Ann Occup Hyg. 2000;44(4):281–9.
Kauppinen T, Vincent R, Liukkonen T, Grzebyk M, Kauppinen A, Welling I, et al. Occupational exposure to inhalable wood dust in the member states of the European Union. Ann Occup Hyg. 2006;50(6):549–61.
Magagnotti N, Nannicini C, Sciarra G, Spinelli R, Volpi D. Determining the exposure of chipper operators to inhalable wood dust. Ann Occup Hyg. 2013;57(6):784–92.
Mohammadyan M, Afzali M. Personal exposure to wood dust among workers in NekaChoob factory in 2013. Iran J Health Sci. 2014;2(4):21–6.
Neghab M, Jabari Z, Shouroki FK. Functional disorders of the lung and symptoms of respiratory disease associated with occupational inhalation exposure to wood dust in Iran. Epidemiol Health. 2018;40:e2018031. https://doi.org/10.4178/epih.e2018031.
Al Zuhair Y, Whitaker C, Cinkotai F. Ventilatory function in workers exposed to tea and wood dust. Occup Environ Med. 1981;38(4):339–45.
Shamssain M. Pulmonary function and symptoms in workers exposed to wood dust. Thorax. 1992;47(2):84–7.
Hosseini DK, Malekshahi Nejad V, Sun H, Hosseini HK, Adeli SH, Wang T. Prevalence of respiratory symptoms and spirometric changes among non-smoker male wood workers. PLoS ONE. 2020;15(3):e224860.
Bislimovska D, Petrovska S, Minov J. Respiratory symptoms and lung function in never-smoking male workers exposed to hardwood dust. Open Access Maced J Med Sci. 2015;3(3):500–5.
Okwari O, Antai A, Owu D, Peters E, Osim E. Lung function status of workers exposed to wood dust in timber markets in Calabar, Nigeria. Afr J Med Med Sci. 2005;34(2):141–5.
Hessel PA, Herbert FA, Melenka LS, Yoshida K, Michaelchuk D, Nakaza M. Lung health in sawmill workers exposed to pine and spruce. Chest. 1995;108(3):642–6.
Bayraktar NM, Karagözler AA, Bayraktar M, Titretir S, Gözükara EM. Investigation of the blood biochemical status of gas station workers. Toxicol Environ Chem. 2006;88(4):587–94.
Rekhadevi P, Mahboob M, Rahman M, Grover P. Genetic damage in wood dust-exposed workers. Mutagenesis. 2008;24(1):59–65.
Karagözler AA, Mehmet N, Batçioglu K. Effects of long-term solvent exposure on blood cytokine levels and antioxidant enzyme activities in house painters. J Toxicol Environ Health Part A. 2002;65(17):1237–46.
Tope A, Bebe FN, Panemangalore M. Micronuclei frequency in lymphocytes and antioxidants in the blood of traditional limited-resource farm workers exposed to pesticides. J Environ Sci Health B. 2006;41(6):843–53.
Gaballah IF, Helal SF, Mourad BH. Early detection of lung cancer potential among Egyptian wood workers. Int J Occup Environ Health. 2017;23(2):120–7.
Funding
This study was supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran, under the grant (7895).
Author information
Authors and Affiliations
Contributions
All authors have reviewed, approved, and consented to the submission, and they are accountable for all aspects of its accuracy and integrity.
Corresponding author
Ethics declarations
Conflict of interest
F. Kargar-Shouroki, M.R. Dehghan Banadkuki, S. Jambarsang and A. Emami declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kargar-Shouroki, F., Dehghan Banadkuki, M.R., Jambarsang, S. et al. The association between wood dust exposure and respiratory disorders and oxidative stress among furniture workers. Wien Klin Wochenschr 134, 529–537 (2022). https://doi.org/10.1007/s00508-022-02048-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-022-02048-5