Summary
Aims
We aimed to determine the contribution of quantitative HBsAg in differentiating chronic infections from chronic hepatitis in HBeAg negative patients with HBV DNA 2000–20,000 IU/ml.
Material and methods
A total of 79 untreated HBeAg negative patients were included. Patients were divided into 3 groups based on HBV DNA levels: group 1 (HBV DNA ≤ 2000 IU/ml), group 2 (HBV DNA: 2000–20,000 IU/ml) and group 3 (HBV DNA > 20,000 IU/ml). We collected serum from all patients for quantitative HBsAg analysis. We compared serum quantitative HBsAg levels with biochemical parameters, HBV DNA and liver biopsy results.
Results
In this study 46 patients were female and the mean age was 42 years. Serum quantitative HBsAg levels were found to be significantly lower in chronic infections compared with chronic hepatitis. There was a positive correlation between quantitative HBsAg and HBV DNA, ALT (alanine aminotransferase), HAI score (histological activity index), fibrosis score and disease stage. The cut-off level of quantitative HBsAg was determined as 4425 IU/ml to differentiate chronic infection from chronic hepatitis. With the test specificity of 95%, we found quantitative HBsAg cut-off values 1026 IU/ml and 20,346 IU/ml for the diagnosis of chronic infection and chronic hepatitis, respectively.
Conclusion
Our study suggests that the quantitative HBsAg ≤ 1000 IU/ml limit value might be used for the diagnosis of chronic infection not only in HBV DNA ≤ 2000 IU/ml but also in patients with HBV DNA between 2000–20,000 IU/ml. In addition, antiviral treatment could be considered in patients with quantitative HBsAg > 20,000 IU/ml and HBV DNA > 2000 IU/ml without further examinations such as liver biopsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis B virus (HBV) is a small enveloped DNA virus belonging to Hepadnaviridae family [1]. Three different viral structures have been shown in investigations from infected patient’s serum under electron microscopy: the Dane particle, spherical particles and tubular particles. While the Dane particle is the only particle containing HBV genome, all three particles express surface antigen (HBsAg) and are the source of HBsAg in serum [2, 3]. Viral sequences integrated into the host cell genome also cause HBsAg expression [4]. The research based on the measurement of HBsAg levels in serum were first studied in the early 1990s and its contribution to virological response was investigated in patients under interferon treatment [5]. With standardization, quantitative HBsAg (qHBsAg) has recently become a valuable parameter in the staging, follow-up, initiation and interruption of treatment of chronic HBV infection and in the prediction of complications [4, 6,7,8,9,10,11]. Relevant studies showed that qHBsAg was related to transcriptional activity of covalently closed circular DNA (cccDNA) which is the most important replication indicator of HBV [12,13,14,15,16].
Long-term complications are very rare in HBeAg negative chronic infection (CI). The guidelines do not recommend routine liver biopsy and initiation of treatment for patients with HBV DNA ≤ 2000 IU/ml and permanently normal alanine aminotransferase levels (PNALT) [7, 17,18,19]. The risk of cirrhosis and hepatocellular carcinoma (HCC) increases approximately 10 times in patients with serum levels of HBV DNA > 20,000 IU/ml compared to CI [20]. While the prognosis and indications for treatment of patients with HBV DNA ≤ 2000 IU/ml and > 20,000 IU/ml have been determined, it is not generally easy to distinguish CI from HBeAg negative chronic hepatitis (CH) in patients with HBV DNA between 2000–20,000 IU/ml. In the long-term follow-up of patients with HBV DNA between 2000–20,000 IU/ml most cases are considered to be CI, while some develop CH. The increase in the frequency of complications, such as chronic liver disease, cirrhosis and HCC with active disease reveals the treatment necessity of patients defined as CH. On the other hand, a group of patients may receive lifelong unnecessary treatment.
In this study, we aimed to determine the contribution of qHBsAg to differentiating CI from CH in HBeAg negative patients with HBV DNA between 2000–20,000 IU/ml.
Material and methods
This study was approved by the ethical committee of Istanbul University Cerrahpasa Medical Faculty (83045809-604.01.02) and was supported financially by Istanbul University Scientific Research Projects Coordination Unit (project code: TTU-2017-24508).
Patients
A total of 79 untreated HBeAg negative patients followed in Istanbul University Cerrahpasa Medical Faculty, Infectious Disease Department were included. The inclusion criteria were HBsAg positivity for at least 6 months, HBeAg negativity, Anti-HBe positivity, being naive for treatment and detectable HBV DNA. The exclusion criteria were coinfection with Hepatitis C Virus (HCV), Hepatitis D Virus (HDV) and Human Immunodeficiency Virus (HIV), alcoholic liver disease, liver cirrhosis or HCC. The HBeAg/AntiHBe serostatus, measurement of HBV DNA and serum alanine aminotransferase (ALT) levels (1-year period with 3‑ month intervals) and histopathological findings (liver biopsy within 6 months from the time of blood collection) were used for determination of the phase of chronic HBV infection. All patients were followed up at least for 1 year at 3‑month intervals.
Patients were divided into 3 groups based on HBV DNA levels: group 1 (HBV DNA ≤ 2000 IU/ml), group 2 (HBV DNA: 2000–20,000 IU/ml) and group 3 (HBV DNA > 20,000 IU/ml) (Fig. 1).
Group 1:
The group included 43 patients with HBV DNA levels below 2000 IU/ml, with PNALT and/or no evidence of active hepatitis in liver biopsy and were considered as CI.
Group 2:
The group included 19 patients with HBV DNA levels between 2000–20,000 IU/ml. All patients had liver biopsy and were followed up for at least 1 year. Based on liver biopsy 10 of them were compatible with CI.
Group 3:
The group included 17 patients with HBV DNA above 20,000 IU/ml. They had high ALT levels and/or active hepatitis in the biopsy and were considered as CH.
Laboratory analysis
The sera from all the patients taken during routine outpatient follow-up and tested for hepatitis serological markers (HBsAg, Anti-HBs, HBeAg, Anti-HBe, anti-HCV, anti-HDV, anti-HIV), complete blood count, routine biochemical tests (ALT, aspartate aminotransferase, alkaline phosphatase, gamma glutamyl transferase, albumin, globulin, total bilirubin, prothrombin time and alpha-fetoprotein), HBV DNA and qHBsAg. The ALT fluctuation was considered in patients with ALT higher than upper limit of normal (ULN > 40 IU/ml) detected no clear etiology during follow-up. Liver biopsy was recommended to all patients but only performed in those who consented. The qHBsAg levels were compared with the results of routine biochemical tests and HBV DNA levels on the same day and liver biopsies performed within 6 months from the time of blood collection. As HBV genotype D is observed predominantly in Turkey, we did not determine genotype. Liver biopsies were analyzed by using standard criteria according to the modified Ishak scoring system [21].
Blood samples taken from all patients for qHBsAg were centrifuged at 2000 rpm for 15 min and the serum was separated. These were stored at −20 °C until analyzed. The qHBsAg levels were studied with the chemiluminescent microparticle immunoassay (CMIA) technique using Abbott Architect® HBsAg II kit (Abbott Diagnostics, Chicago, IL, USA).
Statistical analysis
The SPSS 21.0 program (Statistical Package for Social Science, Chicago, IL, USA) was used for statistical analysis of the data. Continuous variables with normal distribution were expressed by mean ± standard deviation (SD) and continuous variables with skewed distribution by median (interquartile range, IQR). Categorical variables were expressed by frequency and percentage. The χ2-test was used to compare categorical variables. The Mann-Whitney U-test was used in binary groups to compare continuous variables that do not show normal distribution, Kruskal-Wallis test was used in groups with more than two groups. In more than two groups, the statistically significant results were analyzed in pairs using the Mann-Whitney U-test by using the Bonferroni correction. Student’s t independent test was used for the analysis of continuous variables with normal distribution. When the number of groups was more than two, one-way ANOVA and the Tukey test as post hoc test were used for statistically significant results. In the correlation analysis, Pearson correlation test (r) was used for the normally distributed numerical parameters, and Spearman’s Rho test (rho) was used for the analysis of skewed distribution and categorical data. p < 0.05 was considered statistically significant.
The diagnostic accuracy and the cut-off values of qHBsAg were evaluated by receiver operating characteristics (ROC) curves. Highest sensitivity and specificity sum was selected for the cut-off level. In order to increase in the specificity of CI and CH diagnoses, additional cut-off values were determined with specificity of at least 95% with the highest sensitivity.
Results
A total of 79 treatment naive, HBeAg negative patients with detectable HBV DNA were recruited. The mean age was 42 ± 10.7 years and 46 patients were females. The patient characteristics are illustrated in Table 1. The median follow-up period after diagnosis of HBV was 4 years (IQR 7 years, range 1–27 years). There was no significant difference in terms of age and gender between groups, 53 were defined as CI and 26 were CH.
The mean qHBsAg was significantly lower in group 1 than in group 3 (3.14 log IU/ml and 3.90 log IU/ml, respectively). The mean qHBsAg level was 3.44 log IU/ml in group 2 (Table 2, Fig. 2). The ALT fluctuation and histological activity index (HAI) were significantly higher in the group 3 compared to other groups (Table 1). Statistically, ALT fluctuation was significantly correlated with both HAI score (rho=0.42, p = 0.004) and disease stage (rho=0.41, p = 0.001). While there was no patient whose ALT level exceeded 2 times in group 1, 1 patient in group 2 and 9 patients in group 3 had ALT more than 2 times higher ULN. Fibrosis score was significantly higher in group 3 compared to group 1.
A qHBsAg ≤ 1000 IU/ml was found in 41.9% of patients in group 1 and 21.1% of patients in group 2. Only one patient in group 3 had qHBsAg ≤ 1000 IU/ml. Positive correlation was found between qHBsAg and HBV DNA (r = 0.4, p = 0.001), ALT (rho = 0.42, p = 0.001), HAI score (rho = 0.53, p = 0.001), fibrosis score (rho = 0.34, p = 0.02), disease stage (rho = 0.54, p = 0.001) (Fig. 3). In group 2, four patients with qHBsAg < 1000 IU/mL had no findings of active hepatitis in liver biopsy.
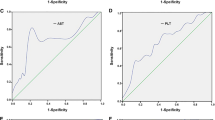
Specificity, sensitivity, positive predictive values (PPV) and negative predictive values (NPV) of qHBsAg levels were determined for diagnosis of CI and CH phase. The cut-off value of qHBsAg for distinguishing between CI and CH was 4425 IU/ml, whereas sensitivity and specificity were 79% and 81%, respectively. When the specificity of the test was ≥ 95% for diagnosis of CI, the cut-off value was 1026 IU/ml (specificity 96%, PPV 96%). When approved the cut-off value of 116 IU/ml, the specificity and PPV increased to 99% but the sensitivity and NPV decreased to 13% and 36%, respectively. For the diagnosis of CH, the cut-off value was 20,346 IU/ml (specificity 95%, PPV 90%) when selected the specificity of the test ≥ 95%. When approved cut-off value of 29,158 IU/ml, the specificity and PPV of the test increased to 99%. As shown in Fig. 4, area under the ROC curve (AUROC) of qHBsAg was 83.4% (95% confidence interval = 74%–93%, p = 0.001). Biopsies of 2 patients with qHBsAg > 20,000 IU/ml in group 2 was compatible with CH.
Discussion
Several recent studies have shown that qHBsAg is a dynamic parameter in both the natural course of the disease and the treatment process [6, 13, 22, 23]. The benefits of qHBsAg have been discussed about staging the disease, predicting complications, monitoring the treatment process and making a decision of treatment cessation [4, 6, 8,9,10,11]. On the other hand, many studies have suggested that qHBsAg is closely associated with intrahepatic viral markers, particularly transcriptional activity of cccDNA [12,13,14,15]. Although nucleos(t)id analogues reduce HBV DNA to an undetectable level, further reduction of qHBsAg with pegylated-interferon therapy is thought to explain this relationship [12].
The present study demonstrated that qHBsAg was lower in CI than in CH that is similar to relevant studies [6, 11, 12, 24, 25]. Many studies showed positive correlation between qHBsAg and HBV DNA previously [6, 26,27,28,29]; however, this clear correlation could not be shown between qHBsAg and ALT, HAI and fibrosis. Sali et al. found positive correlation between qHBsAg and ALT in patients with chronic hepatitis B but no correlation between qHBsAg and HAI and fibrosis [27]. The other study found no correlation between qHBsAg and ALT [30]. An article in 2016 found no correlation between qHBsAg and ALT, HAI and fibrosis score [28]. In our study, qHBsAg was found to be positively correlated with HBV DNA, ALT, HAI score, fibrosis score and disease stage. In a study by Brunetto et al. in 2010, diagnostic accuracy of the coexistence of HBV DNA ≤ 2000 IU/mL and qHBsAg ≤ 1000 IU/mL viewed at any time, was 94.3% compared with 1‑year follow-up [6]. In the current study, qHBsAg ≤ 1000 was found in 18 of the 43 patients in group 1. Only 1 patient had qHBsAg < 1000 IU/ml in group 3 and whose HBV DNA level fluctuated between 11,000–222,000 IU/ml during the 5‑year follow-up and his liver biopsy was scored as fibrosis 1/6 and HAI 5/18. In group 2, 4 out of 19 patients had qHBsAg ≤ 1000 IU/ml and they had no findings of active hepatitis in liver biopsy. Our data indicated that the limit value of 1000 IU/ml for qHBsAg might be used for the diagnosis of CI in patients with HBV DNA between 2000–20,000 IU/ml.
Although qHBsAg cut-off levels are determined in patients who do not need treatment, there is no accepted limit value for patients who should be treated. One study recommends that all patients who do not meet the criteria for HBV DNA ≤ 2000 IU/ml and qHBsAg ≤ 1000 IU/ml should be further examined by liver biopsy [13]. In another study, qHBsAg > 1000 IU/ml and HBV DNA > 200 were defined as high-risk patients for reactivation. Its NPV and sensitivity were 96% and was 92%, respectively. In fact, it is suggested that patients with CH may be noticed during remission period and treatment can be started in these patients earlier [31]. On the other hand, HCC can be seen in some patients despite low levels of HBV DNA and one study showed that HCC is more common in patients with high qHBsAg despite low HBV DNA [32].
In our study, cut-off level of qHBsAg was calculated as 4425 IU/ml for distinguishing between CI and CH. It showed 83.4% diagnostic accuracy, 79% sensitivity, 81% specificity, 89% PPV. When the specificity of the test was ≥ 95%, the limit value for the diagnosis of CI was 1026 IU/ml, PPV was 96% and it was consistent with the qHBsAg limit value accepted by the guidelines [17]. The specificity of qHBsAg ≥ 20,346 IU/ml was found to be 95% in the diagnosis of CH. In these levels of qHBsAg, especially in patients with HBV DNA > 2000 IU/ml, it could be helpful to start antiviral therapy without further examinations such as liver biopsy.
This study has some limitations including retrospective design, low number of patients and data from a single center. These limitations should be considered before making any causal inferences; however, we supposed that it would be of great benefit to share our experience, as it provides a valuable insight on staging the disease and starting the treatment which still remains a topic of controversy.
Conclusion
In patients with HBV DNA between 2000–20,000 IU/ml, qHBsAg which is more stable than HBV DNA may contribute to the existing parameters in disease staging. Our study suggests that the qHBsAg ≤ 1000 IU/ml limit value may be used for the diagnosis of CI not only in HBV DNA ≤ 2000 IU/ml but also in patients with HBV DNA between 2000–20,000 IU/ml. In addition, antiviral treatment could be considered in patients with qHBsAg > 20,000 IU/ml and HBV DNA > 2000 IU/ml without further examinations such as liver biopsy.
References
Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68.
Liang TJ. Hepatitis B : the virus and disease. 2009. pp. 13–21.
Gerlich WH. Medical Virology of Hepatitis B: how it began and where we are now. Virol J. 2013;10:239.
Tseng TC, Kao JH. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: New trick of old dog. J Gastroenterol. 2013;48:13–21.
Janssen HL, Kerhof-Los CJ, Heijtink RA, Schalm SW. Measurement of HBsAg to monitor hepatitis B viral replication in patients on alpha-interferon therapy. Antiviral Res. 1994;23:251–7.
Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483–90.
Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
Zoulim F, Carosi G, Greenbloom S, Mazur W, Nguyen T, Jeffers L, et al. Quantification of HBsAg in nucleos ( t ) ide-naïve patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol. 2015;62:56–63.
Fasano M, Lampertico P, Marzano A, Di Marco V, Niro GA, Brancaccio G, et al. HBV DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years. J Hepatol. 2012;56:1254–8.
Brunetto MR, Marcellin P, Cherubini B, Yurdaydin C, Farci P, Hadziyannis SJ, et al. Response to peginterferon alfa-2a ( 40KD ) in HBeAg-negative CHB : On-treatment kinetics of HBsAg serum levels vary by HBV genotype. J Hepatol. 2013;59:1153–9.
Seto W, Wong DK, Fung J, Huang F, Liu KS, Lai C, et al. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Eur Soc Clin Infect Dis. 2014;20:1173–80.
Brunetto MR, Moriconi F, Bonino F. Hepatitis B virus surface antigen levels: A guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–50.
Larsson SB, Eilard A, Malmström S, Hannoun C, Dhillon AP, Norkrans G, et al. HBsAg quantification for identification of liver disease in chronic hepatitis B virus carriers. Liver Int. 2013;34:e238–45. https://doi.org/10.1111/liv.12345.
Wisedopas N, Poovorawan Y, Tangkijvanich P. Kinetics of serum HBsAg and Intrahepatic cccDNA during Pegylated interferon terapy in patients with HbeAg-positive and HbeAg-negative chronic hepatitis B. J Med Virol. 2017;89:130–8.
Bréchot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127:S56–S61.
Höner zu Siederdissen C, Maasoumy B, Cornberg M. What is new on HBsAg and other diagnostic markers in HBV infection? Best Pract Res Clin Gastroenterol. 2014;31:281–9.
Association E. EASL Clinical Practice Guidelines : management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85.
Terrault NA, Bzowej NH, Chang K‑M, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98.
Iloeje U, Yang H, Su J, Jen C, You S, Chen C. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–86.
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9.
Larsson SB, Hannoun C, Lindh M, Malmstro S. Hepatitis B viral DNA decline at loss of HbeAg is mainly explained by reduced cccDNA load—down-regulated transcription of PgRNA has limited impact. Plos One. 2012;7:e36349.
Weng M, Zeng W, Wu X, Zhang Y, Jiang M, Wang Z, et al. Quantification of serum hepatitis B surface antigen in predicting the response of pegylated interferon alfa-2a in HBeAg-positive chronic hepatitis B with prior lamivudine exposure. Virol J. 2013;10:1.
Janssen HLA, Sonneveld MJ, Brunetto MR. Quantification of serum hepatitis B surface antigen : is it useful for the management of chronic hepatitis B ? Gut. 2012;61:641–5.
Chan HL, Thompson A, Martinot-peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, et al. Review hepatitis B surface antigen quantification : why and how to use it in 2011—A core group report. J Hepatol. 2011;55:1121–31.
Alghamdi A, Aref N, El-Hazmi M, Al-Hamoudi W, Alswat K, Helmy A, et al. Correlation between hepatitis B surface antigen titers and HBV DNA levels. Saudi J Gastroenterol. 2013;19:252–7.
Sali S, Sharafi H, Hoda S, Moayed S, Etesam F. Can serum level of HBsAg differentiate HBeAg-negative chronic hepatitis B from inactive carrier state ? Diagn Microbiol Infect Dis. 2015;82:114–9.
Balkan A, Namıduru M, Balkan Y, Mete AÖ, Karaoğlan İ. Are serum quantitative hepatitis B surface antigen levels , liver histopathology and viral loads related in chronic hepatitis B—infected patients ? Saudi J Gastroenterol. 2016;22:208–14.
Nguyen T, Thompson AJV, Bowden S, Croagh C, Bell S, Desmond PV, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B : a perspective on Asia. J Hepatol. 2010;52:508–13.
Thompson AJV, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–44. https://doi.org/10.1002/hep.23571.
Michelle M‑P, Lapalus M, Laouénan C, Boyer N, -Pierre Ripault M, Asselah T, et al. How to distinguish HBeAg negative chronic hepatitis B, with high risk of reactivation, from inactive carriers: Is there a place for HBsAg quantification? Hepatology. 2012;56:434A–5A.
Tseng T, Liu C, Yang H, Su T, Wang C, Chen C, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–9.
Funding
This study was supported financially by Istanbul University Scientific Research Projects Coordination Unit (project code: TTU-2017-24508).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Yıldız Kaya, B. Mete, A. Kaya, I. I. Balkan, N. Saltoglu and Ö. F. Tabak declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the ethical committee of Istanbul University Cerrahpasa Medical Faculty (83045809-604.01.02)/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors meet the International Committee of Medical Journal Editors (ICMJE) authorship criteria.
Rights and permissions
About this article
Cite this article
Yıldız Kaya, S., Mete, B., Kaya, A. et al. The role of quantitative HBsAg in patients with HBV DNA between 2000–20,000 IU/ml. Wien Klin Wochenschr 133, 647–653 (2021). https://doi.org/10.1007/s00508-021-01854-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-021-01854-7