Summary
Nectins are immunoglobulin-like molecules that are involved in cell to cell adhesion by forming tight junctions and homophilic/heterophilic interactions. This study aimed to analyze serum nectin‑2 and nectin‑4 levels in lung cancer patients and to evaluate the prognostic, diagnostic and predictive strengths. Data from 74 lung cancer patients were retrospectively examined and enzyme-linked immunosorbent assays (ELISA) were used to measure serum nectin‑2 and nectin‑4 concentrations. A total number of 40 age and sex-adjusted healthy controls were also enrolled in the study. The median serum nectin‑2 and nectin‑4 levels of the patients were significantly higher than those of controls (p < 0.001); however, neither biomarker was found to be associated with clinicopathological parameters, (p > 0.05), and furthermore they were found not to be correlated with either overall survival or progression-free survival (p > 0.05). Even though both markers showed high diagnostic values, serum nectin‑2 was found superior to both serum nectin‑4 and serum nectin-2 + nectin‑4 combinations in the diagnosis of lung cancer according to higher sensitivity, specificity and predictive values. Serum nectin‑2 and nectin‑4 might be used in lung cancer diagnosis but the diagnostic importance of nectin‑2 is higher. The prognostic and predictive strengths in cancer are controversial. Furthermore, the interactions with tumor microenvironments and the potentials as therapeutic targets for malignancies have yet to be elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the foremost cause of cancer death among men and it is ranked second in cancer deaths among women globally [1]. In 2018, it is foreseen that 234,030 people (121,680 men and 112,350 women) will be newly diagnosed with lung cancer and, of these patients 154,050 (83,550 men and 70,500 women) will eventually die [2]. As for other types of cancers, several biomarkers have emerged as predictive, prognostic and diagnostic markers for lung cancer, especially for non-small cell lung cancer (NSCLC), such as ALK fusion oncogene, EGFR mutations and ROS1 gene rearrangements [3,4,5]. The need for accurate diagnosis and individualized treatment for lung cancer, just like for any cancer, necessitates the identification of such biomarkers to spare patients from unwanted morbidity and mortality while providing them with the most effective treatment.
The nectin family comprises a group of molecules (nectin‑1, nectin‑2, nectin‑3 and nectin-4) resembling immunoglobulins acting independently of Ca++ and they are mainly involved in cell to cell adhesion by forming tight junctions and homophilic/heterophilic interactions. They are in close relationship with actin cytoskeleton through afadin, which is an F‑actin-binding protein, and they form complexes with adjacent cells and each other to regulate numerous cellular events, such cellular adhesion, movement, and polarization [6,7,8]. They are ubiquitously expressed and have adhesive roles in adherence junctions, formed by transmembrane proteins, such as cadherins which are dependent on calcium that bind cells together within tissues [9, 10]. Nectin‑1, 2, and 3 are widely expressed, of which nectin‑1 and nectin‑2 are frequently associated with immune system organs and nectin‑3 is primarily expressed in the testes and placenta but nectin‑4 is expressed mainly in the embryo and placenta [11,12,13]. The association between nectins and various cancers as well as the potentials as therapeutic targets have also been investigated and it was documented that nectin‑2 and nectin‑4 are overexpressed in malignancies such as breast, ovarian, lung and colorectal cancers [14,15,16,17,18,19].
In the present study the serum levels of nectin‑2 and nectin‑4 were examined by enzyme linked immunosorbent assay (ELISA) in lung cancer patients in order to understand the diagnostic, prognostic and predictive values of these biomarkers.
Patients, materials and methods
Patients
A total number of 74 lung cancer patients (29 patients had squamous cell cancer, 34 patients had adenocancer, and 11 patients had small cell cancer) who had been admitted, treated and followed-up at the Institute of Oncology, Istanbul University between June 2014 and March 2016 were included in this study. The disease was staged according to the American Joint Committee on Cancer (AJCC) and International Union against Cancer (IUCC) staging systems. Detailed clinical history and physical examination along with a series of blood tests were completed prior to initiating treatment. The patients with Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and appropriate blood chemistry tests and complete blood count (i.e. absolute neutrophil count above 1500/μL and platelet count above 100,000/μL) were treated with multidisciplinary methods, such as surgery, radiotherapy (RT) alone, sequential chemotherapy/RT and concurrent chemotherapy/RT, as per the international guidelines and protocols indicated. The chemotherapy regimens were cisplatin-based and chemotherapy regimens used with radiation therapy were both cisplatin-based and carboplatin/paclitaxel. Clinical, laboratory, and radiological assessments were repeated every 6 weeks during chemotherapy and every 12 weeks following the completion of the treatment. The revised RECIST (Response Evaluation Criteria in Solid Tumors) criteria version 1.1 was used to assess the response of treatment. A total number of 40 age and sex-adjusted healthy controls were also enrolled in the study. Informed consent was obtained from all patients and the study was reviewed and approved by the regional ethics committee. Regional Ethics Committee and Number: Istanbul University, Institute of Oncology Regional Ethics Committee; Number: 2015/321.
Processing of serum samples and measurement of serum nectin-2 and nectin-4 levels
Blood samples were collected from the patients and the healthy controls on the first visit and then allowed to coagulate at room temperature. Following centrifugation at 4000 rpm for 10 min at room temperature, the sera were frozen immediately and stored at −80 °C until the time of the analysis. Nectin-2/human poliovirus receptor related protein (PVRL2/CD112) and nectin-4/human poliovirus receptor related protein (PVRL4) levels in the samples were measured using a double-antibody sandwich ELISA (Shanghai Sunred Biological Technology Co. Ltd). Serum samples and standards were placed into the wells that had been previously covered with human nectin‑2 and nectin‑4 monoclonal antibodies, then biotinylated-Fab monoclonal capture antibody and streptavidin horseradish peroxidase (HRP) conjugates were applied to form immune complexes at 37 ˚C for 1 h and unbound particles were washed away. The chromogen solution (colorless) was added and incubated at 37 ˚C for 10 min as maximum protection from light was provided and finally the colorless solution turned blue; the intensity of the conversion was proportional to the amount of nectin‑2 and nectin‑4 in the sample. The acidic stop solution was added and the color turned yellow and the colored end-product was measured using an automated ELISA microplate reader at 450 nm (ChroMate® 4300 microplate awareness technology, Palm City, FL, USA). The results were expressed as ng/ml.
Statistical analysis
Comparisons between continuous variables were done using Mann-Whitney U-test or Kruskall-Wallis test. The odds ratio (OR) was measured with logistic regression test. The progression-free survival (PFS) was calculated from the date of first admission to the radiologic or clinical progression. The overall survival (OS) was measured from the date of diagnosis to the date of death from any cause or to the last follow-up date. The Kaplan-Meier method was used to calculate the cumulative proportion of survival. When p ≤ 0.05 then statistically significant differences were assumed. The receiver operator characteristic (ROC) curves were drawn and the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for nectin‑2 and nectin‑4 and their combination. The statistical analysis was carried out using SPSS 21.0 software (SPSS, Chicago, IL, USA).
Results
A total of 74 lung cancer patients between June 2014 and March 2016 were included in this study. The median age was 60 years (ranging from 28 years to 78 years). The number of men was predominant (n = 62, 84%). Most of the patients were smokers (n = 63, 85%), without chronic obstructive pulmonary disease (COPD, n = 56, 76%), and presented with better ECOG performance scores (n = 64, 86%). The predominant pathology was adenocancer (n = 34, 46%) with advanced stages (n = 48, 65%). Most of the patients did not undergo surgery (n = 61, 82%), received chemotherapy (n = 61, 82%) without interruptions of any cause (n = 55, 74%), and the majority had a disease course without either local recurrence (n = 54, 73%) or metastasis (n = 48, 65%) and most of the people were still alive at the end of the analysis (n = 47, 64%) (Table 1).
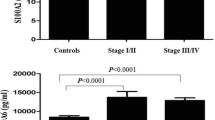
Serum nectin‑2 and nectin‑4 levels were significantly higher in the patients than the control group (median levels: 5.0 ng/mL vs. 0.650 ng/mL, p < 0.0001 and 2.45 ng/mL vs. 1.15 ng/mL, p < 0.001, respectively) (Table 2 and Figs. 1 and 2). Table 3 displays the correlation between serum nectin‑2 and nectin‑4 values and various clinicopathologic variables. As for nectin‑2, of these variables only the stage in NSCLC showed a significant correlation with the level of the biomarker. On the other hand, nectin‑4 level was correlated with the stage in NSCLC, the history of surgery, the tumor size and the presence of metastasis (Table 3).
Neither nectin‑2 nor nectin‑4 had a significant statistical association with OS and PFS in any of the patient groups, i.e. all patients, metastatic and nonmetastatic patients (p > 0.05) (Table 4). Significant relationships between OS and other clinicopathologic variables, such as pathology (squamous cell cancer vs small cell cancer and adenocancer vs small cell cancer), stage (for both non-small cell cancers and small cell cancers), history of surgery, history of radiotherapy, presence of metastasis, were found (Table 4). As for PFS, there were significant relationships with some other clinicopathologic variables, such as history of smoking, pathology (squamous cell cancer vs small cell cancer and adenocancer vs small cell cancer), stage (only for NSCLC), history of surgery, history of chemotherapy, interruption of chemotherapy (Table 4).
The cut-off values and the sensitivity, positive and negative predictive values of nectin‑2 and nectin‑4 in lung cancer patients were determined by using ROC curves. The cut-off levels were decided by accepting a sensitivity levels of about 90% using ROC curves and the cut-off points were set for nectin-2 = 1.45 ng/mL and nectin-4 = 1.5 ng/mL. The areas under the curve were for nectin-2 = 0.802 (95% confidence interval, CI: 0.68–0.90) and nectin-4 = 0.816 (95% CI: 0.72–0.96) (Fig. 3). According to these cut-off points, the sensitivity, specificity, PPV and NPV of nectin‑2, nectin‑4 and their combination were calculated (Table 5).
Discussion
This study found that serum nectin‑2 and nectin‑4 concentrations in 74 lung cancer patients were significantly higher than those of 40 healthy controls (5.0 ng/mL vs. 0.650 ng/mL, p < 0.0001 and 2.45 ng/mL vs. 1.15 ng/mL, p < 0.001, respectively) (Table 2; Figs. 1 and 2). While only the stage in NSCLC showed a significant correlation with the serum level of nectin‑2, nectin‑4 level was correlated with the stage in NSCLC, the history of surgery, the tumor size and the presence of metastasis (Table 3). Neither biomarker was found prognostic in groups (all, metastatic, nonmetastatic patients) regarding survival and progression.
Serum nectin‑2, with higher sensitivity and specificity than serum Nectin‑4 (91.9% vs. 70% and 92.5% vs. 85%, respectively), was determined to be a more effective biomarker than serum nectin‑4 not only in identifying individuals with the disease but also those without the disease. The combination of serum nectin‑2 and nectin‑4 (sensitivity 80% and specificity 87%) was found to be more accurate in diagnosis of lung cancer than nectin‑4 alone but it was still inferior to serum nectin‑2 alone (Table 5). When these three entities were compared regarding PPV and NPV, serum nectin‑2 alone had the highest values; PPV 95.8% and NPV 86%. When combined with serum nectin‑4 it lost some strength; PPV 93% and NPV 70%, but the combination still had superiority to serum nectin‑4 alone; PPV 90% and NPV 60%, (Table 5). All of these results show that people with a positive serum nectin‑2 test actually have the disease and on the other hand people with negative serum nectin‑2 test are disease-free. As for serum nectin‑4 the test still has some strength in identifying the people without the disease (specificity 85%); however, it was found to be far inferior to serum nectin‑2 in identifying individuals with the disease (sensitivity 70%) and 90% of the people with a positive serum nectin‑4 actually had the disease (PPV 90%), but on the other hand only 60% of individuals with a negative serum nectin‑4 test were cancer-free (NPV 60%).
Nectin‑2, also known as CD112, has already been identified as an adhesion molecule that plays important parts in cell junctions and interacts with not only other nectin family members but also with numerous other molecules, which result in downstream signalling associated with vascular endothelial growth factor receptor (VEGFR-2) [20]. The expression of nectin‑2 on outgrowth endothelial cells (OEC), which are a subpopulation of endothelial progenitor cells (EPC) that circulate in the blood and promote proliferation and angiogenesis, indicates that nectin‑2 also influences cell proliferation and angiogenesis by regulating OEC. It was also reported that nectin‑2 knockdown resulted in a decrease of VE-cadherin, another adhesion molecule that regulates endothelial cell to cell interactions and restricts proliferation by holding VEGFR-2 at the membrane thus preventing internalization and signalling cascades, and VEGFR‑2 levels in OECs [20, 21]. Given these findings, nectin‑2 on OECs is believed to impair cell migration and inhibit tube formation and angiogenesis mediated by OECs. The observation of the compensatory upregulation of p-Erk (a cell proliferation signal) and nectin‑1, nectin‑3 and nectin-like molecule 4 (Necl-4) (promoters of VEGFR-mediated signalling causing proliferation, migration and tube formation) in nectin‑2 deficiency might explain the inhibitory effects of nectin‑2 on OECs [20].
Serum nectin‑2 level was found to be diagnostic in colorectal cancer patients and its poor prognostic value on PFS was observed in non-metastatic patients but not in metastatic patients [19]. Similarly, nectin‑2 and nectin‑4 expressions were found to be associated with the aggressiveness and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinoma of the gall bladder and pancreatic ductal adenocarcinoma [22,23,24]. Nectin‑2 expression was determined as an independent poor prognostic indicator for pancreatic ductal carcinoma. While the loss of Necl‑4 expression (one of the five members of the Necl family, which are immunoglobulin-like adhesion molecules interacting with nectins) was independently associated with poorer prognostic features, such as larger tumor, lymphatic involvement, an advanced disease and advanced histological grade, and a short survival in pancreatic ductal adenocarcinoma and colorectal adenocarcinoma, the diffuse nectin‑3 expression on the other hand was correlated with a favorable prognosis in pancreatic adenocarcinoma, and yet it is associated with poorer prognostic features in lung adenocarcinoma, such as higher incidence of pleural invasion and metastasis, provided the tumor expressed membranous nectin‑3 and did not co-localize with E‑cadherin [24,25,26,27]. These confusing findings about the roles of nectin family members in malignancies might stem from the differences of tumor histology. Nevertheless, these findings may also suggest that early stage aggressive malignancies with strong metastasis potentials and the offspring metastases that share the same histopathological features with the primary tumor have a high tendency to express higher nectin‑2 and nectin‑4 levels. Thus, the authors believe that early stage higher grade malignancies may express higher levels of nctin‑2 and nectin‑4 compared to advanced stage but lower grade malignancies.
Since serum levels of nectin‑4 were associated with the numbers of metastases and the therapeutic efficacy in breast and lung cancers, it was suggested that nectin‑4 be considered as a diagnostic and prognostic biomarker both in tissue and serum and as a therapeutic target in malignancies [17, 18]. Similarly, membranous nectin‑4 expression was correlated with a decreased metastasis-free survival in node-negative luminal‑A early breast cancer patients, thus it might be used as a reliable indicator for distant metastasis and as a potential target for treatment in these patients [28].
Nectin‑4 was found to be a potential target for antibody drug conjugates (ADC) therapy for malignancies expressing nectin‑4 [29]. The preclinical results showed growth inhibition and tumor regression and these findings suggested that a nectin‑4 targeted cytotoxic agent delivery may enhance the effect. In this study nectin‑4 overexpression was demonstrated in human tumor specimens, such as breast, bladder, pancreas and lung tissue; however, the question of whether the source of the enhanced nectin levels is the tumor cells or endothelial cells has yet to be answered. Since this study analyzed the serum of the patients for nectin‑2 and nectin‑4 instead of tumor specimens it is harder to answer this question. Probably it is a combined result from both the tumor cells and endothelial cells. A special study on this question should be designed.
The present study showed that both serum nectin‑2 and nectin‑4 levels were significantly higher in lung cancer patients. Nectin‑2 was associated only with the stage in NSCLC, whereas nectin‑4 was correlated with the stage in NSCLC, the history of surgery, the tumor size and the presence of metastasis. These findings suggest that both markers might be considered as having diagnostic values for lung cancer, and nectin‑4 might be more promising at determining the tumor behavioral pattern than nectin‑2. Even though the serum values of both markers are elevated in cancer patients, nectin‑2 is of more diagnostic importance according to the sensitivity and specificity. It was determined that nectin‑2 was more accurate not only in the diagnosis in individuals with lung cancer but also in ruling out those without the disease. Neither biomarker was found to be prognostic for either OS or PFS. The serum nectin-2 + nectin‑4 combination did not surpass nectin‑2 alone in diagnostic value, although the combination was found to be superior to nectin‑4 alone. This diagnostic superiority of nectin‑2 continued according to the predictive values. Notwithstanding the superiority of nectin‑2, the diagnostic values of nectin‑4 and nectin-2 + nectin‑4 combination were not low. The small sample size and short follow-up period might have undermined the analysis and be considered as major limitations of the present study. For instance, it would be interesting to analyze small cell cancer patients and NSCLC patients separately provided the number of patients were sufficient. The other limitation was that the control group consisted of healthy individuals that were merely adjusted for age and sex. The control could have been adjusted for conditions like COPD or smoking history as well. In such a non-cancer control cohort a tumor marker for lung cancer development would seem more relevant than using a group of completely healthy individuals.
In conclusion, it is believed that although both serum nectin‑2 and nectin‑4 are diagnostic in lung cancer, nectin‑2 is a stronger diagnostic indicator. It is also suggested that neither biomarker is either prognostic or predictive since they do not show any correlation with either survival or chemosensitivity. The present study contributes to the literature in that to the best of our knowledge this study is one of the very few studies examining serum nectin‑2, nectin‑4 and their combined levels in lung cancer patients. Further trials in larger patient populations are necessary to detect the clinical importance of these biomarkers in lung cancer patients.
References
Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19.
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7–30.
Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in Crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–8.
Douillard JY, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. 2014;110:55–52.
Besse B, Adjei A, Baas P, Meldgaard P, Nicolson M, Paz-Ares L, et al. Panel Members; ESMO. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475–1474.
Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–15.
Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol. 2007;19:593–592.
Yamada A, Fujita N, Sato T, Okamoto R, Ooshio T, Hirota T, et al. Requirement of nectin, but not cadherin, for formation of claudin-based tight junctions in annexin II-knockdown MDCK cells. Oncogene. 2006;25:5085–5082.
Morita H, Nandadasa S, Yamamoto TS, Terasaka-Iioka C, Wylie C, Ueno N. Nectin‑2 and N‑cadherin interact through extracellular domains and induce apical accumulation of F‑actin in apical constriction of Xenopus neural tube morphogenesis. Development. 2010;137:1315–25.
Yamada A, Irie K, Fukuhara A, Ooshio T, Takai Y. Requirement of the actin cytoskeleton for the association of nectins with other cell adhesion molecules at adherens and tight junctions in MDCK cells. Genes Cells. 2004;9:843–55.
Nakanishi H, Takai Y. Roles of nectins in cell adhesion, migration and polarization. Biol Chem. 2004;385:885–882.
Reymond N, Fabre S, Lecocq E, Adelaïde J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001;276:43205–15.
Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, et al. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C‑C′-C″-D beta-strands of the nectin1 V domain. J Biol Chem. 2002;277:27006–13.
Matsushima H, Utani A, Endo H, Matsuura H, Kakuta M, Nakamura Y, et al. The expression of nectin-1alpha in normal human skin and various skin tumours. Br J Dermatol. 2003;148:755–62.
Athanassiadou AM, Patsouris E, Tsipis A, Gonidi M, Athanassiadou P. The significance of Survivin and Nectin‑4 expression in the prognosis of breast carcinoma. Folia Histochem Cytobiol. 2011;49:26–33.
Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol. 2010;134:835–45.
Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestier C, Reymond N, et al. Nectin‑4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer. 2007;7:73.
Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, et al. Identification of nectin‑4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69:6694–703.
Karabulut M, Gunaldi M, Alis H, Afsar CU, Karabulut S, Serilmez M, et al. Serum nectin‑2 levels are diagnostic and prognostic in patients with colorectal carcinoma. Clin Transl Oncol. 2016;18:160–71.
Son Y, Lee B, Choi YJ, Jeon SA, Kim JH, Lee HK, et al. Nectin‑2 (CD112) is expressed on outgrowth Endothelial cells and regulates cell proliferation and Angiogenic function. PLoS ONE. 2016; https://doi.org/10.1371/journal.pone.0163301.
Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR‑2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604.
Miao X, Yang ZL, Xiong L, Zou Q, Yuan Y, Li J, et al. Nectin‑2 and DDX3 are biomarkers for metastasis and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Int J Clin Exp Pathol. 2013;6:179–90.
Liang S, Yang Z, Li D, Miao X, Yang L, Zou Q, et al. The clinical and pathological significance of Nectin‑2 and DDX3 expression in pancreatic ductal Adenocarcinomas. Dis Markers. 2015; https://doi.org/10.1155/2015/379568.
Izumi H, Hirabayashi K, Nakamura N, Nakagohri T. Nectin expression in pancreatic adenocarcinoma: nectin‑3 is associated with a poor prognosis. Surg Today. 2015;45:487–94.
Kawanishi A, Hirabayashi K, Yamada M, Takanashi Y, Hadano A, Kawaguchi Y, et al. Clinicopathological significance of Necl‑4 expression in pancreatic ductal adenocarcinoma. J Clin Pathol. 2016; https://doi.org/10.1136/jclinpath-2016-204028.
Jang SM, Han H, Jun YJ, Jang SH, Min KW, Sim J, et al. Clinicopathological significance of CADM4 expression, and its correlation with expression of E‑cadherin and Ki-67 in colorectal adenocarcinomas. J Clin Pathol. 2012;65:902–6.
Maniwa Y, Nishio W, Okita Y, Yoshimura M. Expression of nectin 3: novel prognostic marker of lung adenocarcinoma. Thorac Cancer. 2012;3:175–81.
Lattanzio R, Ghasemi R, Brancati F, Sorda RL, Tinari N, Perracchio L, et al. Membranous Nectin‑4 expression is a risk factor for distant relapse of T1–T2, N0 luminal—A early breast cancer. Oncogenesis. 2014; https://doi.org/10.1038/oncsis.2014.32.
Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, et al. Enfortumab Vedotin antibody-drug conjugate targeting Nectin‑4 is a highly potent therapeutic agent in multiple Preclinical cancer models. Cancer Res. 2016;76:3003–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Erturk, S. Karaman, N. Dagoglu, M. Serilmez, D. Duranyildiz and F. Tas declare that they have no conflict of interest relating to the publication of this manuscript.
Ethical standards
K. Erturk, S. Karaman, N. Dagoglu, M. Serilmez, D. Duranyildiz, and F. Tas declare that the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study. Regional Ethics Committee and Number: Istanbul University, Institute of Oncology Regional Ethics Committee; Number: 2015/321.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erturk, K., Karaman, S., Dagoglu, N. et al. Serum nectin-2 and nectin-4 are diagnostic in lung cancer: which is superior?. Wien Klin Wochenschr 131, 419–426 (2019). https://doi.org/10.1007/s00508-019-01537-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-019-01537-4