Abstract
Psoriasis is a chronic inflammatory skin disease. It is associated with changes in skin microbiome. The aim of this study was to evaluate how Lake Hévíz sulfur thermal water influences the composition of microbial communities that colonizes skin in patients with psoriasis. Our secondary objective was to investigate the effects of balneotherapy on disease activity. In this open label study, participants with plaque psoriasis underwent 30-min therapy sessions in Lake Hévíz, at a temperature of 36 °C, five times a week for 3 weeks. The skin microbiome samples were collected by swabbing method from two different areas (lesional skin-psoriatic plaque and non-lesional skin). From 16 patients, 64 samples were processed for a 16S rRNA sequence-based microbiome analysis. Outcome measures were alpha-diversity (Shannon, Simpson, and Chao1 indexes), beta-diversity (Bray–Curtis metric), differences in genus level abundances, and Psoriasis Area and Severity Index (PASI). Skin microbiome samples were collected at baseline, and immediately after treatment. Based on the visual examination of the employed alpha- and beta-diversity measures, no systematic difference based on sampling timepoint or sample location could be revealed in these regards. Balneotherapy in the unaffected area significantly increased the level of Leptolyngbya genus, and significantly decreased the level of Flavobacterium genus. A similar trend was revealed by the results of the psoriasis samples, but the differences were not statistically significant. In patients with mild psoriasis, a significant improvement was observed in PASI scores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The microbiome is an ecological system made up of commensal, symbiotic, and pathogenic microorganisms living in the human body. The microbial flora normally occurring in different regions of the human skin has been investigated under the Human Microbiome Project (HMP) since 2008 (Grice et al. 2008). Different body parts have distinct flora; however, it is fairly consistent within the human population, although individual differences exist. Compared to culture-based techniques, genomic studies confirmed the presence of many more bacteria. Bacteria found on the skin can be classified into four major phylums: Actinobacteria, Firmicutes, Bacteroidetes, Proteobacteria; the differences lie in their distribution. While in the intestines, the dominant phylums are Firmicutes and Bacteroidetes, the dominant phylum of the skin is Actinobacteria (Whittle et al. 2019).
Sequencing-based metagenomics is suitable for determining the exact composition of complex microbial communities without having to isolate and culture individual members of the community. Tests are based on the fast and high throughput determination of DNA extracted from communities using next-generation sequencing (NGS), which can be used to examine the genome of the entire community, including non-culturable microbes. Sequencing enables comprehensive examination of all genes of all microorganisms present, even in complex samples (Rogers and Bruce 2010).
Balneotherapy has been used for a long time in the treatment of dermatological diseases (Huang et al. 2018; Cacciapuoti et al. 2020). Available literature mainly discusses balneotherapy for psoriasis and atopic dermatitis (Péter et al. 2017; Darlenski et al. 2021). The keratolytic effect of sulfur water is well known, that is why it is used for the treatment of psoriasis. Hydrogen sulfide (H2S) has a specific molecular effect on normal human keratinocytes (Gross-Amat et al. 2020). In addition to balneotherapy, balneophototherapy is also a well-established treatment procedure for patients with dermatological conditions, but data are already available even on the combined beneficial effects of concomitant biological therapy and balneotherapy (Peinemann et al. 2021; Cozzi et al. 2015; Halevy et al. 2001).
According to a recently published study, diversity of skin microbiota correlates with PASI score (Assarsson et al. 2018). Moderate to severe psoriasis and mild psoriasis differ in their microbiota composition (Hu et al. 2022). When comparing skin microbiome of healthy controls and patients with psoriasis, increased amounts of Firmicutes and decreased amounts of Actinobacteria were found in patients with psoriasis, which mostly appeared on the lesional skin and was at least partially reversed with systemic treatment (Yerushalmi et al. 2019). Presence of Prevotella and Staphylococcus showed significant association with lesional skin while the presence of Anaerococcus and Propionibacterium was associated with non-lesional skin. There was no significant difference in the amounts of bacteria cultured from the skin of healthy controls and patients with psoriasis (Langan et al. 2019). In the literature, only one human balneotherapy study was found assessing the effects of thermal water on skin microbiome in psoriasis patients (Martin et al. 2015).
The objectives of the study
The primary objective of the study is to assess and compare the microbiome composition of psoriasis-affected and unaffected skin and to investigate the effects of sulfur thermal-mineral water of Lake Hévíz on the microbiome of psoriatic plaques and unaffected skin areas.
The secondary objective of the study is to assess the effects of balneotherapy on the activity of psoriasis.
Patients and methods
The study protocol followed the principles of the Helsinki Declaration. This study was approved by the relevant Scientific and Research Ethics Committee (i.e., ETT TUKEB; its approval Number: 31262–6/2019/EKU), Clinical trial registration ID: NCT05431959. The study was also approved by the Institutional Research Ethics Committee.
Inclusion criteria
This study enrolled male and female patients between 18 and 70 years of age with plaque psoriasis diagnosed at least 6 months before the pre-treatment visit by a dermatologist.
Exclusion criteria
Exclusion criteria included subjects that are on systemic therapy for psoriasis, balneotherapy within 3 months prior to enrollment, and local or systemic steroid therapy within 1 month prior to enrollment. General contraindications to balneotherapy include decompensated cardiovascular status, uncontrolled endocrine and metabolic disorders, severe diseases affecting the internal organs, acute febrile infections, pregnancy, and decompensated mental illnesses.
Recruitment of the patients
After verbal agreement, the rheumatologists of the St. Andrew Hospital for Rheumatic Diseases invited the patients with plaque psoriasis to participate the study, bearing in mind the inclusion and exclusion criteria. Before enrollment, participants were informed verbally and writing about the purpose, procedures of the study. Prior to enrollment, subjects read the Patient Information Sheet and signed the Informed Consent Form. The rheumatologist cross-checked the inclusion and exclusion criteria before the study.
Intervention
The study took place at Spa Hévíz and St. Andrew Hospital for Rheumatic Diseases (H-8380 Hévíz, Dr. Schulhof Vilmos sétány 1, Hungary).
Participants underwent 30-min balneotherapy sessions in Lake Hévíz, at a temperature of 36 °C, five times a week for 3 weeks. The enrolled patients received the treatment as outpatients. They were able to continue their daily activities and work. Participants did not receive any other physiotherapy. During the study, further systemic or local therapy was not allowed. Patients were assessed two times, just before treatment (pre-treatment) and after three weeks (post-treatment). The members of the Lake Hévíz Emergency Medicine Team were available during of balneotherapy sessions in case of emergency. The rheumatologists were on call to deal with side effects or other treatment-related problems. After consulting a dermatologist, the examining rheumatologist prepared a standardized photo documentation, which formed the basis of evaluating the Psoriasis Area and Severity Index (PASI). The samples were collected in the Laboratory Department of St. Andrew Hospital by clinical laboratory specialist. Before the study, the study personnel and the laboratory performing the analysis of the samples standardized and practiced the methodology of the sample collection.
Thermal water composition
The spring of Lake Hévíz is located at the bottom of the vertical sand wall, at 38.5 m depth. Water output of the lake is 420 L/s, and the water transposes in 3–3.5 days. The temperature of the lake reaches 37–38° in the summer, and is never lower than 22–23 °C during winter. The thick layer of mud covers the bed of the lake, which contains both organic and inorganic substances. The thermal mineral water of Lake Hévíz containing carbonate, sulfur, calcium, magnesium, hydrogen carbonate, and very low radon. During the study, the temperature of the lake was 36 ℃. The total mineral content of the water was 754 mg/L, the sulfide content was 3.2 mg/L. The mineral composition of Lake Hévíz water is shown in Table 1.
Materials
Samples were collected from 2 different areas of the patient’s body (1. lesional skin, psoriatic plaque—elbow, 2. control non-lesional skin—right antecubital fossa), pre- and post-treatment with a Swab Collection and DNA Preservation System (Norgen, ON, Canada). Microbial DNA was isolated using the Microbiome DNA Isolation Kit (Norgen, ON, Canada). The purification is based on spin column chromatography. First, the swab collection tube was incubated at 65 °C with the presence of Lysis Additive in order to efficiently and rapidly homogenize the sample. The sample was then centrifuged, and the supernatant was transferred to a DNase-free microcentrifuge tube. Binding buffer was added, and the lysate was incubated for 10 min on ice. The lysate was then spun for 2 min to pellet any cell debris, the supernatant was collected, an equal volume of 70% ethanol was added to the lysate and the solution was loaded onto a spin-column. The bound DNA was then washed using the provided binding buffer and wash solution, and the purified DNA was eluted using the elution buffer. The concentration of the isolated DNA was determined using the Qubit dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA).
DNA amplicon library was prepared using the Ion 16S Metagenomics Kit (Life Technologies, CA, USA). The kit includes 2 sets of primers that can be used to amplify the corresponding hypervariable regions of the 16S rDNA gene in bacteria: primer set V2-4–8 and primer set V3-6,7–9. The primer sets are paired with Environmental Master Mix v2.0 that is optimized to tolerate high levels of PCR inhibitors and amplify targets from complex samples. After pooling and purification of the amplicons with Agencourt AMPure XP reagent (Beckmann Coulter, CA, USA), end repair was carried out. In the next step, sequencing adapters and sample-specific barcodes were ligated to each amplified DNA sample. After final purification, the concentration of the final library was determined by qPCR method run on QuantStudio instrument (Life Technologies, CA, USA).
Template preparation was performed with Ion OneTouch kit (Life Technologies, CA, USA) on semiautomated Ion OneTouch instrument using an emPCR method. After adding the sequencing primer and polymerase, the Ion Sphere Particle (ISP) beads were loaded into an Ion 520 sequencing chip, and the sequencing runs were performed using the Ion S5 Sequencing kit (Life Technologies, CA, USA) with 850 flows.
Outcome parameters, bioinformatics, and statistical methods
Quality evaluation of raw reads was performed with FastQC 0.11.9 (Andrews 2018). Reads were quality trimmed with Trimmomatic v0.39 (Bolger et al. 2014), using the following parameters: LEADING:3, TRAILING:20, SLIDINGWINDOW:4:20, MINLEN:50. After quality control, fastq files were dereplicated and chimera filtered with vsearch v2.21.1 (Rognes et al. 2016). Taxon classification of the reads was performed with Kraken2 v2.1.2 (Wood et al. 2019) to the GreenGenes database (DeSantis et al 2006). Analysis of the sample bacteriome compositions was conducted in R environment with the aid of phyloseq (McMurdie and Holmes 2013) and microbiome (Lahti et al. 2019) R Bioconductor packages. Both Bacteria and Archea kingdoms were used for downstream analysis, with reads agglomerated at the genus level. Community compositions were evaluated in each sample with the Shannon (Shannon 1948), Simpson (Simpson 1949), and Chao1 (Chao 1984) alpha-diversity measures. Beta-diversity was measured with Bray–Curtis (Bray and Curtis 1957) dissimilarities and visualized through NMDS ordination (Kruskal 1964).
Differences in genus abundances were evaluated for the most dominant genera, determined with the core function of the microbiome R package (Lahti et al. 2019), with detection 100 and prevalence 0.1. Statistical analysis of differential abundance of these dominant genera was performed with paired DESeq2 (Love et al. 2014) analysis. Three comparisons were made: normal samples compared pre-treatment and post-treatment, psoriasis samples compared pre-treatment and post-treatment, and the comparison of normal and psoriasis samples pre-treatment.
Psoriasis Area and Severity Index (Fredriksson and Pettersson 1978) data were entered into and analyzed with MS Excel software. Statistical comparisons were made using single-sample (paired) t tests. The level of significance was set at p < 0.05.
Results
Recruitment of patients began in July 2021 and the study was launched at the end of August 2021. Of the 16 patients included in the study, 16 completed more than 80% of the therapy course. Demographic and baseline clinical characteristics of patients are shown in Table 2.
The balneotherapy was well tolerated and no adverse reactions were observed.
Psoriasis area and severity index
Within-group comparison to baseline showed significant improvement of Psoriasis Area and Severity Index (95% CI − 3.3 to − 0.8) p = 0.006011. Five patients had disease activity above Psoriasis Area and Severity Index (PASI) score of 10; in response to balneotherapy, disease activity decreased in two patients in this group, while in patients with PASI score < 10, all patients improved with one exception.
Alpha- and beta-diversity
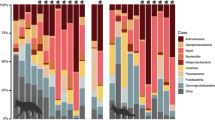
Genus level alpha-diversity of samples were evaluated with Shannon-, Simpson and Chao1 indexes (Figs. 1, 2, and 3). Based on these metrics, neither sampling timepoint (pre- or post-treatment), nor sample location (lesional skin or non-lesional skin) had shown systematic differences in sample genus composition complexities.
Inter-sample genus composition differences were assessed by the Bray–Curtis beta-diversity metric. Bray–Curtis dissimilarities were visualized in the 2-dimensional space by NMDS ordination (Fig. 4). Similarly to alpha-diversity, treatment and sample location have not affected genus composition differences. Samples from the same patient have clustered relatively close to each other in some cases (e.g., patient 02), or only the samples before treatment can be found close together (e.g., in the case of patient 03 or 04). In most cases, however, samples from the same patient can be located far from each other in the 2-dimensional space.
Differential abundance of the dominant bacterial genera
Differences in genus abundances of samples were evaluated on the most dominant genera (determined at detection 100 and prevalence 0.1 of the core function in the microbiome R Bioconductor package). Differences in genus abundances were compared between control samples pre-treatment and post-treatment, psoriasis samples pre-treatment and post-treatment, and control and psoriasis samples pre-treatment. Significant differences at the FDR level were found in Leptolyngbya (log2FC = 4.5804, p value (FDR corrected) < 0.0001) and Flavobacterium (log2FC = − 1.7835, p value (FDR corrected) = 0.0496) genus in control, non-lesional skin regions between treatments (positive log2 Fold Change indicates higher relative abundance post-treatment). Considering the rest of the dominant genera found in our analysis, we could not reveal any significant differences. More details in abundance differences can be found in Tables 3, 4, and 5.
Discussion
Climatotherapy and balneotherapy have already been proven as effective means of achieving clinical improvement in psoriasis. Severe adverse events following this type of therapy are rare (Timis et al. 2021). The results of our study confirm the beneficial effects of balneotherapy in plaque psoriasis. In healthy skin areas, the amounts of Leptolyngbya genus dramatically increase, whereas the amounts of Flavobacterium genus decrease as a result of bathing. The same tendency is observed in lesional psoriatic areas; however, significance has disappeared after correcting the p-values for multiple comparisons.
Leptolyngbya is a genus of Cyanobacteria phylum. Flavobacterium is a genus of Bacteroidota phylum. Only very few human studies were published on Leptolyngbya and Flavobacterium genera. Leptolyngbya is mainly found in aquatic environments, including marine, in ground and river water, and its antioxidant and anticancer effects have been shown in in vitro studies (Senousy et al. 2020). Examination of a Tunisian thermal spring showed that the Leptolyngbya species have abundant natural antioxidant properties which may have prophylactic and therapeutic effects on many diseases and toxicities (Trabelsi et al. 2016). Studying the microbiome composition of the thermal mud from Balaruc spa revealed that Cyanobacteria extracts may have anti-inflammatory therapeutic potential, including inhibition of the secretion of proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8 (Demay et al. 2020). Five types of Cyanobacteria strains were isolated and demonstrated antioxidant, anti-inflammatory, and anti-proliferative effects, which seems promising in the local treatment of psoriasis (Lopes et al. 2020). In a recently published paper, it was suggested that Cyanobacteria may clinically cause a so-called thermal crisis or balneointoxication, but our work has proven the opposite (Cobo et al. 2022).
Even fewer human studies were published on Flavobacteria genus than on Leptolyngbya genus. Their pathological role is unclear, but by being Gram-negative, they can be the source of several infections (Beathard et al. 2021; Mosayebi et al. 2011). It is also interesting to note that the Flavobacterium genus content in the intestine of rheumatoid arthritis patients is higher than that of healthy controls (Yu et al. 2022). It is known that Flavobacterium species are regularly found together with Cyanobacteria. They can inhibit or enhance the growth of Cyanobacteria and degrade compounds synthesized by Cyanobacteria. The ecological implications of these close interactions remain largely unknown (Péquin et al. 2022).
Our study was among the first to investigate the effects of balneotherapy on skin microbiome. In a previous study, the bacterial and fungal characteristics of the skin of healthy volunteers were examined during Dead Sea climatotherapy. After sun exposure and Dead Sea bathing for a 21-day period, diversity of the healthy human skin bacterial microbiome remained undisturbed, while fungal diversity significantly decreased (Brandwein et al. 2018). French authors investigated the effects of 3-week balneotherapy (high-pressure filiform shower, bath, facial, and body spray treatment) on skin microbiome in patients with psoriasis. Our results confirm what they observed, specifically that the bacterial communities were similar on psoriatic and non-lesional skin before treatment and at the end of the third week. They noticed that balneotherapy on both unaffected and adjacent affected skin significantly increased the level of Xanthomonas genus, and a lesser increase in the level of Corynebacterium genus. The Xanthomonas strain belongs to the Xanthomonadaceae family, which is part of the Proteobacteria phylum community known for its keratolytic effect. Disease activity of psoriasis improved after balneotherapy treatment (Martin et al. 2015). This study was conducted using medicinal water containing bicarbonate, calcium, silicate, and selenium, while our study was carried out with sulfur-containing water.
In the recent decades, the microbial diversity of thermal spring waters has been extensively investigated by a combination of traditional and new molecular biological approaches. Studies of microbial populations inhabiting mat communities of hot springs in Yellowstone National Park have been conducted, which showed the dominance of Cyanobacteria and Chloroflexi in the area (Ward et al. 1998, 2006). The major phylotypes, including Proteobacteria, Bacteroidetes, Cyanobacteria, and Firmicutes have also been reported in other thermal springs at various geographical locations (Hedlund et al. 2012; Kambura et al. 2016; Ghilamicael et al. 2017; Saghatelyan et al. 2021).
The development of molecular biological techniques has provided more information about the microbial communities of thermal springs and their taxonomic diversity, their adaptation mechanisms, and functional roles. Based on the literature biogeography and geological history, along with abiotic factors such as temperature, pH, and mineralization, these factors will collectively contribute to the dynamics and structure of microbial populations (Li et al. 2015; Saghatelyan et al. 2021; Pedron et al. 2022).
Cyanobacteria obtain their energy through photosynthesis and some cyanobacterial species are involved in the global nitrogen cycles by fixing atmospheric nitrogen. Environmental factors influence the dynamic, physiological characteristics, and metabolic profiles of Cyanobacteria. This results in their ability to adapt and survive in diverse ecosystems. Cyanobacteria produce many secondary metabolites, which are considered to be rich sources for drug discovery and development (Li et al. 2020). Thermal springs harbor potential thermotolerant Cyanobacteria strains for biotechnological applications and many new thermophilic bacterial strains have been isolated from these springs and evaluated for their biotechnological potential (Amarouche-Yala et al. 2014; Saghatelyan et al. 2021; Keshari et al. 2022).
Avène Thermal Spring Water from the Montagne Noire in France is used to treat inflammatory skin diseases. Over a 4-year sampling period, in-depth prospection of Avène thermal spring water reveals a stable microbial community, with a relatively constant richness of species. Nitrospirae and Proteobacteria phyla were the most prevalent (Bourrain et al. 2020). A biological concentrate, from culture of an Avène aquatic microflora isolate namely Aquaphilus dolomiae, showed immunomodulatory, anti-inflammatory, antipruritic, and tolerogenic activities in atopic dermatitis pharmacology models (Nguyen et al. 2017).
The bacterial communities of Lake Hévíz were studied between 2009 and 2015, using various microbial methods such as denaturing gel electrophoresis, direct cell count determination, cultivation, molecular cloning, pyrosequencing. Significant seasonal differences were noticed regarding the total bacterial community composition. Due to the high output of the spring, no clear vertical stratification was observed in the bacterial communities. From the water of Lake Hévíz besides the aerobic or facultative anaerobic heterotrophic bacteria (e.g., Aquiluna rubra, Rheinheimera aquatic, Polynucleobacter cosmopolitanus, Polynucleobacter acidiphobus, Gemmobacter lanyuensis), dominant and permanent presence of chemolithotrophic (e.g., Thiobacillus) and photolithotrophic (e.g., Cyanobacteria and Chloroflexi) autotrophic bacteria were identified (Krett et al. 2016).
First published in the literature, Italian authors characterized the microbial community of sulfurous-bromine-iodine thermal water, flowing from springs to points of use, and of muds during the various maturation stages using next-generation sequencing technologies. Despite the slight fluctuations in genera variety and abundance caused by temperature variations along the pipelines, their data highlighted the presence of a typical microbial community, mainly composed of sulfur-cycling bacteria (Desulfomonile, Thermodesulfovibrio, Geothermobacterium, Thermus, Thiofaba, and Syntrophomonas genera). The three predominant genera in the microbial community of mud were Pelobacter, Desulfomonile, and Thiobacillus. The abundance of these genera varied during the maturation process. The results showed that the microbiome of mature mud was dominated by Pelobacter genus capable of lipid biosynthesis, suggesting that these bacteria may play a role in the anti-rheumatic properties of thermal mud (Paduano et al. 2018).
Overall, our study highlights the importance of skin microbiome and the beneficial effects of balneotherapy in psoriasis patients. Further studies with larger number of patients are needed to clarify how waters with different mineral content, concentration, and bacterial communities affect the skin microbiome system.
Limitation of the study
Only 16 patients were enrolled into our study. This sample size is obviously not enough for a detailed statistical analysis, but the obtained trend clearly confirmed the effects of balneotherapy on skin microbiome.
Conclusion
In this study, we evaluated the effect of balneotherapy on skin microbiome in patients with plaque psoriasis. The composition of microbial communities were similar on psoriatic plaque and non-lesional skin at baseline and after balneotherapy. Balneotherapy in the unaffected area significantly increased the level of Leptolyngbya genus, and significantly decreased the level of Flavobacterium genus. A similar trend was revealed in the lesional skin. Balneotherapy with sulfur-containing water was proved to be an effective treatment modality for the management of mild plaque psoriasis. With the results of our study, we can get closer to clarifying the mechanism of action of balneotherapy, but further studies are needed with waters containing other minerals (radon, salt, iodine, etc.).
Data Availability
All data is in the article. The data is not available on a public interface.
References
Amarouche-Yala S, Benouadah A, El Ouahab Bentabet A, López-García P (2014) Morphological and phylogenetic diversity of thermophilic cyanobacteria in Algerian hot springs. Extremophiles 18(6):1035–47. https://doi.org/10.1007/s00792-014-0680-7
Andrews S. (2018) FastQC – a quality control tool for high throughput sequence data. Babraham Bioinformatics, The Babraham Institute, Cambridge, available at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 15 Jan 2019
Assarsson M, Duvetorp A, Dienus O, Söderman J, Seifert O (2018) Significant changes in the skin microbiome in patients with chronic plaque Psoriasis after treatment with Narrowband Ultraviolet B. Acta Derm Venereol 98(4):428–436. https://doi.org/10.2340/00015555-2859
Beathard WA, Pickering A, Jacobs M (2021) Myroides cellulitis and bacteremia: a case report. IDCases 24:e01061. https://doi.org/10.1016/j.idcr.2021.e01061
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Bourrain M, Suzuki MT, Calvez A, West NJ, Lions J, Lebaron P. (2020) In-depth prospection of avene thermal spring water reveals an uncommon and stable microbial community. J Eur Acad Dermatol Venereol 34(Suppl 5):8–14. https://doi.org/10.1111/jdv.16599
Brandwein M, Fuks G, Israel A, Al-Ashhab A, Nejman D, Straussman R, Hodak E, Harari M, Steinberg D, Bentwich Z, Shental N, Meshner S (2018) Temporal stability of the healthy human skin microbiome following dead sea climatotherapy. Acta Derm Venereol 98(2):256–261. https://doi.org/10.2340/00015555-2769
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:325–349
Cacciapuoti S, Luciano MA, Megna M, Annunziata MC, Napolitano M, Patruno C, Scala E, Colicchio R, Pagliuca C, Salvatore P, Fabbrocini G (2020) The role of thermal water in chronic skin diseases management: a review of the literature. J Clin Med 9(9):3047. https://doi.org/10.3390/jcm9093047
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11(4):265–270
Cobo F, Barca S, Flores C, Caixach J, Cobo MC, Vieira-Lanero R (2022) Can cyanotoxins explain the clinical features of the thermal crisis in balneotherapy? Harmful Algae 115:102240. https://doi.org/10.1016/j.hal.2022.102240
Cozzi F, Raffeiner B, Beltrame V, Ciprian L, Coran A, Botsios C, Perissinotto E, Grisan E, Ramonda R, Oliviero F, Stramare R, Punzi L (2015) Effects of mud-bath therapy in psoriatic arthritis patients treated with TNF inhibitors. Clinical evaluation and assessment of synovial inflammation by contrast-enhanced ultrasound (CEUS). Joint Bone Spine 82(2):104–8. https://doi.org/10.1016/j.jbspin.2014.11.002
Darlenski R, Bogdanov I, Kacheva M, Zheleva D, Demerdjieva Z, Hristakieva E, Fluhr JW, N Tsankov (2021) Disease severity, patient-reported outcomes and skin hydration improve during balneotherapy with hydrocarbonate- and sulphur-rich water of psoriasis. J Eur Acad Dermatol Venereol 35(3):e196-e198. https://doi.org/10.1111/jdv.16908
Demay J, Halary S, Knittel-Obrecht A, Villa P, Duval C, Hamlaoui S, Roussel T, Yéprémian C, Reinhardt A, Bernard C, Marie B (2020) Anti-inflammatory, antioxidant, and wound-healing properties of Cyanobacteria from thermal mud of Balaruc-Les-Bains, France: a multi-approach study. Biomolecules 11(1):28. https://doi.org/10.3390/biom11010028
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72(7):5069–5072
Fredriksson T, Pettersson U (1978) Severe psoriasis—oral therapy with a new retinoid. Dermatologica 157:238–244
Ghilamicael AM, Budambula NLM, Anami SE, Mehari T, Boga HI (2017) Evaluation of prokaryotic diversity of five hot springs in Eritrea. BMC Microbiol 17:203. https://doi.org/10.1186/s12866-017-1113
Grice EA, Kong HH, Renaud G, Young AC, NISC comparative sequencing program; Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA (2008) A diversity profile of the human skin microbiota. Genome Res 18(7):1043–50. https://doi.org/10.1101/gr.075549.107
Gross-Amat O, Guillen M, Gimeno JP, Salzet M, Lebonvallet N, Misery L, Auxenfans C, Nataf S (2020) Molecular Mapping of Hydrogen Sulfide Targets in Normal Human Keratinocytes. Int J Mol Sci 21(13):4648. https://doi.org/10.3390/ijms21134648
Halevy S, Giryes H, Friger M, Grossman N, Karpas Z, Sarov B, Sukenik S (2001) The role of trace elements in psoriatic patients undergoing balneotherapy with Dead Sea bath salt. Isr Med Assoc J 3(11):828–32
Hedlund BP, Cole JK, Williams AJ, Hou W, Zhou E, Li W et al (2012) A review of the microbiology of the Rehai geothermal field in Tengchong, Yunnan Province, China. Geosci Front 3:273–288. https://doi.org/10.1016/j.gsf.2011.12.006
Hu J, Lu W, Li X, Yang J, Tan M, Hu K, Wang Q, Deng S, Liu Y, Chen J, Zhu W, Kuang Y (2022) Microbiota differences of skin and pharyngeal microbiota between patients with plaque and guttate psoriasis in China. Front Microbiol 13:937666. https://doi.org/10.3389/fmicb.2022.937666
Huang A, Seité S, Adar T (2018) The use of balneotherapy in dermatology. Clin Dermatol 36(3):363–368. https://doi.org/10.1016/j.clindermatol.2018.03.010
Kambura AK, Mwirichia RK, Kasili RW, Karanja EN, Makonde HM, Boga HI (2016) Bacteria and archaea diversity within the hot springs of lake magadi and little magadi in Kenya. BMC Microbiol 16:136. https://doi.org/10.1186/s12866-016-0748-x
Keshari N, Zhao Y, Das SK, Zhu T, Lu X (2022) Cyanobacterial community structure and isolates from representative hot springs of Yunnan Province, China Using an Integrative Approach. Front Microbiol 13:872598. https://doi.org/10.3389/fmicb.2022.872598
Krett G, Nagymáté Z, Márialigeti K, Borsodi AK (2016) Seasonal and spatial changes of planktonic bacterial communities inhabiting the natural thermal Lake Hévíz, Hungary. Acta Microbiol Immunol Hung 63(1):115–30. https://doi.org/10.1556/030.63.2016.1.9
Kruskal JB (1964) Nonmetric multidimensional scaling a numerical method. Psychometrika 29:115–129
Lahti L et al. (2019) microbiome R package. URL: http://microbiome.github.io
Langan EA, Künstner A, Miodovnik M, Zillikens D, Thaçi D, Baines JF, Ibrahim SM, Solbach W, Knobloch JK (2019) Combined culture and metagenomic analyses reveal significant shifts in the composition of the cutaneous microbiome in psoriasis. Br J Dermatol 181(6):1254–1264. https://doi.org/10.1111/bjd.17989
Li H, Yang Q, Li J, Gao H, Li P, Zhou H (2015) The impact of temperature on microbial diversity and AOA activity in the Tengchong Geothermal Field, China. Sci Rep 5:17056. https://doi.org/10.1038/srep17056
Li Y, Naman CB, Alexander KL, Guan H, William H, Gerwick WH (2020) The Chemistry, Biochemistry and Pharmacology of marine natural products from Leptolyngbya, a chemically endowed Genus of Cyanobacteria. Mar Drugs 18(10):508. https://doi.org/10.3390/md18100508
Lopes G, Clarinha D, Vasconcelos V (2020) Carotenoids from Cyanobacteria: a biotechnological approach for the topical treatment of psoriasis. Microorganisms 8(2):302. https://doi.org/10.3390/microorganisms8020302
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):1–21
Martin R, Henley JB, Sarrazin P, Seité S (2015) Skin microbiome in patients with psoriasis before and after balneotherapy at the thermal care center of La Roche-Posay. J Drugs Dermatol 14(12):1400–5
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8(4):e61217
Mosayebi Z, Movahedian AH, Soori T (2011) Flavobacterium sepsis outbreak due to contaminated distilled water in a neonatal intensive care unit. J Hosp Infect 78(3):214–5. https://doi.org/10.1016/j.jhin.2010.11.022
Nguyen T, Castex-Rizzi N, Redoulès D (2017) Immunomodulatory, anti-inflammatory, anti-pruritus and tolerogenic activities induced by I-modulia®, an Aquaphilus dolomiae culture extract, in atopic dermatitis pharmacology models. Ann Dermatol Venereol 144:S42–S49. https://doi.org/10.1016/S0151-9638(17)31042-6
Paduano S, Valeriani F, Romano-Spica V, Bargellini A, Borella P, Marchesi I (2018) Microbial biodiversity of thermal water and mud in an Italian spa by metagenomics: a pilot study. Water Sci Technol: Water Supply 18:1456–1465. https://doi.org/10.2166/ws.2017.209
Pedron R, Esposito A, Cozza W, Paolazzi M, Cristofolini M, Segata N, Jousson O (2022) Microbiome characterization of alpine water springs for human consumption reveals site- and usage-specific microbial signatures. Front Microbiol 13:946460. https://doi.org/10.3389/fmicb.2022.946460
Peinemann F, Harari M, Peternel S, Chan T, Chan D, Labeit AM, Gambichler T (2021) Indoor balneophototherapy for chronic plaque psoriasis: Abridged Cochrane Review. Dermatol Ther 34(1):e14588. https://doi.org/10.1111/dth.14588
Péquin B, Tremblay J, Maynard C, Wasserscheid J, Greer CW (2022) Draft whole-genome sequences of the polar cyanobacterium Leptolyngbya sp. Strain Cla-17 and Its Associated Flavobacterium. Microbiol Resour Announc 11(7):e00059–22. https://doi.org/10.1128/mra.00059-22
Péter I, Jagicza A, Ajtay Z, Boncz I, Kiss I, Szendi K, Kustán P, Németh B (2017) Balneotherapy in Psoriasis rehabilitation. In Vivo 31(6):1163–1168. https://doi.org/10.21873/invivo.11184
Rogers GB, Bruce KD (2010) Next-generation sequencing in the analysis of human microbiota: essential considerations for clinical application. Mol Diagn Ther 14(6):343–50. https://doi.org/10.1007/BF03256391
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Saghatelyan A, Margaryan A, Panosyan H, Birkeland NK (2021) Microbial diversity of terrestrial geothermal springs in Armenia and Nagorno-Karabakh: a review. Microorganisms 9(7):1473. https://doi.org/10.3390/microorganisms9071473
Senousy HH, Ellatif SA, Ali S (2020) Assessment of the antioxidant and anticancer potential of different isolated strains of cyanobacteria and microalgae from soil and agriculture drain water. Environ Sci Pollut Res Int 27(15):18463–18474. https://doi.org/10.1007/s11356-020-08332-z
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423 & 623–656, July & October
Simpson EH (1949) Measurement of diversity. Nature 163:688
Timis TL, Florian IA, Mitrea DR, Orasan R (2021) Mind-body interventions as alternative and complementary therapies for psoriasis: a systematic review of the English literature. Medicina 57:410. https://doi.org/10.3390/medicina57050410
Trabelsi L, Mnari A, Abdel-Daim MM, Abid-Essafi S, Aleya L (2016) Therapeutic properties in Tunisian hot springs: first evidence of phenolic compounds in the cyanobacterium Leptolyngbya sp. biomass, capsular polysaccharides and releasing polysaccharides. BMC Complement Altern Med 16(1):515. https://doi.org/10.1186/s12906-016-1492-3
Ward DM, Ferris MJ, Nold SC, Bateson MM (1998) A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev 62:1353–1370. https://doi.org/10.1128/MMBR.62.4.1353-1370
Ward DM, Bateson MM, Ferris MJ, Kühl M, Wieland A, Koeppel A et al (2006) Cyanobacterial ecotypes in the microbial mat community of mushroom spring (Yellowstone National Park, Wyoming) as species-like units linking microbial community composition, structure and function. Philos Trans R Soc B Biol Sci 361:1997–2008. https://doi.org/10.1098/rstb.2006.1919
Whittle E, Leonard OM, Harrison R, Gant WT, Tonge DP (2019) Multi-method characterization of the human circulating microbiome. Front Microbiol 9:3266. https://doi.org/10.3389/fmicb.2018.03266
Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20(1):1–13
Yerushalmi M, Elalouf O, Anderson M, Chandran V (2019) The skin microbiome in psoriatic disease: a systematic review and critical appraisal. J Transl Autoimmun 2:100009. https://doi.org/10.1016/j.jtauto.2019.100009
Yu D, Du J, Pu X, Zheng L, Chen S, Wang N, Li J, Chen S, Pan S, Shen B (2022) The gut microbiome and metabolites are altered and interrelated in patients with rheumatoid arthritis. Front Cell Infect Microbiol 11:763507. https://doi.org/10.3389/fcimb.2021.763507
Acknowledgements
We would like to thank Ágnes Gárdos, MD and Attila Elek, MD for their help in patient selection. This study was supported by the grants from Ministry of Innovation and Technology (2020-4.1.1.-TKP2020-MOLOKRIV) as well as Eötvös Lóránd Research Network (SE-ELKH ENDOMOLPAT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulisch, Á., Mándó, Z., Sándor, E. et al. Evaluation of the effects of Lake Hévíz sulfur thermal water on skin microbiome in plaque psoriasis: An open label, pilot study. Int J Biometeorol 67, 661–673 (2023). https://doi.org/10.1007/s00484-023-02443-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-023-02443-1