Abstract
Due to the high milk production of Holstein cows, many countries have chosen to import semen to improve local dairy herds. This strategy would be more effective if this semen was used in the same environment conditions in which the bulls were selected. If the effect of genotype by environment (G × E) interaction is not considered, the estimated breeding values (EBVs) may vary, potentially reducing the selection response. We evaluate the impact of heat stress on selection for milk yield and composition of Holstein cows using random regression models. To verify the interference of heat stress in milk yield (MY) and composition traits (fat, protein, total saturated, and total unsaturated fatty acids content in milk), temperature–humidity index (THI) on test-day milk records was used. The threshold value to divide the environments using test-day information from Brazilian Holstein cows was 72 units of THI, i.e., < 72 represented no heat stress and > 72 represented heat stress. Legendre polynomials of second-order (Leg 2) model and two lactation points (33 and 122 DIM) were used to estimate heritabilities and EBVs for five important dairy traits. The heritabilities of milk components and fatty acids were low (0.09–0.29), regardless of lactation period and degree of heat stress, with the exception of protein content (0.30–0.35). Fat content was the only milk component that was reduced according to the degree of heat stress and lactation period. The EBVs tended to decrease in heat stress conditions, thus animals with high genetic potential demonstrated evidence of G × E interaction. However, acclimatization of dairy cows to heat stress in the farm production systems may have been responsible for the low differences among genetic parameters and EBVs with and without heat stress found in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The climate changes around the world are considerably increasing the temperatures over the years, especially in tropical regions, causing changes in agriculture and livestock production. Extreme temperatures linked to factors such as relative humidity affect the animals’ comfort zone due to the increase of body temperature, which influences the homeostatic balance (Boettcher et al. 2015; Vasconcelos et al. 2020). Heat stress is the most significant impact related to climate change in livestock production, as it reduces feed intake (Kappes et al. 2022), reproductive efficiency (Menegassi et al., 2016), and increases respiration rate (Ferreira et al. 2021a) and peripheral blood (Silveira et al. 2021). Many factors contribute to the vulnerability of livestock to heat stress such as species genetic potential, age, physiological, and nutritional status (Thornton et al. 2021; Ferreira et al. 2021b).
Aiming to increase dairy productivity, developing tropical countries are being encouraged to import genetic material to improve local herds. However, this strategy would be more effective if the imported genetic material was derived from bulls evaluated under the same environmental conditions and herd management. Increasing intensification of dairy systems in developing tropical regions with temperate-breed genetic stock could lead to greater vulnerability of animals to rising temperatures. Moreover, it is expected that progenies of animals evaluated in temperate environments will present a different performance in tropical climate, not only because of the differences in climate itself but also because the genotypes have different responses according to the environment to which they are exposed (Hammami et al. 2008).
In that sense, an unfavorable genotype by environment interaction could reduce the benefits of a strategy based on imported semen (Banos and Smith 1991). Milk production losses have already been mentioned if the G × E interaction is ignored, especially when using imported semen, as in many cases exporting countries have a different production environment compared to importing countries (Payne and Hodges 1997; Hayes et al. 2003). For example, Silva et al. (2021) demonstrated genotype × environment interaction for Holstein cattle milk yield in temperate (Portugal) × tropical environment (Brazil). Furthermore, international bull evaluations showed that there is low genetic correlation between countries using the production system pasture and countries using a more industrialized production system, such as New Zealand and the USA (Strandberg et al. 2009).
One of the tools used to measure heat stress in farm animals is the use of the temperature–humidity index (THI; NRC 1971), which is already used to estimate genetic parameters related to heat tolerance (Sungkhapreecha et al. 2021; Carrara et al. 2021; Mbuthia et al. 2021). Given the above, the aim of this study was to evaluate the impact of heat stress on selection for milk yield and composition of Brazilian Holstein cows using a random regression model.
Material and methods
Data set
We used 49 monthly records of milk yield (MY; kg day−1), fat (%), protein (%), total saturated fatty acids (SFA; %), and total unsaturated fatty acids (UFA; %) from 2896 Holstein cows with calving between 1 and 6 from four commercial Brazilian farms (Table 1), and collected between May 2012 and July 2016. All the farms had three-times-a-day milking and nutritional conditions were standardized in farms, such that there were no different nutritional conditions within a farm. The farms considered in this study are well established and their productions are stable during the year. This way, the calving is not concentrated in one period of the year. In addition, the selection criteria adopted by the farms are milk production, morphological traits, productive life, somatic cell count, and livability.
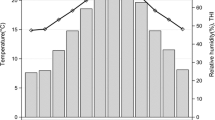
The meteorological data recorded during the study are presented in Fig. 1. The milk components were determined by mid-infrared spectrometry (Delta Instruments CombiScope™ Filter, Advanced Instruments, Inc., Norwood, USA). Quality control excluded animals with days in milk lower than five or higher than 305; without valid measurements (mean ± 3 SD); without calving date or lactation order, and age information; and with age higher than 9 years. The contemporary groups (CGs) were formed by the combination of calving periods: dry — April to September, and rainy — October to March, calving year based on the start of calving periods, farm, and collection month. CGs containing lower than five individuals were eliminated. The pedigree consisted of 8789 animals, 4197 dams, and 576 sires of 7 generations, considering the first generation as one. All animals were considered in the analyses.
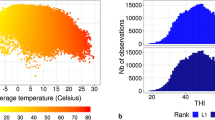
THI was determined from air temperature (AT, °C) and relative humidity (RH, %), following the National Research Council (NRC 1971):
The AT and RH were obtained from the Space Administration/Prediction of Worldwide Energy Resources database (NASA/POWER; Power 2016) based on the geographic coordinates of each dairy farm. The heat stress degree (t) was obtained based on the THI and defined as the number of units of mean THI above 72 (NRC 1971; Bohmanova et al. 2008).
Genetic analysis
Criterion model settings
The analyses were carried out by using a random regression model to describe the phenotype expression in relation to genotype based on the environmental changes. In this case, the environments are not classified into groups. The phenotypes will change gradually and continuously over an environmental gradient, being the regression performance of genotypes in each thermal environment the most used method for this type of analysis (Kolmodin et al. 2002).
To model covariance functions, first-degree (linear) Legendre polynomials were used. Studies with dairy cows showed that lower orders for Legendre polynomials could be sufficient to fit the data. In addition, previous studies have shown that lower-order polynomials are preferable (in terms of better fit) to milk yield and components in this same evaluated population (Carrara et al. 2021).
Model considering the genotype × environment interaction
To verify the presence of G × E interaction for MY, fat, protein, SFA, and UFA, a random regression model was used as suggested by Meyer (1998) and Bohmanova et al. (2008).
where yij is the vector of the response variable i measured at the animal j; μ is the global average; F is the vector of fixed effects, composed by the contemporary groups; βm is the regression coefficient for the Legendre polynomial m; α0im and ρ0im are random regression coefficients for the direct additive genetic effect and permanent environment for animal i regardless of the level of heat stress t; α1im and ρ1im are random regression coefficients for the direct additive genetic effect and permanent environment effect, respectively, considering the environmental sensitivity of the animal i to heat stress t; kβ, kα, and kρ are the orders of the polynomials (linear); \(\phi\) m is the nth Legendre polynomial function; and ɛij is the random residual effect.
Variance components were obtained using WOMBAT software (Meyer 2006). The covariance functions were estimated for the additive genetic and permanent environmental effects using first-degree (linear) orthogonal Legendre polynomials (Leg 2) taking into account two points of lactation, 33 days in milk (DIM) and 122 DIM. These points were chosen based on the largest number of information in the data set.
The variance covariance structure was:
where A is the relationship matrix; I is the identity matrix; σ2a is the variance of the additive genetic effect; σ2p is the variance of the permanent environmental effect; σαα is the covariance between the intercept and the additive genetic effect; and σpπ is the covariance between the intercept and the effect of permanent environment.
The heritability coefficient (h2) was estimated for each trait as the ratio of the additive genetic variance (σ2a) and the phenotypic variance (σ2y).
where σ2p is the permanent environment variance and σ2e is the residual variance.
Estimated breeding value
Breeding values were estimated by the following equation:
where EBVpj is the breeding value of cow p at DIM j; \({\alpha }_{pi}\) is the regression coefficient of additive breeding value of cow p for trait i; \({\beta }_{pi}\) is the second-order regression coefficient of additive breeding value of cow p to trait i; and w is the lactation stage standardized.
Results and discussion
The threshold value to divide the environments using test-day information from Brazilian Holstein cows was 72 units of THI, i.e., < 72 represented no heat stress and > 72 represented heat stress. Leg 2 model and two lactation points (33 and 122 DIM) were used to estimate heritabilities and EBVs for five important dairy traits.
Genetic variance components and heritabilities
The estimates of variance components and heritability for MY and composition traits obtained for both thermal environments (no heat stress and heat stress) are given in Table 2. The additive genetic component decreased under heat stress environments for fat and SFA. Furthermore, protein and UFA showed higher additive genetic values in the heat stress environment. Regarding the lactation periods evaluated (33 and 122 DIM), only the additive genetic variance reduced for fat. We believe that this reduction is explained by different causes, in which at 33 days, they are more related to heat stress, as milk production is higher, while at 122 days, it may be related to the reduction of natural milk in the lactation curve of the cows. It is also worth mentioning that fat is the component of milk with the greatest variation, being mainly influenced by diet, breed, feed intake, and milk production (Stürmer et al. 2018; Silveira et al. 2022).
Greater heritabilities (> 0.30) were observed in both lactation stages (33 and 122 DIM) and in the environment with no heat stress for protein, indicating that this trait can be able to provide a good response to selection. Similar results were obtained by Jamrozik and Schaeffer (1997; 0.33 to 0.69), Biassus et al. (2011; 0.10 to 0.35), and Borquis et al. (2013; 0.21 to 0.37) for protein. The heritability for protein was lower than those estimated by Jamrozik and Schaeffer (1997) (0.34 to 0.68) and Soyeurt et al. (2008) (0.23 to 0.44), especially in heat stress environments. Generally, first-parity cows show higher heritability estimates than higher-order-parity cows; therefore, the use of cows with different parities in the database may be responsible for causing these differences in estimates (Bastin et al. 2013; Petrini et al. 2016).
Low to moderate heritabilities (0.10 to 0.28) were estimated for SFA and fat, similar to Bobe et al. (2008) (0.27), Penasa et al. (2015) (0.24), and Petrini et al. (2016) (0.25) indicating that these traits can be able to provide an improvement in response to selection although slow. Higher heritabilities for SFA were reported by Soyeurt et al. (2007; 0.36), Soyeurt et al. (2008) (0.42), and Bastin et al. (2011) (0.43). Different heritability estimates among studies on the same breed can be explained by differences in the sample size, type of records used in each study (e.g., first or multiple lactations), and estimation methods.
In both thermal environments, SFA heritabilities were higher than UFA meaning that SFA are more heritable than UFA. The reason for this difference could be related to fatty acids (FA) origin. Fatty acids in milk are derived from synthesized de novo by the mammary gland and from the blood (Knutsen et al. 2018). SFA are de novo synthesized in the mammary gland from blood precursors and regulated by two enzymes. On the other hand, UFA is provided by diet and is hydrogenated in the rumen by bacteria and transported by the blood (Gion et al. 2011) then their concentration in milk is close to the absorbed quantities in the intestine. Bastin et al. (2011) suggest that de novo synthesis FA are under stronger genetic control than FA originating from the diet than from body fat mobilization.
Fat composition of cow milk is influenced by metabolic status and stage of lactation of the cow, as a negative energy balance directly impacts the presence of UFA. The mobilization of fat reserves has been found to increase the content of UFA in milk. In general, the estimates of heritabilities decreased in the heat stress environment. Therefore, the environment may have influenced the selection response in an animal breeding program. These differences in the heritabilities for FA can be justified by the fact that these traits are highly influenced by environmental conditions (Penasa et al. 2015).
Genotype by environment interaction
The EBV for MY and composition traits obtained for both environments and DIM (33 and 122 days) are shown in Table 3. In addition, Fig. 2 shows the top ten animals for each trait according to the EBV values at different environmental conditions.
It is noted that the EBVs varied over the two environments, as well as a re-ranking of individuals, especially for fat and SFA. The EBV for fat and SFA reduced significantly with the heat stress interference, evidencing the presence of G × E interaction. Low variation of the EBV of animals for protein and UFA shows that the performance of these animals was relatively stable under different environmental conditions, indicating that breeding values were more robust during the lactation (Bignardi et al. 2015). The cause of this stability is due to high genetic correlations between them, and increasing the stability of genotypes represents a lower environmental sensitivity of these genotypes. Acclimatization of dairy cows to heat stress in the farm production system may have been responsible for low differences found in this study. Moreover, the THI value used (72) may not be enough to find more meaningful G × E interactions. Another important factor to be taken into account is the choice of the day used to obtain the relative humidity and air temperature information to calculate the THI, since heat stress effects can be observed a few days after animal exposure to hot temperature.
For MY, the EBVs were similar in the heat stress environment. The results found in this study differ from a research conducted by Carabaño et al. (2014), in which the animals with the best classifications showed lower EBVs when exposed to environments with higher temperatures, confirming the antagonistic relationship between the level of production and tolerance to heat stress found in other studies (Silva et al. 2021). Milk yield of dairy cows with high genetic merit is reduced when these animals are exposed to an environment with high temperatures (Kadzere et al. 2002), but it is possible that the animals under study have some degree of adaptation. In addition, the high environmental sensitivity can be beneficial in controlled environments; it is possible to maintain the high levels of production of these animals with the aid of physical improvements in the environment, such as radiation, temperature, and humidity control. However, we emphasize that modifying the physics of the thermal environment with the use of refrigerated equipment means increases the production cost per liter of milk, which is often infeasible for dairy farming in tropical conditions.
EBV versus days of lactation (33 × 122 days) were similar for milk composition, but we observed a reduction in EBV for milk yield at 33 compared to 122 DIM under both heat stress conditions (THI ≤ 72 and THI > 72), which is explained by the higher metabolic heat production at the beginning of lactation for milk synthesis, especially in the period of peak lactation (1st phase of the lactation curve), making cows more susceptible to heat stress in this production phase, while dairy cows after peak lactation (2nd and 3rd phases of the lactation curve) produce less metabolic heat and are less susceptible to heat stress.
Finally, animals with lower environmental sensitivity are appropriate to include in an animal breeding program, as they are able to adapt to different environments, producing consistently. Although it is not possible to exclude the effects of G × E interaction by genetic means, these effects could be minimized by using animal breeding strategies. In this context, studies that include G × E in the models allow the evaluation of the phenotypic plasticity of individuals, i.e., to identify robust sires that do not substantially vary their genetic merit in different environments. Thus, the selection of robust animals with high breeding value can contribute to obtaining progenies more adapted to tropical conditions and with satisfactory performance in the different Brazilian climatic environments. While the exclusion effect of G × E interaction by genetic means can be minimized by using animal breeding strategies, in the future sensors, robots may solve the problem using artificial intelligence (AI). Healthy farm animals/dairy cattle with a high degree of accuracy can be used by a camera with AI to achieve a “smart” cowhouse which could enable early detection of injuries and illnesses that could impact the quantity and quality of milk production.
Conclusion
The heritability of milk components and fatty acids is low, regardless of lactation period and degree of heat stress, with the exception of protein. The EBVs at higher temperatures tended to decrease in heat stress conditions, thus animals with high genetic potential demonstrated evidence for G × E interactions in Brazilian Holstein cattle population.
Data availability
Data will be made available on request.
References
Banos G, Smith C (1991) Selecting bulls across countries to maximize genetic improvement in dairy cattle. J Anim Breed Genet 108:174–181. https://doi.org/10.1111/j.1439-0388.1991.tb00172.x
Bastin C, Gengler N, Soyeurt H (2011) Phenotypic and genetic variability of production traits and milk fatty acid contents across days in milk for Walloon Holstein first-parity cows. J Dairy Sci 94:4152–4163. https://doi.org/10.3168/jds.2010-4108
Bastin C, Soyeurt H, Gengler N (2013) Genetic parameters of milk production traits and fatty acid contents in milk for Holstein cows in parity 1–3. J Anim Breed Genet 130:118–127. https://doi.org/10.1111/jbg.12010
Bignardi AB, El Faro L, Pereira RJ, Ayres DR, Machado PF, De Albuquerque LG, Santana ML (2015) Reaction norm model to describe environmental sensitivity across first lactation in dairy cattle under tropical conditions. Trop Anim Health Prod 47:1405–1410. https://doi.org/10.1007/s11250-015-0878-4
Bobe G, Minick JA, Bormann GL, Lindberg AE, Freeman Beitz DC (2008) Short communication: estimates of genetic variation of milk fatty acids in US Holstein cows. J Dairy Sci 91:1209–1213. https://doi.org/10.3168/jds.2007-0252.2
Boettcher PJ, Hoffmann I, Baumung R, Drucker AG, McManus C, Berg P, Stella A, Nilsen LB, Moran D, Naves M, Thompson MC (2015) Genetic resources and genomics for adaptation of livestock to climate change. Front Genet 5:461
Bohmanova J, Misztal I, Tsuruta S, Norman HD, Lawlor TJ (2008) Short communication: genotype by environment interaction due to heat stress. J Dairy Sci 91:840–846. https://doi.org/10.3168/jds.2006-142
Borquis RRA, Neto FRDA, Baldi F, Hurtado-Lugo N, de Camargo GMF, Muñoz-Berrocal M, Tonhati H (2013) Multiple-trait random regression models for the estimation of genetic parameters for milk, fat, and protein yield in buffaloes. J Dairy Sci 96:5923–5932. https://doi.org/10.3168/jds.2012-6023
Brügemann K, Gernand E, von Borstel UU, König S (2011) Genetic analyses of protein yield in dairy cows applying random regression models with time-dependent and temperature x humidity-dependent covariates. J Dairy Sci 94:4129–4139. https://doi.org/10.3168/jds.2010-4063
Carabaño MJ, Bachagha K, Ramón M, Díaz C (2014) Modeling heat stress effect on Holstein cows under hot and dry conditions: selection tools. J Dairy Sci 97:7889–7904. https://doi.org/10.3168/jds.2014-8023
Carrara ER, Petrini J, Salvian M, de Oliveira HR, Rovadoscki GA, Iung LHS, Miquilini M, Machado PF, Mourão GB (2021) Genetic parameters for milk yield and quality traits of Brazilian Holstein cows as a function of temperature and humidity index. J Anim Breed Genet 133:384–395. https://doi.org/10.1111/jbg.12205
Corrales JD, Munilla S, Cantet RJ (2015) Polynomial order selection in random regression models via penalizing adaptively the likelihood. J Anim Breed Genet. 132(4):281–8. https://doi.org/10.1111/jbg.12130
Druet T, Jaffrézic F, Boichard D, Ducrocq V (2003) Modeling lactation curves and estimation of genetic parameters for first lactation test-day records of French Holstein cows. J Dairy Sci 86:2480–2490. https://doi.org/10.3168/jds.S0022-0302(03)73842-9
Ferreira J, Serafim MV, Diógenes GV, Silveira RMF, Antas SU, Costa MG (2021b) Simultaneity between nutrition and thermoregulatory responses in ruminants. Biol Rhythm Res 52(9):1372–1382. https://doi.org/10.1080/09291016.2019.1629166
Hammami H, Rekik B, Soyeurt H, Bastin C, Stoll J, Gengler N (2008) Genotype x environment interaction for milk yield in Holsteins using Luxembourg and Tunisian populations. J Dairy Sci 91:3661–3671. https://doi.org/10.3168/jds.2008-1147
Jamrozik J, Schaeffer LR (1997) Estimates of genetic parameters for a test day model with random regressions for yield traits of first lactation Holsteins. J Dairy Sci 80:762–770. https://doi.org/10.3168/jds.S0022-0302(97)75996-4
Kadzere CT, Murphy MR, Silanikove N, Maltz E (2002) Heat stress in lactating dairy cows: a review. Livest Prod Sci 77:59–91. https://doi.org/10.1016/S0301-6226(01)00330-X
Kappes R, Knob DA, Scheid AL (2022) Rumination time, activity index, and productive performance of Holstein and crossbred Holstein × jersey cows exposed to different temperature-humidity indexes. Int J Biometeorol. https://doi.org/10.1007/s00484-021-02237-3
Knutsen TM, Olsen HG, Tafintseva V, Svendsen M, Kohler A, Kent MP, Lien S (2018) Unravelling genetic variation underlying de novo-synthesis of bovine milk fatty acids. Sci Rep 8:2179. https://doi.org/10.1038/s41598-018-20476-0
Kolmodin R, Strandberg E, Madsen P, Jensen J, Jorjani H (2002) Genotype by environment interaction in Nordic dairy cattle studied using reaction norms. Acta Agric Scand Sect A-Anim Sci 52:11–24. https://doi.org/10.1080/09064700252806380
Meyer K (1998) Estimating covariance functions for longitudinal data using a random regression model. Genet Sel Evol 30:221. https://doi.org/10.1186/1297-9686-30-3-221
NRC (1971) A guide to environmental research on animals. National Academy of Sciences, Washington, DC
Penasa M, Tiezzi F, Gottardo P, Cassandro M, De Marchi M (2015) Genetics of milk fatty acid groups predicted during routine data recording in Holstein dairy cattle. Livest Sci 173:9–13. https://doi.org/10.1016/j.livsci.2014.12.014
Petrini J, Iung LHS, Rodriguez MAP, Salvian M, Pértille F, Rovadoscki GA, Cassoli LD, Coutinho LL, Machado PF, Wiggans GR, Mourão GB (2016) Genetic parameters for milk fatty acids, milk yield and quality traits of a Holstein cattle population reared under tropical conditions. J Anim Breed Genet 133:384–395. https://doi.org/10.1111/jbg.12205
Silva DA, Lopes PS, Costa CN, Silva AA, Silva HT, Silva FF, Veroneze R, Thompson G, Carvalheira J (2021) Genotype by environment interaction for Holstein cattle populations using autoregressive and within- and across-country multi-trait reaction norms test-day models. Animal 15(2):100084. https://doi.org/10.1016/j.animal.2020.100084
Silveira RMF, da Silva VJ, Ferreira JB, Oliveira R, Vega WHO, Cavalcanti D, Pereira A, Castro MSM, Toro-Mujica P, de Vasconcelos AM (2022) Diversity in smallholder dairy production systems in the Brazilian semiarid region: farm typologies and characteristics of raw milk and water used in milking. J Arid Environ 203(2021):104774. https://doi.org/10.1016/j.jaridenv.2022.104774
Soyeurt H, Gillon A, Vanderick S, Mayeres P, Bertozzi C, Gengler N (2007) Estimation of heritability and genetic correlations for the major fatty acids in bovine milk. J Dairy Sci 90:4435–4442. https://doi.org/10.3168/jds.2007-0054
Soyeurt H, Dardenne P, Dehareng F, Bastin C, Gengler N (2008) Genetic parameters of saturated and monounsaturated fatty acid content and the ratio of saturated to unsaturated fatty acids in bovine milk. J Dairy Sci 91:3611–3626. https://doi.org/10.3168/jds.2007-0971
Strandberg E, Brotherstone S, Wall E, Coffey MP (2009) Genotype by environment interaction for first-lactation female fertility traits in UK dairy cattle. J Dairy Sci 92:3437–3446. https://doi.org/10.3168/jds.2008-1844
Stürmer M, Busanello M, Velho JP, Heck VI, Haygert-Velho IMP (2018) Relationship between climatic variables and the variation in bulk tank milk composition using canonical correlation analysis. Int J Biometeorol 62(9):1663–1674. https://doi.org/10.1007/s00484-018-1566-7
Sungkhapreecha P, Misztal I, Hidalgo J, Steyn Y, Buaban S, Duangjinda M, Boonkum W (2021) Changes in genetic parameters for milk yield and heat tolerance in the Thai Holstein crossbred dairy population under different heat stress levels and over time. J Dairy Sci 104(12):12703–12712. https://doi.org/10.3168/jds.2021-20151 (Epub 2021 Sep 14 PMID: 34531057)
Thornton P, Nelson G, Mayberry D, Herrero M (2021) Increases in extreme heat stress in domesticated livestock species during the twenty-first century. Global Chance Biol. https://doi.org/10.1111/gcb.15825
Vasconcelos AA, Albuquerque CC, Carvalho JF, de Façanha DAE, Lima FRL, Silveira RMF, Ferreira JB (2020) Development of an animal adaptability index: application for dairy cows. J Therm Biol 89:1–7. https://doi.org/10.1016/j.jtherbio.2020.102543
Ferreira J, Silveira RMF, de Sousa JER, Guilhermino MM, Vasconcelos AM, Façanha DAE, (2021a) Evaluation of homeothermy, acid-base and electrolytic balance of black goats and ewes in an equatorial semi-arid environment J Therm Biol 100 https://doi.org/10.1016/j.jtherbio.2021.103027
Gion A, Larroque H, Brochard M, Lahalle F, and Boichard D (2011) Genetic parameter estimation for milk fatty acids in three French dairy cattle breeds. Interbull Meet. 4 p.
Meyer K (2006) “WOMBAT”: digging deep for quantitative genetic analyses by restricted maximum likelihood. Page. World Congress On Genetic Applied To Livestock Production, 8. Belo Horizonte, MG.
Mbuthia JM, Mayer M, Reinsch N (2021) Modeling heat stress effects on dairy cattle milk production in a tropical environment using test-day records and random regression models, Animal, 15 https://doi.org/10.1016/j.animal.2021.100222
Payne WJA, Hodges J (1997). Tropical cattle: origin, breeds and breeding policies. 1 edition. Wiley-Blackwell.
Power, N. 2016. Accessed February 8, 2016. https://power.larc.nasa.gov/data-access-viewer/.
Silveira RMF, Ferreira J, Busanello M, Vasconcelos AM, Jannuzzi Valente FL, Façanha DAE (2021) Relationship between thermal environment and morphophysiological, performance and carcass traits of Brahman bulls raised on tropical pasture: a canonical approach to a set of indicators. J Therm Biol, 96. https://doi.org/10.1016/j.jtherbio.2020.102814
Funding
This research received a financial support from the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, grant numbers: 2010/12929–6; 2012/24788–3; 2012/15948–7; 16/15066–5), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant numbers: 553966/2009–0, 484560/2012–3), fellowships to Paulo F. Machado and Gerson B. Mourão, and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil, finance code 001). The authors are grateful to “Clínica do Leite” and the Department of Animal Science, “Luiz de Queiroz” College of Agriculture (ESALQ), University of São Paulo (USP) for their support and for providing the database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All experimental procedures related to animal experimentation employed in the present study were performed in agreement with the protocol N° 2017.5.1197.11.3 approved by Institutional Animal Care and Use Committee Guidelines from “Luiz de Queiroz” College of Agriculture (ESALQ), University of São Paulo (USP) to ensure compliance with international guidelines for animal welfare.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salvian, M., Silveira, R.M.F., Petrini, J. et al. Heat stress on breeding value prediction for milk yield and composition of a Brazilian Holstein cattle population. Int J Biometeorol 67, 347–354 (2023). https://doi.org/10.1007/s00484-022-02413-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-022-02413-z