Abstract
The objective of this study was to evaluate the seasonal effects of the environment on sperm quality in subtropical region determined by temperature and humidity index (THI). We used 20 Brangus bulls (5/8 Angus × 3/8 Nellore) aged approximately 24 months at the beginning of the study. Semen evaluations were performed twice per season during 1 year. Climate THI data were collected from an automatic weather station from the National Institute of Meteorology. Infrared thermography images were used to determine the temperature of the proximal and distal poles of the testis to assess the testicular temperature gradient (TG). The seasonal effects on seminal and climatic variables were analyzed with ANOVA using MIXED procedure of SAS. Sperm motility in spring (60.1 %), summer (57.6 %), and autumn (64.5 %) showed difference compared to winter (73.0 %; P < 0.01). TG was negatively correlated with THI at 18 days (spermiogenesis) (−0.76; P < 0.05) and at 12 days (epididymal transit) (−0.85; P < 0.01). Ocular temperature (OcT) had a positive correlation with THI at 18 days (0.78; P < 0.05) and at 12 days (0.84; P < 0.01). Motility showed a negative correlation with THI only at 18 days (−0.79; P < 0.05). During spermiogenesis, the TG had higher negative correlation compared to OcT (−0.97; P < 0.01) and rectal temperature (−0.72; P < 0.05). Spermatozoa with distal midpiece reflex were correlated with THI during transit epididymis (0.72; P < 0.05). Seminal parameters are not affected when THI reaches 93.0 (spermiogenesis) and 88.0 (epididymal transit). We concluded that infrared thermography can be adopted as an indirect method in order to assess the effect of environmental changes in TG and OcT of Brangus bulls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to climate change and the uses of crossbreeding animals at the tropical regions in Brazil, adaptation of bull breeds to the ambient conditions becomes an important aspect for the expression of their genetic potential (Burns et al. 2010; McManus et al. 2011). The increase in the frequency of environmental events such as extreme heat waves can lead to severe reproductive failure in bulls due to heat stress (Hansen 2009, 2013). Factors such as wind speed, radiation, air temperature, and humidity change constantly, causing the effects on the thermal balance (Silva 1999; Marai and Haeeb 2010). In fact, any alteration on thermoregulation mechanisms involved in heat dissipation leads to decrease regulation efficiency and to increase of scrotum-testicular temperature that will affect the semen quality and bull fertility (Marai et al. 2008; Wolfenson and Thatcher 2012; Hansen 2013).
Researchers typically evaluate variables such as temperature and air humidity based on a thermal comfort index that is represented as a temperature-humidity index (THI) (Bouraoui et al. 2002; Marai and Haeeb 2010; Menegassi et al. 2015). Moreover, THI over 72 is well known to characterize a stressful environment for dairy cattle (West et al. 2003; Correa-Calderon et al. 2004; Bohmanova et al. 2007). However, studies of the effect of THI on the sperm quality of bulls during breeding season still scarce, mainly due to difficulties in conducting experiments in the natural environment. In these conditions, Menegassi et al. (2015) found negative correlation of the scrotal temperature gradient measured by infrared thermography in the proximal and distal poles and observed that THI of 83 in summer decrease sperm quality, but not in a sufficient intensity to promote morphological damages.
Infrared thermography was largely developed from the late 1980s (Coulter 1988) and has been used as a noninvasive method to evaluate of physiological response to the environmental effects of heat (Paim et al. 2013). Differently from other studies, we intend to determine, in more details, a possible cause of reduction in semen quality based on sequential spermatogenesis analyses throughout the year, using THI as a main factor involved in thermoregulation. In this context, the objective of this study was to evaluate the environmental seasonal effects determined by THI on sperm quality of beef bulls in subtropical regions.
Materials and methods
Animals and weather data
Twenty Brangus breed bulls (5/8 Angus × 3/8 Nelore) with 24 months of age were used in this experiment. All procedures were approved by the Federal University of Rio Grande do Sul Ethical Committee for care and use of experimental animals (Project 26250, CEUA/UFRGS). Bulls were collected twice in each season during the four seasons as follows: October 29, 2013 and November 30, 2013 (spring); January 20, 2014 and February 19, 2014 (summer); May 20, 2014 and June 19, 2014 (autumn); and July 11, 2014 and August 14, 2014 (winter). All animals were kept outdoor in the same environmental conditions and fed in a diet system based on cultivated and natural pasture. Bulls received mineral supplementation and free access to water and shelter. The experiment was conducted in Southern Brazil at the farm located at 54° 19′ 12″ W longitude and 30° 20′ 11″ S latitude, at 114-m altitude and climate classified as Cfa, according to Köppen-Geiger.
Calculation of temperature-humidity index
The temperature and humidity data were collected every hour from the automatic weather station at the National Institute of Meteorology (INMET 2014), situated at 54° 31′ 09″ W longitude and at 30° 34′ 14″ S latitude at 115-m altitude. The THI data were estimated from the following equation described by The National Research Council (1971):
THI = (1.8 × T db + 32) − (0.55 − 0.0055 × RH) × (1.8 × T db − 26), where, T db = dry-bulb air temperature (°C) and RH = relative humidity in decimal form.
Two semen collections per season were considered, with an interval of 30 days among them, being considered the spermatogenesis phase of 18 days and the epididymal transit of 12 days, according to criteria adopted by Pineda and Faulkner (1980). The THI was calculated twice for each season of the year.
Reproductive evaluation
All animals had a breeding soundness examination at the beginning of the experiment. The examination consisted of a comprehensive general clinical examination, specific reproductive examination, and seminal evaluation. The rectal temperature from each bull was registered in all evaluations, measured with a digital thermometer for 1 min prior to semen collection. A total of eight ejaculates (two per season) were collected from each bull using an automatic operated electroejaculator Pulsator IV (Lane Manufacturing Denver, CO, USA). For semen collection, bulls were restrained to facilitate rectal probe insertion and feces were evacuated manually removed. An electroejaculator probe with three ventrally oriented longitudinal electrodes was used to deliver a sequence of electrical impulses to each bull.
Mass activity was determined by placing a 10 μL drop of semen on a prewarmed microscope slide, and the edge of the drop was examined using an optical microscope at ×40 magnification. Mass motion (MM) received a score ranging from 0 to 5: 0 = no swirl, + = no swirl with generalized oscillation of individual sperm only, ++ = very slow distinct swirl, +++ = slow distinct swirl, ++++ = moderately fast distinct swirl and eddies, and +++++ = fast distinct swirls and eddies with the appearance of good quality semen. Sperm motility (M) was examined under a bright-field microscope (LEICA CME, Buffalo, NY, USA) at a magnification of ×100 with a 5 μL aliquot of semen placed on a warmed (37 °C) slide and covered with a coverslip. Sperm M was evaluated as the percentage of sperm movement (0 to 100 %). Vigor (VIG) was evaluated using a scale from 0 to 5 based on sperm progressive movement, where 0 = none, 1 = very weak, 2 = weak, 3 = intermediate, 4 = strong, and 5 = very strong.
In addition, each semen aliquot was diluted in buffered saline-formaldehyde (1:10), and sperm morphology was analyzed using a phase-contrast microscope. Spermatozoa were also evaluated with eosin-nigrosin staining using a bright-field microscope. Major sperm defects (MaD) were considered as the following: acrosome defect, abnormal head, double head, abnormal small head, proximal protoplasmic droplet, midpiece defect, accessory tail, and strongly bent tail. Minor sperm defects (MiD) included distal protoplasmic droplet, abaxial implantation, bent tail, and detached head. The total defects (TD) were considered in 200 sperm cells from each animal, and a sperm classification was performed as previously described by the Bull Breeding Soundness Evaluation of the Western Canadian Association of Bovine Practitioners (Barth 2000).
Infrared thermography measurement and analysis
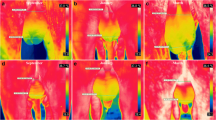
The mean temperature of the scrotal surface of each bull was evaluated by positioning the FLIR T 300 infrared camera 1 m from each testicular pair oriented perpendicular to the scrotum. The gradient between the images at 76,800 pixel (320 × 240) resolution with a thermal sensitivity of under 0.05 °C was evaluated. The temperatures from the proximal (proximal pole temperature; PPT) and distal (distal pole temperature; DPT) poles of the scrotum were measured using a line 1 pixel high located from side to the scrotum image for each region. The thermal gradient (TG) variation between these two extremities was also evaluated in this manner. To evaluate the bull’s thermal status, a lateral image 1 m from the animals’ head was recorded, and a circle was drawn around the orbital region including the ocular globe, the skin surrounding the ocular cavity and the lacrimal gland (OcT). The images generated from the radiation emitted by the body (thermograms) were later analyzed by Quick Report software 1.2. The infrared thermography measurement and analysis were conducted as previously described by Menegassi et al. (2015).
Statistical analyses
Statistical analysis was performed using mixed models (SAS Inst. Inc., Cary, NC, USA) with fixed effects for season and random effects for day and bull evaluation. The statistical model regarding the analysis of the variables studied was represented by Y ijk = μ + T i + β j + γ k + ε ijk, where Y ijk is the dependent variable, μ is an average inherent to all observations, T i represents the effect of season, β j is the random effect of animal (bull), γ k is the random effect of day evaluation (repeated measured on time), and ε ijk represents the experimental error. The day evaluations were considered to be repeated measures, where each day represented the measurement on the same animal. Data normality and homoscedasticity were verified using the Shapiro-Wilk test (P > 0.05). When necessary, data were transformed (logarithmic and square root transformations were used). The means of the seasons were compared using Tukey HSD test, considering a significance level of 5 % (P < 0.05). Pearson correlation analyses were used to correlate the temperature-humidity index and physiological and seminal parameters of bulls (P < 0.05).
Results
The daily mean temperature and relative humidity were used for the calculation of the daily values of THI for 1 year (Fig. 1). As expected, the THI was higher in the summer (91.0) compared to other seasons (P < 0.01). THI from the winter (67.2) season was similar to autumn (69.3). TG decreased in the summer (2.1) compared to autumn (6.6), spring (3.6), and winter (4.4) seasons (P < 0.01). Climate and physiological changes, spermiogenesis, and seminal parameters from bulls subjected to different THI are shown in Table 1.
Daily mean values of ambient air temperature (°C), relative humidity of air (%), and temperature-humidity index (THI) during the experimental period. Grey arrow indicates the day of semen collection, dashed grey lines indicate 18 days (spermiogenesis) and 12 days (epididymis transit) period between semen collections, and black lines indicates THI mean during each period of 30 days (two black lines per season)
Sperm M in spring, summer, and autumn (60.1 %, 57.6 % and 64.5 %, respectively) showed difference compared to winter (73.0 %; P < 0.01). When analyzed the main morphological changes, the presence of distal protoplasmic drops were significantly higher in spring and summer compared to autumn (P < 0.01). In addition, spermatozoa with distal midpiece reflex (DMR) were strongly correlated with THI during the epididymal transit (0.72; P < 0.05), but no effect on minor and total defects was observed in our study along the bull breeding soundness evaluation (Table 2).
TG was negatively correlated with spermiogenesis (18 days) period (−0.76; P < 0.05) and epididymal transit (12 days) period THI (−0.85; P < 0.01). OcT had a positive correlation with 18 days (0.78; P < 0.05) and with 12 days THI (0.84; P < 0.01). Motility showed a negative correlation with 18 days THI (−0.79; P < 0.05). During spermiogenesis (18 days period), the TG was negatively correlated with OcT (−0.97; P < 0.01) and RT (−0.72; P < 0.05) (Table 3). During epididymal transit (12 days period), the TG was negatively correlated with THI (−0.85; P <0.01) and OcT (−0.84; P < 0.05) (Table 4).
Discussion
This study shows that infrared thermography can be used as an indirect technique to assess the effects of heat stress on reproductive parameters of bulls, showing that the scrotal TG decreases in summer compared to autumn, winter, and spring. The successful evaluation of seasonal effects on thermoregulation and reproductive changes is crucial to identify these alterations in bovine physiology and health.
In regions with higher THI values, feeding behavior and survival rates tend to be more affected due to the longer periods of animal environmental exposure caused by heat stress (Hahn and Mader 1997). In these scenarios, the index above the critical value combined with high temperatures may cause difficulty of body heat dissipation at night. The use of experimental models that take in consideration the radiation and wind speed is decisive to protect the animals by ensuring their thermal comfort through the mitigation of the environment effects (Mader et al. 2010). In this experiment, the THI average at 30 days prior to semen collection in summer was 91.0 and did not cause significant morphological changes in the semen parameters over the seasons. Therefore, it could be that THI of 93.0 and 88.0 observed during 18 (spermiogenesis) and 12 days (epididymis transit) prior to semen collection were not detrimental for semen quality and for morphological changes. In agreement, Menegassi et al. (2015) found no morphological changes during spermiogenesis with THI higher than 83.8 in Bradford bulls.
The sperm M differed significantly only in winter compared to spring and summer. The TG had a negative interaction with the THI, OcT, and RT. All those aspects occurred during both spermiogenesis and epididymal transit. A higher percentage of DMR on sperm associated with increase of THI and decrease of M was observed in the summer, suggesting alterations on spermatozoa maturation stage during this season. Otherwise, in Angus, DMR can be associated with low concentration of testosterone or related with heat stress (Cassady et al. 1953; Barth and Bowman 1994). Recently, Barth (2000) found a hereditary predisposition of DMR in semen of Angus and Jersey bulls. Thus, it is important to highlight that crossbreed bulls (6 out of 20) used in our study had the same Brangus parental origin.
Motility was significantly higher in winter than in other seasons, demonstrating that the testicular structure is less affected by the ambient temperature than the epididymis structure. This idea is reinforced with the strong interaction between THI and DMR (0.72; P < 0.05) during the epididymis transit. High ambient temperatures may increase DMR during sperm maturation compromising mitochondrial activity. Cytoplasmic droplet might be associated with DMR that prevents the drop output (Barth 2000). In the human epididymis, the excess residual cytoplasm contains elevated levels of cytoplasm enzymes that produce pathological amounts of reactive oxygen species (ROS) (Rengan et al. 2012). A study performed in tropical climates using Simmental and Nellore bulls demonstrated variations in morphology, M, VIG, and MM during all seasons for 1 year and its association with a higher levels ROS production and lower activity of antioxidant enzymes in the Simmental semen (Nichi et al. 2006). Effects of heat stress on bull reproductive performance are frequently conducted by the simulation of the natural environment in climatic chambers or by scrotal insulation (Kastelic et al. 1996; Fernandes et al. 2008). Indeed, climatic chambers are artificial environmental in which the animals present reactions often much different from those have in open fields conditions. Thus, this study emphasized the data provide from natural conditions in which animals are submitted to environmental stress during production and reproductive performance.
It is known that metabolic alterations occur as a result of thermal stress and may lead to the exhaustion of the cellular energy reserves. The same metabolic pathways may change according to the source of energy supply, leading to new adaptive strategies in homoeothermic metabolism (Burns et al. 2010; Baumgard and Rhoads 2013; Rhoads et al. 2013). Although significant differences in sperm defects by season was not found, the presence of distal cytoplasmic droplet in 3.90 % (spring) and 3.24 % (summer) and a negative correlation of the DMR and THI during the spermatozoa passage thought the epididymis was determined. Sperm cells may have adaptation strategies due heat stress to maintain their metabolic functions.
No correlation between THI and RT for all seasons was observed. In agreement, Silva et al. (2007) and Ávila et al. (2013) found no difference when THI and RT were evaluated in Holstein and Jersey cows’ temperature in different regions of Brazil. Rectal temperature is a parameter for animal body temperature, and the thermoregulation mechanism adopted to keep the animals temperature stable are more effective than the mechanisms involved to regulate the temperature on animals surface as in the scrotal skin or ocular region. Accordingly, Menegassi et al. (2015) studied bulls’ reproduction on spring in environment with THI of 77.5 and when Ávila et al. (2013) studied Holstein cows with THI of at 72.0 in the same season, neither one found a correlation between THI and RT.
Studies on livestock thermal comfort where THI values were higher than 79.0 demonstrated deleterious effects in milk production (Rosemberg et al. 1983) indicating that this parameter should be used for the adoption of mitigation measures. Moreover, Dikmen and Hansen (2009) defined a THI limit of 78.2 for milk cow’s production as a key factor that decrease milk production. However, these experiments were conducted in dairy cows or under the artificial conditions of climatic chambers. According to this data, it was noticed that bulls have adopted different behavioral strategies to dissipate heat in an environment with THI of 93.0 or higher temperature index. Consequently, the RT had a small oscillation and the morphological quality of semen did not suffer substantially changes in order to reprove animals for breeding programs.
The present study demonstrated that infrared thermography can be used as an indirect technique to evaluate the effect of environmental stress on bull reproductive traits. Additionally, physiological changes in Brangus bulls during summer based on low scrotal TG and high OcT measurements was observed. The main morphological changes that occurred in bulls’ semen when the THI reached highest value (summer) were related to the epididymal transit and not with the final phase of the spermatogenesis. However, these changes were not significant to eliminate bulls on the BBSE.
Conclusion
Seminal characteristics of crossbreeding bulls are not affected when THI reaches 93.0 (spermiogenesis) and 88.0 (epididymis transit). It could be concluded that bulls evaluated throughout the seasons demonstrated no seminal damage under natural conditions of environment with high THI values and that infrared thermography can be adopted as an indirect method in order to assess the effect of environmental changes in TG and OcT of Brangus bulls.
References
Ávila AS, Jácome IMTD, Faccenda A, Pannazolo DM, Muller ER (2013) Avaliação e correlação de parâmetros fisiológicos e índices bioclimáticos de vacas Holandesas em diferentes estações. Revista Eletrônica em Gestão, Educação e Tecnologia Ambiental 14:2878–2884. doi:10.5902/2236117010747
Barth AD (2000) Bull breeding soundness evaluation manual, 2nd edn. The Western Canadian Association of Bovine Practitioners, Saskatoon, 74 pp
Barth AD, Bowman PA (1994) The sequential appearance of sperm abnormalities after scrotal insulation or dexamethasone treatment in bulls. Can Vet J 35:93–102
Baumgard LH, Rhoads RP (2013) Effects of heat stress on post- absorptive metabolim and energetics. Annu Rev Anim Biosci 1: 311–317
Bohmanova J, Misztal I, Cole JB (2007) Temperature-humidity indices as indicators of milk production losses due to heat stress. J Dairy Sci 90:1947–1956. doi:10.3168/jds.2006-513
Bouraoui R, Lahmar M, Majdoub A, Djemali M, Belyea R (2002) The relationship of temperature-humidity index with milk production of dairy cows in a Mediterranean climate. Anim Res 51:479–491. doi:10.1051/animres:2002036
Burns BM, Fordyce G, Holroyd RG (2010) A review of factors that impact on the capacity of beef cattle females to conceive, maintain a pregnancy and wean a calf—implications for reproductive efficiency in northern Australia. Anim Reprod Sci 122:1–22. doi:10.1016/j.anireprosci.2010.04.010
Cassady RB, Myers RM, Legates JE (1953) The effect of exposure to high ambient temperature on spermatogenesis of the dairy bull. J Dairy Sci 36:14–20
Correa-Calderon A, Armstrong D, Ray D, DeNise S, Enns M, Howinson C (2004) Thermoregulatory responses of Holstein and Brown Swiss heat-stressed dairy cows to two different cooling systems. Int J Biometeorol 48:142–148. doi:10.1007/s00484-003-0194-y
Coulter GH (1988) Thermography of bull testes, 12th Technical Conference of Artificial Insemination and Reproduction. National Association of Animal Breeders, Columbia, pp 58–63
Fernandes CE, Dode MA, Pereira D, Silva AE (2008) Effects of scrotal insulation in Nellore bulls (Bos taurus indicus) on seminal quality and its relationship with in vitro fertilizing ability. Theriogenology 70:1560–1568. doi:10.1016/j.theriogenology.2008.07.005
Hahn GL, Mader TL (1997) Heat waves in relation to thermoregulation, feeding behavior and mortality of feedlot cattle. In: 5th international livestock environment symposium. ASAE, Minneapolis, pp 563–567
Hansen PJ (2009) Effects of heat stress on mammalian reproduction. Philos Trans: Biol Sci 364(1534):3341–3350. doi:10.1098/rstb.2009.0131
Hansen PJ (2013) Cellular and molecular basis of therapies to ameliorate effects of heat stress on embryonic development in cattle. Anim Reprod 10(3):322–333
Instituto Nacional de Meteorologia - INMET (2014) Normais Climatológicas. Available from: http://www.inmet.gov.br. Updated 2014 Aug 16; Cited 2015 Feb 10
Kastelic JP, Cook RB, Coulter GH, Wallins TE (1996) Environmental factors affecting measurement of bovine scrotal surface temperature with infrared thermography. Anim Reprod Sci 41:153–159. doi:10.1016/0378-4320(95)01460-8
Mader TL, Johnson LJ, Gaughant JB (2010) A comprehensive index for assessing environmental stress in animals. J Anim Sci 88:2153–2165. doi:10.2527/jas.2009-2586
Marai IFM, Haeeb AAM (2010) Buffalo’s biological functions as affected by heat stress — a review. Livest Sci 127:89–109. doi:10.1016/j.livsci.2009.08.001
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2008) Reproductive performance traits as affected by heat stress and its alleviation in sheep. Trop Subtrop Agroecosyst 8:209–234
McManus C, Louvandini H, Carneiro HC, Lima PRM, Neto JC (2011) Production indices for dual purpose cattle in central Brazil. Rev Bras Zootec 40(7):1576–1586. doi:10.1590/S1516-35982011000700025
Menegassi SRO, Barcellos JOJ, Dias EA, Koetz C Jr, Pereira GP, Peripolli V, McManus C, Canozzi MEA, Lopes FG (2015) Scrotal infrared digital thermography as a predictor of seasonal effects on sperm traits in Braford bulls. Int J Biometeorol 59(3):357–364. doi:10.1007/s00484-014-0847-z
National Research Council (1971) A guide to environmental research on animals. National Academy of Science, Washington
Nichi M, Bols PEJ, Züche RM, Barnabe VH, Goovaerts IGF, Barnabe RC, Cortada CMN (2006) Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology 66:822–828
Paim TP, Borges BO, Lima PMT, Gomes EF, Dallago BSL, Fadel R, Menezes AM, Louvandini H, Canozzi MEA, Barcellos JOJ, McManus C (2013) Thermographic evaluation of climatic conditions on lambs from different genetic groups. Int J Biometeorol 57:59–66. doi:10.1007/s00484-012-0533-y
Pineda MH, Faulkner LC (1980) Biology of sex. In: McDonald LE (ed) Veterinary endocrinology and reproduction. Lea & Febiger, Philadelphia, pp 208–234
Rengan AK, Agarwal A, Van der Linde M, Plessis SS (2012) An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol 10:92. doi:10.1186/1477-7827-10-92
Rhoads RP, Baumgard LH, Suagee JK (2013) Metabolic priorities during heat stress with an emphasis on skeletal muscle. J Anim Sci 91:2492–2503
Rosemberg NJ, Blad BL, Verma SB (1983) Microclimate: the biological environment. Wiley, New York
Silva RG (1999) Estimativa de balanço térmico por radiação em vacas holandesas exposta ao sol e à sombra em ambiente tropical. Rev Bras Zootec 28:1403–1411. doi:10.1590/S151635981999000600031
Silva RG, Morais DAEF, Guilhermino MM (2007) Evaluation of thermal stress indexes for dairy cows in tropical regions. Braz J Anim Sci 36(4):1192–1198. doi:10.1590/S1516-35982007000500028
West JW, Mullinix BG, Bernard JK (2003) Effects of hot, humid weather on milk temperature, dry matter intake and milk yield of lactating dairy cows. J Dairy Sci 86:232–242. doi:10.3168/jds.S0022-0302(03)73602-9
Wolfenson D, Thatcher WW (2012) Strategies for improvement of thermal and reproductive responses under heat stress. In: Collier RJ, Collier JL (eds) Environmental physiology of livestock, 1st edn. Wiley-Blackwell, United Kingdom, pp 181–190
Acknowledgments
This study was supported by The Brazilian Council of Scientific and Technological Development (Project CNPq/Universal No. 456724/2014-1) and The Coordination for the Improvement of Higher Education Personnel/CAPES, Brazil (Project CAPES/PNPD No. 2842/2010). The authors thank Agropecuária JMT situated in São Gabriel/RS for providing the animals and technical assistance for this study.
Conflict of interest
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menegassi, S.R.O., Pereira, G.R., Dias, E.A. et al. The uses of infrared thermography to evaluate the effects of climatic variables in bull’s reproduction. Int J Biometeorol 60, 151–157 (2016). https://doi.org/10.1007/s00484-015-1013-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-015-1013-y