Abstract
Key message
Models of bark thickness and trunk cavity occurrence improve allometry assessments and provide good indicators of the probability of tree decay or vitality—knowledge useful for old-growth tree conservation and management.
Abstract
This study aimed to model the attributes of Araucaria angustifolia that influence allometry assessments and its biomechanics. We used samples collected during dendrochronological studies to measure bark thickness and data from old-growth attribute surveys to assess the occurrence of trunk cavities (or hollows) associated with heartwood decay. First, nonlinear mixed-effects models were calibrated to predict bark thickness. Then the probability of hollow occurrence was evaluated with binomial regression using generalized linear models. Bark thickness increased with tree size, attaining an asymptote of 11.9 cm. This finding suggests that thickness above the asymptote may not offer additional protection to trees. Because bark has an influence on allometry assessments, we provide an accurate model to improve wood volume estimates. Hollows are associated with tree size and affect mechanical stability, and due to their empty space, it can cause bias in allometry assessment of biomass and wood volume. Suitable predictors of the probability of tree decay or vitality are provided. The predictors evaluated can also be used as a tree-level indicator of quality in selection systems. Our results highlight the importance of accounting for bark thickness and trunk hollows in carbon, conservation, and management surveys of Araucaria forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Araucaria angustifolia (Bertol.) Kuntze is a native conifer to southern and southeastern Brazil that occurs in cold and humid highland plateaus (500–2400 masl), into Argentina and Paraguay (Hueck 1953; Wrege et al. 2017). It is an economically important species within its natural range and has been a source of timber and food for many decades (Eisfeld et al. 2020; Souza et al. 2012). A. angustifolia is a key component of the mixed evergreen forest, also known as the Araucaria Forest, a forest type characterized by the dominance of this species. This forest type is mainly found in southern Brazil, where A. angustifolia has been described as a keystone species due to its role in maintaining the forest’s ecological balance (Mattos 1994). However, due to its intense exploitation for wood products and conversion of forest land to agriculture, it is currently facing a critical situation. The species is restricted to a few populations related to each other, with low haplotype diversity, which expanded possibly from a single refugium with human aid (Lauterjung et al. 2018; Robinson et al. 2018). This status has led to the species being classified as critically endangered in the International Union for Conservation of Nature’s Red List of Threatened Species (Thomas 2013). On the other hand, the wood of the species is highly valued for its quality and is used for a variety of purposes, including furniture making, construction, and paper production (Lorenzi 2002), while its pine nuts are one of the most important non-timber forest products in Brazil (Silva et al. 2020).
Timber tree species are susceptible to a wide range of defects that can reduce the amount of wood with desirable qualities (Moreau et al. 2023). A common example of defect in A. angustifolia is trunk cavities or tree hollows, characterized as an attribute of old-growth trees (Scipioni et al. 2022). A hollow tree may fail if the cavity is ~ ≥ 70% of the trunk radius (Mattheck et al. 2006; Mattheck 2007). Thus, hollows can compromise the mechanical stability of trees, making them more susceptible to breakage (Sterken 2005). Cavities also affect estimates of tree biomass and volume due to the empty space in trunks (Fearnside 1992). These biases are propagated to predictions at stand and landscape level, thereby compromising carbon assessments (Nogueira et al. 2006) and volume yield of timber harvested (Almeida et al. 2022). On the other hand, hollow trunks are microhabitats that provide ecological benefits and have positive effects on the biodiversity of forest ecosystems (Martin et al. 2022; Vuidot et al. 2011).
Thick bark is also a notable feature of A. angustifolia. Bark is all plant tissue external to the vascular cambium and includes conducting and non-conducting tissue (Roth 1981; Zimmermann and Brown 1980). As bark acts to protect living tissue, its thickness is thought to be mainly driven by its defensive function (Paine et al. 2010; Pausas 2015). Thus, the thicker bark in the Araucariaceae may be recognized as offering vital protection against insect attack, physical damage from abrasion or fire, and extreme weather events (Costa et al. 2021; Molina et al. 2016). Bark thickness influences both stem transpiration and respiration, reflecting strategies for growth and tolerance to water deficit (Loram-Lourenço et al. 2022), and is even associated with structural support and flexural rigidity of tree trunks (Niklas 1999). Another perspective is that bark can make up a large amount of the volume of a tree (Li and Weiskittel 2011). Although tree measurements are based on the diameter outside the bark, the volume of wood inside the bark is required for most forestry applications (Stängle et al. 2017); therefore, bark thickness and its relationships with tree diameter and volume must be accurately determined (Pemberton 1924; Muhairwe 2000).
As bark thickness and trunk cavities affect tree stability and cause uncertainties in biomass and wood volume estimation, they are essential knowledge both for conservation and management of timber species. The main aim of this study was to determine bark thickness and probability of trunk cavities occurrence in A. angustifolia as a function of tree size across young, mature, and old individuals. Therefore, we examined attributes at tree level that affect allometry assessments and biomechanics.
Materials and methods
Data collection and measurement
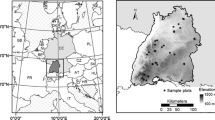
We used samples collected for dendrochronological studies (Scipioni et al. 2021) to measure tree bark thickness. Increment cores were collected from standing trees (Fig. 1d, e) and wood disks were extracted from fallen trees with preserved bark (Fig. 1f, g). Bark thickness was measured with a precision ruler later in the laboratory. These samples were collected at 9 sites, with 318 samples taken from 185 trees (Table S1). The diameter at 1.3 m above the ground (diameter at breast height [DBH]) of these trees was also measured in the field. To account for the probability of trunk cavities, we used data from old-growth attribute surveys (Fig. 1a, b, c) of 159 trees (Scipioni et al. 2022), of which 21 were visibly hollow, sampled at 13 sites (Table S1). DBH and total height (H) of these trees were measured using diametric tape and a laser dendrometer (Criterion RD 1000), and the number of reiterated trunks (NRT) was quantified visually (Fig. 1b). The dendrochronological samples (increment cores) were also used to assess possible trunk cavities not visible at the stem outer surface for all of the other sampled trees (n = 138). Further information on data collection, measurements, and sampling site location are available in Table S1 and Scipioni et al. (2021, 2022).
Modeling bark thickness
We evaluated the relationships between tree size (DBH) and bark thickness (BT, cm) and percentage (BP, %) using scatterplots. We then calibrated nonlinear biological models of bark thickness using tree diameter as a predictor. The data set was randomly categorized into training data (85%, 273 trees) and test data (15%, 45 trees) for calibration and model performance evaluation. Biophysical and growth rate models (Howell et al. 2022) were fitted for bark thickness modeling using the training data. We tested the following models: exponential (Eq. 1); Gompertz (Eq. 2); logistic (Eq. 3); Mitscherlich (Eq. 4); power (Eq. 5); and Weibull (Eq. 6).
where BT was bark thickness (cm), DBH was diameter at breast height (cm), a, b, and c were parameter estimates, and ε was the residual error. The nonlinear models were calibrated with the maximum likelihood method using the nls function in the nlme package (Pinheiro et al. 2020) in R version 4.0.3 (R Core Team 2021).
The best model was selected based on the Akaike information criterion (AIC), root mean square error (RMSE), and coefficient of determination (R2) computed using the square of the correlation between observed and estimated values. Therefore, the model that maximized R2 and minimized AIC and RMSE was selected. In addition, graphical analysis was performed to verify whether the models provided adequate biological representation (Vanclay and Skovsgaard 1997) and to examine the assumptions of normality and homogeneity of variances.
After selecting the best model functional form, the random effect of sites was incorporated using nonlinear mixed-effects (NLME) modeling (Eq. 7) with the nlme function in the homonymous package in R.
where BT was bark thickness modeled as a non-linear function of DBH (f), α was site as random effect, and ε was the residual error. This procedure allows model parameters to vary between sites (Hulshof et al. 2015) and accounts for data non-independence (Banin et al. 2012). This provided a significantly better fit compared to the previous fixed-effects model (likelihood ratio test, p < 0.001). The final NLME model was also evaluated based on AIC, RMSE, R2 criteria, and graphical analysis of statistical assumptions.
Bark thickness and diameter inside bark (DWB) measured for the test data were compared to estimates from the final NLME to examine model performance. To test whether BT and DWB differed significantly between measurements and estimates, we applied the non-parametric Wilcoxon rank-sum test (α = 0.05). DWB was calculated by subtracting bark thickness multiplied by two from DBH (Muhairwe 2000).
Modeling the probability of trunk cavities
Binomial regression was used to determine the probability of trunk cavity occurrence in A. angustifolia trees. The presence of hollow trunks was categorized as a binary variable (0 = non-hollow and 1 = hollow). Then generalized linear models (GLMs) were calibrated to model the probability of cavity occurrence as a function of tree size attributes using the binomial distribution and the logit link function (Eq. 8).
where HP was the probability of hollow trunk, X was DBH, H, or NRT, a and b were parameter estimates, and ε was the residual error. Parameters of tree size—DBH, H, and NRT—were used as single predictors in the GLM. The model’s performance was evaluated based on the AIC and the percentage of variance explained by the model. The significance of βi coefficients indicated by the t-statistic was used to test the effect of predictors on the probability of hollowness. The GLMs were calibrated using base functions in R version 4.0.3 (R Core Team 2021).
We then used height–diameter allometry to examine the mechanical support capacity of trees with basal cavities. The height–diameter relationship may reflect a trade-off between growth and survival (King et al. 2006) and plays a key role in studies related to trees mechanical support (McMahon 1973). We fitted the power allometric model to height and diameter data for 159 trees, separately for non-hollow (n = 138) and hollow (n = 21) trees. NLME modeling was again applied to account for site effects as a random factor (Eq. 9) using the nlme function in the homonymous package in R language.
where H was total height (m) of non-hollow or hollow trees, DBH was diameter at breast height (cm), a and b were parameter estimates, α0 and α1 were random effect terms, and ε was the residual error. Lastly, we compared the estimated coefficients (b) with the predictions of metabolic theory (Niklas and Spatz 2004) that height increases consistently with diameter raised to 2/3 power, and, therefore, the slope coefficient of the model is 0.667. The NLME power model fitting and evaluation followed the same statistical procedure used in NLME bark thickness models.
Results
Bark thickness
Bark thickness exhibited asymptotic growth as a function of tree size (Fig. 2a), with a tendency to stabilize in trees with DBH > 150 cm. The percentage of bark showed exponential decay (Fig. 2b), decreasing at a constant rate. Scatterplots indicated that some small trees had thick bark, and consequently, a high percentage of bark in DBH, while the percentage of bark of large trees was low even with thicker bark. Among the models tested, the logistic model (3) maximized R2 and minimized AIC and RMSE (Table 1) and provided significant parameters (p < 0.001). In addition, the errors in this model were normally distributed (Fig. S1a) and the variance was homogeneous (Fig. S1b). Therefore, the logistic model was selected as the best for modeling bark thickness. The sigmoidal shape of the logistic model provided adequate biological representation and identified an asymptote of 11.9 cm for bark thickness as tree size increased (Fig. 3).
After the logistic model for bark estimation was applied, the random effect of site was significant (likelihood ratio test, p < 0.001), denoting a likely influence of location or sampling on predictions. The NLME logistic model, in addition to accounting for non-independence, improved predictions since it maximized R2 and minimized AIC and RMSE (Table 2) compared to the previous fixed-effects model. The NLME model also fulfilled the assumptions of normality and homogeneity of variances (Fig. S1). In the tests of the model for new predictions (test data), we did not find differences between estimated and observed values of bark thickness (Fig. S2a, p = 0.91) and diameter without bark (Fig. S2b, p = 0.93), demonstrating its accuracy for assessing A. angustifolia trees.
Probability of trunk cavities
Binomial GLMs showed that tree size and reiterated trunks had a significant positive effect (p < 0.001) on the occurrence of basal trunk cavities (Table 3). Diameter was selected as the best predictor of trunk cavity occurrence (lower AIC and higher explained variance), followed by height and the number of reiterated trunks. The probability of trunk cavity occurrence tended to increase with increases in all predictors (Fig. 4). Trees with DBH up to 70 cm did not have hollows, while trees with DBH > 200 cm showed a high probability of cavity occurrence (> 75%). Only trees over 25 m tall have a trunk basal cavity, with the probability of a cavity occurrence greater than 75% for those surpassing 40 m in height. Although the presence of reiterated trunks contributed less to explaining the presence of trunk cavities (higher AIC and lower explained variance), it still is a good predictor of the probability of cavity occurrence as it is easily assessed visually.
Only trees with a DBH of > 280 cm and six or more reiterated trunks showed the maximum probability of trunk cavity occurrence (Fig. 4). In fitting the NLME power model to test the influence of basal hollows on the mechanical stability of trees (Fig. 5), only trees without such basal cavities showed an allometric coefficient adhering to theoretical predictions of metabolic theory (0.667, p < 0.001). In contrast, trees with trunk cavities had a lower allometric coefficient than predicted (0.608, p < 0.001), denoting a lower rate of height growth.
Discussion
Relationship between bark thickness and tree size
According to the selected model, bark thickness tended to increase with tree size, reaching an asymptote of 11.9 cm that corresponded to an average DBH of 200 cm. Although the thickness pattern of A. angustifolia bark has been investigated previously (Costa et al. 2021), an asymptote has not been reported to date due to the small size of trees in previous studies compared to the sample in the present study (DBH range of 10–325 cm). An asymptotic increase in thickness is expected for tree bark, because beyond a certain limit, the additional thickness may not confer further survival advantages (Paine et al. 2010; Wilson and Witkowski 2003)—although the tree protection mechanisms provided by bark are complex (Molina et al. 2016). Furthermore, the asymptotic bark thickness may be related to lower metabolic activity as trees grow (McMahon 1973, King 2006), limiting bark production due to lower phellogen activity. However, asymptotic growth is mainly expected in species with bark shedding (Roth 1981) such as A. angustifolia (Costa et al. 2021). In turn, the biologically realistic allometric functions (Howell et al. 2022) for the bark thickness × DBH relationship of this species had not yet been explored. Logistic and Gompertz growth rate models provided a better fit for and biological representation of this relationship, relative to Mitscherlich and Weibull models (which generated nonsignificant coefficients) and power model which indicated continuous growth. However, all models performed similarly in the case of smaller trees (DBH < 120 cm).
The results revealed that large trees have a low percentage of bark, unlike small trees whose bark is less thick but occurs at a higher percentage. This suggests that in A. angustifolia, the bark may have an important structural role in small trees (Niklas 1999) and greater protective capacity in large trees (Paine et al. 2010). These results also demonstrate that bark can affect allometric estimates of both large and small trees. However, the selected model showed high accuracy in training and test procedures, especially when site effects were accounted for by mixed-effects modeling. The site effects emphasize the need for a more comprehensive sampling across Araucaria forests, to produce model estimates applicable at larger spatial and environmental scales. Bark thickness can be influenced by several conditions at the tree and site level; thus, bark models are common in forest surveys to estimate inside-bark diameters (Stängle et al. 2017). Although these models have been applied to few tree species in the Brazilian Atlantic Forest, they are well known elsewhere for other timber species and stand types, such as European mixed-species and pure spruce stands (Stängle et al. 2017), Australian eucalyptus in natural and planted stands (Muhairwe 2000), and North American conifers in even-aged naturally regenerated stands (Li and Weiskittel 2011).
Although bark thickness varies along the trunk according to height (Muhairwe 2000; Stängle et al. 2017), only bark measured at breast height was evaluated in the present study as the aim was to test its influence on diameter—the main forestry predictor of tree allometry (Chave et al. 2014). Other tree-level variables such as height, crown base height, branch arrangement, and sociological position can also affect A. angustifolia bark thickness (Costa et al. 2021). This highlights the need for further studies to elucidate the environmental, ecological, and silvicultural drivers of A. angustifolia bark thickness, and its influences on biomass and wood volume estimation. In addition, we believe that A. angustifolia may be the species with the thickest bark in Brazilian humid forests; this emphasizes the importance of selecting an appropriate model for accurate estimates of bark thickness and diameter inside bark.
Relationships between probability of trunk cavities and tree attributes
Our results highlight that tree structural attributes such as trunk cavities and reiterated trunks become characteristic of large old-growth A. angustifolia trees (Scipioni et al. 2022), but are absent in smaller, younger trees; these findings are consistent with the hypothesis that the probability of a decline in wood quality increases as trees get old (Moreau et al. 2023). Thus, older age is an important driver of Araucaria cavity occurrence, since mature and old trees are strongly related to loss of vigor (reduced growth), greater wood decay, and mortality (King et al. 2006; Dey et al. 2017). The vigor reduction is also notable in A. angustifolia, which decrease its survival (Cysneiros et al. 2023) and relative growth rate for large and old trees (Stepka et al. 2021). Although tree size and age are the main drivers of trunk cavity occurrence, such hollows may originate from combinations of multiple factors not addressed in our analysis, such as the presence of crown damage, historical fire occurrences, unmanaged/stocked forests, higher average temperature (Moreau et al. 2023), spacing between neighboring trees, and bark damage (Vanclay 2022), in addition to species traits, such as wood density (Eleuterio et al. 2020) and vitality (Vuidot et al. 2011). Therefore, further studies considering the effects of competition (crown spacing, tree damage, etc.) and environmental factors (climate, soil, susceptibility to wildfires, etc.) are still needed to explain the occurrence of trunk cavities in A. angustifolia.
Three theories about variations in tree height allometry (Banin et al. 2012; Hulshof et al. 2015) may partly explain the observed differences in A. angustifolia height:diameter relationships between smaller trees without trunk cavities and larger trees that commonly have basal trunk hollows. (1) The mechanical constraints theory states that trees may stop growing once reaching a certain height to avoid buckling (McMahon 1973); thus perhaps the population of A. angustifolia trees that is large and old enough to develop cavities has already almost reached their size limit as a strategy of not breaking or toppling under their own weight, and thus has a different tree height:diameter allometry than the population of smaller non-hollow trees. Further, a related direct explanation is that as older and larger-diameter trees become likely to develop basal cavities with heartwood decay, there are associated reductions in mechanical stability of basal trunks and roots that results in greater stem breakage and tip-overs during storm events, thereby eliminating some of the tallest trees from the population of trees with cavities, and flattening their height:diameter relationship. (2) The hydraulic limitation theory suggests that the challenges of maintaining hydraulic conductance in canopy leaves and lower availability of water in sapwood cross-sections restrict apical height growth of taller trees (McDowell and Allen 2015; Ryan et al. 2006), ultimately causing a height asymptote (Niklas and Spatz 2004); by extension, perhaps large A. angustifolia trees that have basal cavities may grow even less height-wise than undamaged trees due to reduced vertical water transport capacities from the reduced basal xylem area (Mattheck et al. 2006; Sterken 2005), thus the lower allometric coefficients for trees with basal hollows. (3) The metabolic theory predicts that tree height increases as a function of diameter (Niklas and Spatz 2004), in which lower allometric coefficients may indicate a decrease in the height growth capacity of old trees with trunk cavities, reflecting successful adjustments to long-term survival.
General implications
Our results provide useful information for allometry assessment of A. angustifolia since bark thickness (Cochran 1982; Muhairwe 2000) and trunk cavities (Nogueira et al. 2006) cause overestimation of biomass and wood volume (Fearnside 1992; Hauck et al. 2023). Furthermore, as trunk hollows compromise the mechanical stability of trees, their potential predictors can be used as indicators of wood production quality. The findings of this study can be used to prescribe a maximum tree size beyond which it may not be advantageous to keep the trees from an economic perspective, due to elevated risk of developing tree cavities and associated wood defect (Dey et al. 2017; Mattheck et al. 2006; Moreau et al. 2023) and consequent reduction in forest timber stocks (Liu et al. 2013; Almeida et al. 2022). We found that in addition to diameter, the number of reiterated trunks was another predictor that can be used for tree quality assessment. Besides being able to be easily analyzed visually, it is an indicator of trees with a higher probability of developing cavities with decay, and thus is a potential indicator of commercial wood defect. These findings have significant implications for commercial tree management via selection systems (Moreau et al. 2023; Vanclay 2022).
However, it is also crucial to recognize the essential ecological roles of tree cavities and hollow trunks in providing unique and irreplaceable microhabitats that are necessary and beneficial for a wide range of biodiversity (Gibbons and Lindenmayer 2002; Martin et al. 2022; Michel and Winter 2009). Ecologically the reiterated trunks can be an indicator of high tree vitality after a top-damaging event, representing an old-growth structure related to crown recovery that also provides unique and rare microhabitats that are essential to support biodiversity (Scipioni et al. 2022). Cavities and reiterated trunks are such important forest structural features on the landscape that they must be recognized even in managed stands, as they provide increasingly scarce structural attributes in forest landscapes (Larrieu et al. 2018; Lindenmayer et al. 2012), particularly in Araucaria forests of south Brazil. In addition, the largest and oldest trees, despite being characterized by the presence of trunk cavities and other old-age commercial “defects” (Hauck et al. 2023), are well-known to store disproportionately large amounts of carbon in their woody structures (Lutz et al. 2018; Stephenson et al. 2014)—and reiterated trunks represent an additional tree carbon pool. Thus, managing forests to include conservation of old and large-diameter trees is an important way to enhance a variety of valuable ecosystem services (Lutz et al. 2018), from maintenance of irreplaceable biodiversity to acting as a climate nature-based solution to support forest carbon storage. Certainly, both the ecological benefits and economic trade-offs of tree cavities and hollow trunks must be considered in forest management and conservation.
Conclusion
Bark thickness of Araucaria angustifolia increased asymptotically with tree size, stabilizing only in large trees. Although thick bark provides effective protection against abiotic and biotic damage, tree biomass and wood volume estimates can be biased if bark is not considered. However, the model selected in this study provided accurate estimates of bark thickness to improve allometry assessments. Tree cavities and trunk hollows are associated with tree size and may affect tree biomechanics. The attributes evaluated in this study (diameter at breast height, height, and number of reiterated trunks) are suitable predictors of the probability of tree decay and related essential biodiversity values. Finally, the tree-level indicators provided here can aid both timber management and forest conservation efforts, since these indicators can support decisions to retain or eliminate trees in selection forestry systems aiming that also aim to maintain important ecosystem services.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the authors upon request.
References
Almeida VB, Silva JNM, Steinbrenner MKA (2022) Impact of occurrence of hollow trees on the wood volume yield of timber harvested in the Saracá-Taquera National Forest, Pará State, Brazil. Pesq Flor Bras 42:e201902058. https://doi.org/10.4336/2022.pfb.42e201902058
Banin L, Feldpausch TR, Phillips OL et al (2012) What controls tropical forest architecture? Testing environmental, structural and floristic drivers: determinants of tropical forest architecture. Glob Ecol Biogeog 21:1179–1190. https://doi.org/10.1111/j.1466-8238.2012.00778.x
Chave J, Réjou-Méchain M, Búrquez A et al (2014) Improved allometric models to estimate the aboveground biomass of tropical trees. Glob Change Biol 20:3177–3190. https://doi.org/10.1111/gcb.12629
Cochran PH (1982) Estimating wood volume for Douglas fir and white fir from outside bark measurements. For Sci 28:172–174. https://doi.org/10.1093/forestscience/28.1.172
Costa EA, Liesenberg V, Finger CAG et al (2021) Understanding bark thickness variations for Araucaria angustifolia in southern Brazil. J for Res 32:1077–1087. https://doi.org/10.1007/s11676-020-01163-1
Cysneiros VC, Pelissari AL, Pellico Netto S, Machado SA, Figueiredo Filho A (2023) Population structure of Araucaria angustifolia under distinct forest protection status: implications for management and conservation. J for Res 34:1437–1446. https://doi.org/10.1007/s11676-023-01603-8
Dey DC, Dwyer J, Wiedenbeck J (2017) Relationship between tree value, diameter, and age in high-quality sugar Maple (Acer saccharum) on the Menominee Reservation, Wisconsin. J for 115:397–405. https://doi.org/10.5849/jof.2016-026R1
Eisfeld RL, Arce JE, Sanquetta CR, Braz EM (2020) Is it forbidden the wood use of Araucaria angustifolia? An analysis on the current legal budget. Floresta 50:971–982. https://doi.org/10.5380/rf.v50i1.60023
Eleuterio AA, Jesus MAD, Putz FE (2020) Stem decay in live trees: heartwood hollows and termites in five timber species in Eastern Amazonia. Forests 11:1087. https://doi.org/10.3390/f11101087
Fearnside PM (1992) Forest biomass in Brazilian Amazônia: comments on the estimate by Brown and Lugo. Interciência 17:1
Gibbons P, Lindenmayer D (2002) Tree hollows and wildlife conservation in Australia. CSIRO Publ. https://doi.org/10.1071/9780643090033
Hauck M, Csapek G, Dulamsuren C (2023) The significance of large old trees and tree cavities for forest carbon estimates. For Ecol Manage 546:121319. https://doi.org/10.1016/j.foreco.2023.121319
Howell SR, Song G-ZM, Chao K-J, Doley D (2022) Functional evaluation of height–diameter relationships and tree development in an Australian subtropical rainforest. Aust J Bot 70:158–173. https://doi.org/10.1071/BT21049
Hueck K (1953) Distribuição e habitat natural do Pinheiro do Paraná (Araucaria angustifolia). Botânica 10:3–24
Hulshof CM, Swenson NG, Weiser MD (2015) Tree height-diameter allometry across the United States. Ecol Evol 5:1193–1204. https://doi.org/10.1002/ece3.1328
King DA, Davies SJ, Noor NSM (2006) Growth and mortality are related to adult tree size in a Malaysian mixed dipterocarp forest. For Ecol Manage 223:152–158. https://doi.org/10.1016/j.foreco.2005.10.066
Larrieu L, Paillet Y, Winter S, Bütler R, Kraus D, Krumm F, Lachat T, Michel AK, Regnery B, Vandekerkhove B (2018) Tree related microhabitats in temperate and Mediterranean European forests: a hierarchical typology for inventory standardization. Ecol Ind 84:194–207. https://doi.org/10.1016/j.ecolind.2017.08.051
Lauterjung MB, Bernardi AP, Montagna T et al (2018) Phylogeography of Brazilian pine (Araucaria angustifolia): integrative evidence for pre-Columbian anthropogenic dispersal. Tree Genet Genomes 14:36. https://doi.org/10.1007/s11295-018-1250-4
Li R, Weiskittel AR (2011) Estimating and predicting bark thickness for seven conifer species in the Acadian Region of North America using a mixed-effects modeling approach: comparison of model forms and subsampling strategies. Eur J Forest Res 130:219–233. https://doi.org/10.1007/s10342-010-0423-y
Lindenmayer DB, Laurance WF, Franklin JF (2012) Global decline in large old trees. Science 338:1305–1306. https://doi.org/10.1126/science.1231070
Liu Y, Yu G, Wang Q, Zhang Y, Xu Z (2013) Carbon carry capacity and carbon sequestration potential in China based on an integrated analysis of mature forest biomass. Sci China Life Sci 57:1218–1229. https://doi.org/10.1007/s11427-014-4776-1
Loram-Lourenço L, Farnese FS, Alves RDFB et al (2022) Variations in bark structural properties affect both water loss and carbon economics in neotropical savanna trees in the Cerrado region of Brazil. J Ecol 110:1826–1843. https://doi.org/10.1111/1365-2745.13908
Lorenzi H (2002) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil. 4th edn. Instituto Plantarum, Nova Odessa
Lutz JA, Furniss TJ, Jonhson DJ et al (2018) Global importance of large-diameter trees. Glob Ecol Biogeo. https://doi.org/10.1111/geb.12747
Martin M, Paillet Y, Larrieu L et al (2022) Tree-related microhabitats are promising yet underused tools for biodiversity and nature conservation: a systematic review for international perspectives. Front for Glob Change 5:818474. https://doi.org/10.3389/ffgc.2022.818474
Mattheck C, Bethge K, Tesari I (2006) Shear effects on failure of hollow trees. Trees Struct Funct 20:329–333. https://doi.org/10.1007/s00468-005-0044-0
Mattheck C (2007) Field guide for visual tree assessment. Forschungszentrum Karlsruhe Gm
Mattos JR (1994) O Pinheiro Brasileiro. Lages
McDowell NG, Allen CD (2015) Darcy’s law predicts widespread forest mortality under climate warming. Nat Clim Chang 5:669–672
McMahon TA (1973) Size and shape in biology. Science 179:1202–1204
Michel AK, Winter S (2009) Tree microhabitat structures as indicators of biodiversity in Douglas-fir forests of different stand ages and management histories in the Pacific Northwest, U.S.A. For Ecol Manage 257:1453–1464. https://doi.org/10.1016/j.foreco.2008.11.027
Molina JGA, Hadad MA, Patón Domínguez D, Roig FA (2016) Tree age and bark thickness as traits linked to frost ring probability on Araucaria araucana trees in northern Patagonia. Dendrochronologia 37:116–125. https://doi.org/10.1016/j.dendro.2016.01.003
Moreau G, Cecil-Cockwell MJL, Achim A et al (2023) Quantifying the probability of decline in quality: implications for selection management in Northern Hardwood Forests. Forests 14:280. https://doi.org/10.3390/f14020280
Muhairwe CK (2000) Bark thickness equations for five commercial tree species in regrowth forests of Northern New South Wales. Aust for 63:34–43. https://doi.org/10.1080/00049158.2000.10674811
Niklas KJ (1999) The mechanical role of bark. Am J Bot 86:465–469
Niklas KJ, Spatz H-C (2004) Growth and hydraulic (not mechanical) constraints govern the scaling of tree height and mass. PNAS 101:15661–15663. https://doi.org/10.1073/pnas.0405857101
Nogueira EM, Nelson BW, Fearnside PM (2006) Volume and biomass of trees in central Amazonia: influence of irregularly shaped and hollow trunks. For Ecol Manage 227:14–21. https://doi.org/10.1016/j.foreco.2006.02.004
Paine CET, Stahl C, Courtois EA et al (2010) Functional explanations for variation in bark thickness in tropical rain forest trees: bark thickness in tropical trees. Funct Ecol 24:1202–1210. https://doi.org/10.1111/j.1365-2435.2010.01736.x
Pausas JG (2015) Bark thickness and fire regime. Funct Ecol 29:315–327. https://doi.org/10.1111/1365-2435.12372
Pemberton JE (1924) The relation of bark to diameter and volume in Redwood. J for 22:44–48
Pinheiro J, Bates D, Debroy S, Sakar D, Heisterkamp S, Willigen BV (2020) nlme: linear and nonlinear mixed effects models. R Package Version 3:1–149
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. www.R-project.org/
Robinson M, De Souza JG, Maezumi SY et al (2018) Uncoupling human and climate drivers of late Holocene vegetation change in southern Brazil. Sci Rep 8:12. https://doi.org/10.1038/s41598-018-24429-5
Roth I (1981) Structural patterns of tropical barks. Borntraeger, Berlin
Ryan MG, Phillips N, Bond BJ (2006) The hydraulic limitation hypothesis revisited. Plant Cell Environ 29:367–381. https://doi.org/10.1111/j.1365-3040.2005.01478.x
Scipioni MC, Fontana C, Morales J et al (2021) Effects of cold conditions on the growth rates of a subtropical conifer. Dendrochronologia 68:125858. https://doi.org/10.1016/j.dendro.2021.125858
Scipioni MC, Higuchi P, Fockink GD, Allen CD (2022) Old-growth structural attributes associated with the last giant subtropical conifers in South America. Austral Ecol 47:1271–1285. https://doi.org/10.1111/aec.13212
Silva TC, Araújo ECG, Lins TRS, Reis CA, Sanquetta CR, Rocha MP (2020) Non-timber forest products in Brazil: a bibliometric and a state of the art review. Sustainability 12:7151. https://doi.org/10.3390/su12177151
Souza AF, Cortez LSR, Longhi SJ (2012) Native forest management in subtropical South America: long-term effects of logging and multiple-use on forest structure and diversity. Biodivers Conserv 21:1953–1969. https://doi.org/10.1007/s10531-012-0287-1
Stängle SM, Sauter UH, Dormann CF (2017) Comparison of models for estimating bark thickness of Picea abies in southwest Germany: the role of tree, stand, and environmental factors. Ann Forest Sci 74:16. https://doi.org/10.1007/s13595-016-0601-2
Stephenson NL, Das AJ, Condit R et al (2014) Rate of tree carbon accumulation increases continuously with tree size. Nature 507:90–93. https://doi.org/10.1038/nature12914
Stepka TF, Mattos PP, Figueiredo Filho A, Braz EM, Machado SA (2021) Growth of Araucaria angustifolia, Cedrela fissilis and Ocotea porosa in different locations within the Araucaria forest in the southern Brazil. For Ecol Manage 486:118941. https://doi.org/10.1016/j.foreco.2021.118941
Sterken P (2005) A guide for tree-stability analysis. Edited by Autor, Plasencia.
Thomas P (2013) Araucaria angustifolia. The IUCN Red List of Threatened Species. http://www.iucnredlist.org. Accessed 27 Jan 2015.
Vanclay JK (2022) Hollow occurrence and tree spacing in Eucalyptus tereticornis. Aust for 85:105–115. https://doi.org/10.1080/00049158.2022.2137956
Vanclay JK, Skovsgaard JP (1997) Evaluating forest growth models. Ecol Model 98:1–12. https://doi.org/10.1016/S0304-3800(96)01932-1
Vuidot A, Paillet Y, Archaux F, Gosselin F (2011) Influence of tree characteristics and forest management on tree microhabitats. Biol Conserv 144:441–450. https://doi.org/10.1016/j.biocon.2010.09.030
Wilson BG, Witkowski ETF (2003) Seed banks, bark thickness and change in age and size structure (1978–1999) of the African savanna tree, Burkea africana. Plant Ecol 167:151–162. https://doi.org/10.1023/A:1023999806577
Wrege MS, Fritzsons E, Soares MTS et al (2017) Distribution and habitat of Brazilian-pine according to global climate change. Pesq Flor Bras 37:331–346. https://doi.org/10.4336/2017.pfb.37.91.1413
Zimmermann MH, Brown CL (1980) Trees: structure and function. Springer, Berlin
Acknowledgements
The authors thank the National Council for Scientific and Technological Development—CNPq for funding support (Project Grant: 423912/2018-6), and three anonymous reviewers for their contributions.
Funding
This work was supported by National Council for Scientific and Technological Development (Project Grant: 423912/2018-6).
Author information
Authors and Affiliations
Contributions
VCC: conceptualization, methodology, data analysis, writing—original data preparation; MCS: conceptualization, methodology, data curation and collection, writing—reviewing and editing; CDA: writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare they have no competing of interest.
Additional information
Communicated by T. Rötzer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cysneiros, V.C., Scipioni, M.C. & Allen, C.D. Modeling bark thickness and probability of trunk cavity occurrence relative to tree size in Araucaria angustifolia trees. Trees 38, 1013–1022 (2024). https://doi.org/10.1007/s00468-024-02531-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-024-02531-8