Abstract
Key message
Bark was the major NSC component in twigs across species. Future research should increasingly follow a bark–xylem approach for a better understanding of NSC distribution and its function in the twig.
Abstract
Despite extensive research on non-structural carbohydrates (NSC), the distribution of total NSC (TNC) and its primary components (sugar and starch) to the bark and xylem of twigs remains poorly understood. We determined seasonal NSC dynamics in twig bark and xylem in seven temperate tree species exhibiting different xylem anatomies and leaf habits. Seasonal trends in sugar were similar across species with concentrations peaking at least 15 days earlier in the spring for xylem than bark. However, evergreens exhibited maximum bark starch concentrations in early spring, followed by declines throughout the growing season, whereas deciduous species exhibited early spring declines in bark starch, followed by late growing season increases. Evergreens exhibited limited seasonal variation in xylem starch concentrations, whereas deciduous species exhibited variation in xylem starch concentrations that was similar to variation in bark starch. With a few exceptions, concentrations and seasonal amplitudes for sugar, starch, and TNC were generally higher in bark than xylem. Sugar concentrations were generally higher than starch, especially in the bark, which resulted in variability of TNC concentrations in bark or xylem. NSC concentrations varied significantly between xylem and the entire twig when bark was not explicitly considered. Averaged across species, sugar, starch, and TNC content in bark accounted for 66%, 54%, and 61% of total twig content, respectively, even though bark only accounted for 53% of the total twig mass. We recommend quantifying bark sugar and starch separately from xylem when determining twig NSC concentration and content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-structural carbohydrates (NSC), mainly sugar and starch, can be stored in perennial tree organs for later use (Hoch et al. 2003; Martínez-Vilalta et al. 2016; Tixier et al. 2019). The storage of NSC is important to buffer asynchrony between carbon supply and demand that may occur predictably with seasonal changes in physiological activity (Chapin et al. 1990) or unpredictably under various stressors or disturbances such as shading (Gansert and Sprick 1998), drought (McDowell et al. 2011), leaf damage from fire (Aubrey et al. 2012), or pests and pathogens (Anderegg and Callaway 2012; Puri et al. 2015), or complete top kill (Ruswick et al. 2021). As such, NSC storage is an important trait for a variety of tree life history strategies (Hoch et al. 2003; Mims et al. 2018; Ruswick et al. 2021). Understanding differences in NSC concentrations among species (Barbaroux et al. 2003; Zhang et al. 2014; Mims et al. 2018), and across organs (Gansert and Sprick 1998; Yang et al. 2016; Santos et al. 2021) and seasons within species (Martínez-Vilalta et al. 2016; Aubrey and Teskey 2018; Oswald and Aubrey 2020) is critical for assessing and understanding tree carbon dynamics.
Twigs, the youngest part of the branch, are often used to study NSC concentrations and assess carbon status because they exhibit high metabolic activity (Piper et al. 2016; Wang et al. 2018), resulting in relatively high NSC concentrations that make them one of the largest NSC reservoirs in trees (Barbaroux et al. 2003; Furze et al. 2019; Rosell et al. 2021); however, the distribution of NSC and its components (i.e., sugar and starch) between bark and xylem of twigs is poorly understood relative to distributions among entire organs (Hartmann and Trumbore 2016; Martínez-Vilalta et al. 2016). Anatomically, twigs are composed of bark (periderm and phloem) and xylem, which have different physiological functions and chemical components (Rosell 2019), and exchange between these tissues likely links NSC pools (Sevanto et al. 2014; Cernusak and Cheesman 2015; Vandegehuchte et al. 2015; Morris et al. 2016; Tixier et al. 2019). However, a few studies have explicitly explored differences in NSC between the bark and xylem of twigs (Barbaroux et al. 2003; Puri et al. 2015; Yang et al. 2016), and most studies do not separate them (Maguire and Kobe 2015; Zhang et al. 2015; Salomón et al. 2016) or sample only xylem (Hoch et al. 2003; Schӓdel et al. 2009; Wang et al. 2018).
Increasing evidence suggests that twig bark and xylem tissues differ in their NSC concentrations and the distribution of NSC components. For example, sugar concentrations of twig bark were higher than xylem, while bark starch concentrations were lower than xylem (Drossopoulos and Niavis 1988; Yoshioka et al. 1988; Gansert and Sprick 1998). Moreover, sugar, starch, and total NSC concentrations are usually higher in bark than in xylem for branches and twigs (Ashworth et al. 1993; Li et al. 2002; Puri et al. 2015; Yang et al. 2016; Rosell et al. 2021) with some exceptions (Barbaroux et al. 2003). In addition, the distribution of sugar and starch between bark and xylem in branches may not always be similar (Barbaroux et al. 2003).
Bark and xylem NSC concentrations may respond differently to environmental factors, but these dynamics may go undetected if NSC concentrations are not determined separately for both tissues. For example, NSC concentrations have increased in xylem or bark tissue in response to nutrient, drought, or defoliation treatments while concurrently decreasing or remaining unchanged in the other tissue and the magnitude of difference in NSC concentrations between tissues increased from the controls to the treatments (Yoshioka et al. 1988; Li et al. 2002; Anderegg and Callaway 2012; Puri et al. 2015; Yang et al. 2016), suggesting that NSC responses to environmental factors should be considered separately for each tissue. Indeed, previous reports of increasing, decreasing, or unchanging NSC concentrations in response to drought stress (Hartmann and Trumbore 2016; Hartmann et al. 2018) or other environmental factors may reflect the confounding effect of not considering bark and xylem tissues separately.
Most previous studies focused primarily on NSC concentrations in xylem for current-, more than 2-year-old branches, or with diameter ≤ 1 cm to estimate crown NSC content (Gholz et al. 1991; Hoch et al. 2003; Gruber et al. 2011; Cheng and Wang 2015). Only little attention has been given to bark, with the exception of a few papers where stem bark (Gruber et al. 2013; Zhang et al. 2014) or twig, stem, and root bark (Rosell et al. 2021) have been considered. The reason for focusing on xylem and ignoring bark for these investigations was likely due to the relatively small proportion of bark to total biomass (Pallardy 2008), even though twig bark (Puri et al. 2015; Yang et al. 2016; Rosell et al. 2021) as well as stem bark (Barbaroux et al. 2003; Anderegg and Callaway 2012; Zhang et al. 2014) often exhibit higher NSC concentrations. However, the proportion of twig bark is higher than the proportion of stem bark, because the proportion of bark decreases with increasing diameter (Senelwa and Sims 1999; Cheng and Wang 2015; Rosell et al. 2017). Thus, twig bark may store more NSC than xylem, even though its biomass is smaller. Clearly, sampling only xylem is inadequate to determine NSC content and distribution in twigs (Little 1970; Barbaroux et al. 2003; Furze et al. 2019).
Seasonal patterns of twig bark and xylem NSC concentrations may be similar (Ashworth et al. 1993) or different (Puri et al. 2015) among species, reflecting the tissue’s dynamic contribution to physiological processes throughout the year. Seasonal dynamics of branch NSC concentration and distribution among NSC components are influenced by leaf habit (Hoch et al. 2003; Richardson et al. 2015) and xylem anatomy (Barbaroux et al. 2003). For example, deciduous species may rely more on NSC reserves stored in xylem than evergreen conifer species in temperate climate, because deciduous species refoliate entire canopies in the spring, whereas evergreen species only refoliate a portion of their canopy and may not rely entirely, or at all, on stored NSC to do so (Hoch et al. 2003). For species exhibiting ring-porous anatomy, wood growth prior to leaf expansion in spring is presumably more dependent on the NSC accumulated during the previous growing season, whereas early wood growth of diffuse-porous species is more reliant on carbon acquired from current photosynthesis (Barbaroux et al. 2003; Hoch et al. 2003). However, this understanding is based on branch or stem xylem NSC concentrations without consideration of bark (Zhang et al. 2014), so general patterns of NSC among functional groups remain uncertain. Understanding and generalizing seasonal NSC dynamics and distributions of NSC components among species or functional groups require robust data across multiple species at the tissue level and not just the organ level (Barbaroux et al. 2003; Hoch et al. 2003; Zhang et al. 2014).
Here, we characterize seasonal dynamics of bark and xylem NSC concentrations of 1-year-old twigs in seven temperate tree species with contrasting xylem anatomies (non-, ring-, and diffuse-porous) and leaf habits (evergreen and deciduous). By examining NSC concentrations in twig bark and xylem across key phenological periods of varying NSC supply and demand, we can determine the relative magnitude of bark and xylem NSC components, as well as characterize seasonal fluctuations of sugar and starch concentrations in the different tissues of the same organ. This approach facilitates testing the hypothesis that seasonal NSC dynamics differ between twig bark and xylem tissues. Inclusion of different xylem anatomies and leaf habits allows us to explore whether NSC for different tissues exhibit a more pronounced seasonal variation in deciduous (porous) or evergreen (non-porous) trees. Our specific objectives were to: (1) examine seasonal variation and relative magnitudes of twig bark and xylem NSC concentrations across a range of species; (2) determine if seasonal NSC dynamics were related to xylem anatomy or leaf habit; and (3) determine if xylem concentrations alone provided accurate estimates and inference of twig NSC content and seasonal dynamics.
Materials and methods
Site description, plant materials, and sampling
The experiment was conducted at Maoershan Forest Ecosystem Research Station (45° 40′ N and 127° 66′ E, 400 m a.s.l.), Heilongjiang Province, northeast China. The mean slope is 10°–15°. The regional climate is temperate continental monsoon which is characterized by warm and humid summers, cold and dry winters, a short growing season, and abundant precipitation with the annual average temperature and annual precipitation of 2.2 °C and 591 mm, respectively. The zonal vegetation represents a typical forest type and condition in northeastern China (Wang et al. 2019), which was originally broad-leaved Korean pine forest that has been disturbed by logging, fire, reclamation, and now many different types of natural secondary forests and plantations co-exist.
The study was conducted from early April to late October in 2018. Precipitation events were frequent, and total rainfall from June to August was 682 mm, which was 91 mm above the 10-year mean annual precipitation. We investigated seven tree species: Korean pine (Pinus koraiensis; hereafter Pks), Korean spruce (Picea koraiensis; hereafter Pkn), Manchurian walnut (Juglans mandshurica; hereafter Jm), Mongolian oak (Quercus mongolica; hereafter Qm), Japanese elm (Ulmus japonica; hereafter Uj), white birch (Betula platyphylla; hereafter Bp), and mono maple (Acer mono; hereafter Am). We chose these species for our experiment because they represented different xylem anatomies and leaf habits. Korean pine and Korean spruce are evergreen conifers, whereas the other species are deciduous broad-leaved trees. Among the deciduous broad-leaved species, Mongolian oak and Japanese elm are ring-porous, Manchurian walnut is semi-ring-porous, and mono maple and white birch are diffuse-porous.
Four healthy dominant mature trees with similar DBH of each species were repeatedly sampled on seven occasions across the growing seasons based on key phenological periods, i.e., April (before bud break), mid-May (green leaves/needles emerging for the early leafing species), late-May (leaves/needles fully expanded for the early leafing species, green leaves/needles emerging for the late-leafing species), late-June, and mid-August (leaves/needles fully expanded), mid-September (before leaf senescence), and mid-October (complete defoliation for the deciduous species) during well-watered field conditions. At each sampling date, 1-year-old twigs from the sun-exposed branches growing on the top third of south-facing side of the tree were randomly harvested between 5:00 and 10:00 a.m. to minimize effects of diurnal NSC variations (Morin et al. 2011; Tixier et al. 2018). Previous work has demonstrated insignificant vertical variation of NSC concentrations in twigs of temperate tree species (Zhang et al. 2015). All samples were immediately placed in a cooler on ice after collection, transported to the laboratory within 2 h and placed in a microwave oven at 600 W for 90 s to eliminate enzymatic activity (Hoch et al. 2003). Then, xylem and the secondary phloem, which included the active phloem and bark, were separated from each twig using a knife. Subsequently, dry mass (DM) of all tissue samples was measured after oven drying at 70 ℃ to a constant mass. Dried samples were ground to a fine powder and analyzed for sugar and starch concentrations.
Chemical analyses of NSC concentrations
Sugar and starch concentrations were determined by a modified phenol–sulfuric acid method (Buysse and Merckx 1993; Zhang et al. 2015). In brief, 50 mg of the powdered samples placed in a 50-mL centrifuge tube was extracted with 10 mL of 80% v/v ethanol for 24 h, followed by two centrifugations at 4,000 rpm for 10 min. The supernatant solution was measured for sugar concentrations with a spectrophotometer at 490 nm (UV–VIS, Purkinje General Instrument Co., Beijing, China). After the ethanol evaporated, the residues were boiled for 15 min in 10 mL of distilled water and cooled to room temperature, and then, fungal α amylase (300 U mg−1) was added and the sample was incubated in a water bath at 60 °C for 1 h. The supernatant solution was used for determining the concentrations of starch with the same procedure as described above. The concentrations of sugar and starch were determined by standard curves and presented as percent dry matter (%). Total NSC (TNC) concentrations (%) were calculated as the sum of sugar and starch concentrations, and we derived another variable to represent the proportion of sugar to starch (SST).
Since the seasonal amplitude of NSC is the key toward identifying periodic source–sink disparities, the difference between the maximum and minimum concentrations were calculated to represent tissue storage capacity. Additionally, sugar, starch, and TNC content were calculated as the product of bark and xylem biomass and their concentrations at each sampling time. Due to the strong intra- and inter-specific variation of twig diameter and length, the proportion of NSC contents instead of absolute contents in each tissue was calculated as the ratio of sugar, starch, and TNC content in bark or xylem relative to the total twig.
Data analysis
Repeated-measures analysis of variance (ANOVA) in a mixed model framework was used to test the fixed effects of sampling date (n = 7), tissue (n = 2; xylem or bark), species (n = 7), and their interactions on concentrations of sugar, starch, and TNC (i.e., sugar + starch), the ratio of sugar and starch (SST), as well as the proportion of bark and xylem sugar, starch, and TNC content relative to total twig content. We used a similar model to compare NSC concentrations of the entire twig with NSC of just the xylem. NSC data were arc sin transformed to achieve normal distribution and equal variances (Zar 1996). Individual tree was treated as a random factor. Mean comparisons were made using Duncan’s multiple range test (α = 0.05). Differences in NSC concentrations between the species functional groups (i.e., xylem anatomy or leaf habit) were also tested using Duncan multiple comparison procedure (α = 0.05). Manchurian walnut was the only semi-ring-porous species, and arbitrarily classified as the ring-porous group for the analyses due to their similar phenology. All analyses were performed using SPSS software (SPSS 19.0 for Windows, SPSS Inc., IL, USA).
Results
Seasonal dynamics in bark and xylem NSC concentrations
Twig sugar, starch, TNC, and SST were influenced by sampling date, tissue, and species; however, these individual effects were not independent (i.e., sampling date × tissue × species interactions; Table 1). To simplify communication of results within the context of this complicated interaction, we focus below on describing general patterns related to seasonal dynamics, comparisons of bark and xylem tissues, and comparisons of species with respect to functional groups.
Twig bark and xylem sugar, starch, and TNC concentrations, and SST varied significantly among sampling dates for all species. Generally, bark sugar concentrations increased between mid-April and the end of May (Fig. 1). However, bark sugar concentrations of deciduous broad-leaved species decreased slightly in mid-May, and then, all species exhibited substantial declines in bark sugar concentrations to minimums in June, before increasing until October (Fig. 1). Xylem sugar concentrations exhibited seasonal patterns similar to that of bark sugar, but xylem sugar concentrations peaked at least 15 days earlier in the spring (Fig. 1). Bark starch concentrations for evergreen coniferous species increased to their maximum in mid-May and declined to their minimum in August, whereas xylem starch concentrations remained similar throughout the growing season (Fig. 1). Bark and xylem starch concentrations for deciduous broad-leaved species exhibited their minimum in May and increased to their maximum in September (Fig. 1). Seasonal variability of bark and xylem TNC concentrations was mainly due to fluctuations in sugar concentrations for all species, except xylem TNC concentrations followed xylem starch patterns for Qm and Am (Fig. 1). Seasonal fluctuations of xylem and bark SST generally followed patterns of sugar concentrations (Fig. 1). Total twig (i.e., bark and xylem) NSC concentrations (sugar, starch, and TNC) and SST generally followed similar patterns as observed in bark for all species.
Mean ± SE (n = 4) sugar (SS), starch (ST), and total non-structural carbohydrate (TNC; i.e., SS + ST) concentrations and the ratio of sugar to starch (SST) in annual twigs (filled triangle) and their bark (open circle) and xylem (open square) for Pinus koraiensis (Pks), Picea koraiensis (Pkn), Juglans mandshurica (Jm), Quercus mongolica (Qm), Ulmus japonica (Uj), Betula platyphylla (Bp), and Acer mono (Am) on seven sampling dates from April to October 2018
Twig bark and xylem tissues differed in their NSC concentrations and their relative distribution of NSC components. Bark sugar concentrations were higher than xylem sugar concentrations, especially at the end of May, except for Pks in mid-May, Pkn in mid-April and from June to October, and Bp in October (Fig. 1). Bark starch concentrations were higher than xylem starch concentrations for Pks and Pkn, but xylem starch concentrations were higher than bark starch concentrations for Qm and Am (Fig. 1). Bark and xylem starch concentrations for the other species (Jm, Uj, Bp) were generally more similar throughout the sampling period, or at least their differences were much smaller (Fig. 1). Bark TNC concentrations were higher than xylem TNC concentrations for most species, except xylem TNC concentrations were higher than bark TNC concentrations for Qm for most sampling dates, Pkn in April, and Bp in October (Fig. 1). Bark SST was lower than xylem SST for Pks and Pkn, but higher than xylem SST for Jm, Qm, Uj, and Am, and either higher or lower than xylem SST for Bp, which exhibited bark and xylem SST values generally ≥ 1 throughout the growing season, with a few exceptions (Fig. 1).
The seasonal amplitudes of bark and xylem sugar, starch, and TNC concentrations differed among species (Fig. 2). The largest seasonal bark sugar, starch, and TNC amplitudes occurred in Bp, Jm, and Pks, respectively, whereas the largest seasonal xylem sugar, starch, and TNC amplitudes occurred in Pks, Qm, and Jm, respectively. The smallest seasonal bark sugar, starch, and TNC amplitudes occurred in Qm, Am, and Qm, respectively, whereas the smallest seasonal xylem sugar, starch, and TNC amplitudes occurred in Uj, Pks, and Uj, respectively. The ratio between the maximum and minimum bark and xylem sugar, starch, and TNC concentration was 2.0–2.9, 2.4–4.6, and 2.0–3.4, respectively, when averaged across the seven species. The ratio between the maximum and minimum sugar, starch, and TNC were 1.4–2.9, 1.3–4.6, and 1.2–2.2 times higher in bark than xylem for all species, with a few exceptions. For example, there was no difference between the bark and xylem maximum and minimum sugar concentrations for Pks, starch concentrations for Bp, and TNC concentrations for Jm, and the ratio between the maximum and minimum starch and TNC concentrations for Qm, and starch concentration for Am were about twice as high in xylem as bark (Fig. 2). Overall, bark NSC exhibited greater seasonal fluctuations and higher storage capacity than xylem.
Mean ± SE (n = 4) absolute seasonal difference (i.e., maximum—minimum) of sugar (SS), starch (ST), and total non-structural carbohydrate (TNC; i.e., SS + ST) concentrations in the bark, xylem, and twig for Pinus koraiensis (Pks), Picea koraiensis (Pkn), Juglans mandshurica (Jm), Quercus mongolica (Qm), Ulmus japonica (Uj), Betula platyphylla (Bp), and Acer mono (Am) during experiment period from April to October 2018. The different lowercase letters above the bars indicate significant differences between tissues within a species based on Duncan’s multiple range test (P < 0.05)
Twig and xylem NSC comparisons
We compared NSC concentrations of xylem with NSC concentrations of the whole twig to determine if xylem samples alone could provide accurate representations of whole-twig NSC dynamics. Whole-twig and xylem sugar, starch, and TNC concentrations differed among species at each sampling date (Online Appendix 1); however, there were no clear inter-specific patterns. Xylem sugar, starch, and TNC concentrations averaged across sampling periods differed among species, whereas twig starch and TNC concentrations did not. However, twig sugar concentrations differed among species, and the relative ranking among species was similar to that observed in xylem (Fig. 3). The highest xylem sugar (7.82 ± 0.63%), starch (6.39 ± 1.09%), and TNC concentrations (10.38 ± 0.96%) occurred in Pkn, Qm, and Bp, respectively, whereas the lowest occurred in Uj (3.39 ± 0.27%), Pks (1.79 ± 0.20%), and Uj (6.70 ± 0.53%), respectively. The highest twig sugar (9.20 ± 0.97%), starch (4.95 ± 0.81%), and TNC concentrations (12.22 ± 1.51%) occurred in Bp, Qm, and Pks, whereas the lowest occurred in Qm (5.38 ± 0.35%), Bp (2.84 ± 0.63%), and Am (9.02 ± 0.65%), respectively.
Mean ± SE (n = 4) of sugar (SS), starch (ST), and total non-structural carbohydrate (TNC = SS + ST) concentrations in xylem and twig averaged across the growing season and compared among: species Pinus koraiensis (Pks), Picea koraiensis (Pkn), Juglans mandshurica (Jm), Quercus mongolica (Qm), Ulmus japonica (Uj), Betula platyphylla (Bp), and Acer mono (Am); functional groups based on non-porous (Np), ring-porous (Rp), and diffuse-porous (Dp) xylem anatomies; and functional groups based on evergreen (Ev) and deciduous (De) leaf habits. Vertical dashed lines separate comparisons among species (left), xylem anatomies (center), and leaf habits (right). The different letters above the bars indicate significant differences among species (lowercase), among xylem anatomies (capital), and between leaf habits (lowercase, italicized) based on Duncan’s multiple range test (P < 0.05)
There were no clear patterns in xylem or twig sugar, starch, and TNC concentrations related to xylem anatomies or leaf habits (Fig. 3). Xylem sugar concentrations followed the decreasing sequence of non-porous > diffuse > ring-porous, and evergreen > deciduous; however, the opposite rank order occurred for xylem starch concentrations, and there were no differences in xylem TNC concentrations among xylem anatomies or between leaf habits. However, in the twig, there were no differences in twig sugar, starch, and TNC concentrations among xylem anatomies or between leaf habits. Seasonal patterns of xylem and twig sugar and TNC concentrations were similar among xylem anatomies and between leaf habits throughout the observation period. Xylem and twig starch concentrations were similar among xylem anatomies and between leaf habits during the middle and late growing season; however, there were differences between xylem and twig NSC and its components during spring (Fig. 4). For example, xylem sugar concentrations differed among xylem anatomies and between leaf habits in mid-May, but twig sugar concentrations did not. Similarly, xylem starch concentrations were similar among xylem anatomies and between leaf habits, but twig starch concentrations were as non-porous/evergreen species exhibited higher starch concentrations than ring- and diffuse-porous/deciduous species during May. As for TNC, xylem and twig TNC concentrations followed the similar rank order of non-porous > diffuse > ring-porous, or evergreen > deciduous species; however, the former differed in late-May and the latter differed in mid- and late-May.
Mean ± SE (n = 4) sugar (SS), starch (ST), and total non-structural carbohydrate (TNC; i.e., SS + ST) concentrations in the xylem and twigs for Pinus koraiensis (Pks), Picea koraiensis (Pkn), Juglans mandshurica (Jm), Quercus mongolica (Qm), Ulmus japonica (Uj), Betula platyphylla (Bp), and Acer mono (Am) averaged across xylem anatomies (A) and leaf habits (B). The different lowercase and capital letters above the bars indicate significant differences among the xylem anatomies and between leaf habits, respectively, based on Duncan’s multiple range test (P < 0.05)
Relative contributions of bark and xylem to twig NSC content
The content proportion of sugar, starch, and TNC were significantly affected by tissues and its interaction with sampling date, species, and sampling date × tissue × species interactions, but not the sampling date, species (with the exception of sugar), and their interactions (Table 2). The relative proportion of bark and xylem sugar, starch, and TNC content for all species varied among sampling dates (Online Appendix 2, Fig. 5). Bark accounted for a high proportion of sugar, starch, and TNC contents in twigs for all tree species, especially in May. The bark sugar content for evergreen coniferous species Pks and Pkn varied from 60 to 86%, slightly higher than those of deciduous broad-leaved species which varied from 49 to 80%. The bark starch content for evergreen coniferous species accounted for 71–96% of twig starch content, whereas bark starch content for Qm and Am was < 50%, and 33–74% for the rest three species (Jm, Uj, and Bp). Bark TNC content was 64–90% and 36–78% of total twig NSC content for evergreen coniferous and deciduous broad-leaved species, respectively.
Mean proportional distribution of sugar (SS), starch (ST), total non-structural carbohydrate (TNC) content and biomass in twig bark and xylem of Pinus koraiensis (Pks), Picea koraiensis (Pkn), Juglans mandshurica (Jm), Quercus mongolica (Qm), Ulmus japonica (Uj), Betula platyphylla (Bp), and Acer mono (Am). Data were collected from April to October 2018. The MC represents the mean proportional distribution of NSC content across all observation periods for each species. The different capital letters above the bars indicate significant differences between proportion contributions of bark and xylem to twig biomass based on Duncan’s multiple range test (P < 0.05)
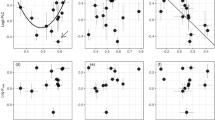
Bark contributed more NSC to the twig than xylem, despite generally accounting for a smaller proportion of twig mass. The relative proportion of twig sugar, starch, and TNC attributed to bark varied from 61% in Jm to 72% in Pks, 29% in Qm to 80% in Pks and Pkn, 45% in Qm to 75% in Pks, respectively, when averaged across the sampling periods. Averaged across the observation period and species, bark sugar, starch, and TNC accounted for 66%, 54%, and 61% of twig sugar, starch, and TNC content, respectively. In comparison, bark biomass accounted for only 53 ± 3.9% of twig biomass when averaged across species. For example, bark biomass for coniferous Pks and Pkn was 63% and 70%, and broad-leaved species were in the range of 43–57% to the twig total biomass (Fig. 5). The contribution of bark sugar and TNC to twig sugar content was either disproportionately higher (e.g., Pks) or proportional (e.g., Pkn) to its biomass, whereas the contribution of bark starch was either disproportionately higher (e.g., Pks), disproportionately lower (e.g., Qm), or proportional (e.g., Bp) to its biomass (Fig. 6).
The proportion of whole-twig sugar (SS), starch (ST), and total non-structural carbohydrate (TNC; i.e., SS + ST) content attributed to bark plotted against the proportion of whole-twig biomass attributed to bark in Pinus koraiensis (Pks), Picea koraiensis (Pkn), Juglans mandshurica (Jm), Quercus mongolica (Qm), Ulmus japonica (Uj), Betula platyphylla (Bp), and Acer mono (Am). Data were collected from April to October 2018. The dotted line extending from the origin represents a 1:1 relationship between the dependent and independent variables
Discussion
We found that twig NSC concentrations exhibited seasonal dynamics across multiple tree species, and for both NSC components (i.e., sugar and starch) the seasonal dynamics in twig NSC followed that of bark NSC more so than xylem NSC, primarily because bark exhibited higher NSC concentrations than xylem and not because of its proportional mass. Seasonal amplitudes of NSC concentrations were generally higher in bark than xylem and sugar concentrations were generally higher than starch in the same tissue, especially bark. Thus, much of the variability in bark or xylem TNC concentrations could be attributed to fluctuations in sugar concentrations. Seasonal NSC dynamics differed as a function of leaf habit and xylem anatomy with evergreen/non-porous species exhibiting maximum starch concentrations in early spring followed by declines throughout the growing season, and deciduous/porous species exhibiting early spring declines and late growing season increases. Although bark only accounted for 53% of twig biomass on average, it accounted for 66%, 54%, and 61% of twig sugar, starch, and TNC on average, respectively. Overall, our findings support the hypothesis that seasonal NSC dynamics differ between twig bark and xylem tissues, thereby highlighting the importance of considering bark and xylem separately for understanding NSC dynamics.
Seasonal NSC variations in twig bark and xylem
The seasonal variation in twig sugar, starch, and TNC concentrations in our study was similar to previous studies (Kramer and Kozlowski 1979; Kozlowski 1992; Mei et al. 2015). Generally, TNC concentrations were highest in spring, decreased after leaf-out, and then accumulated before leaf fall. However, the contribution of bark and xylem to seasonal dynamics of twig NSC and its components were different due to seasonal fluctuations in different tissues and tree life forms (Barbaroux and Bréda 2002; Hoch et al. 2003). We observed seasonal variation in bark and xylem sugar concentrations that were similar to Olea europaea (Drossopoulos and Niavis 1988), Malus pumila Mill. var. domestica ‘Jonagold’ (Yoshioka et al. 1988), Corms sericea (Ashworth et al. 1993), Fagus sylvatica seedlings (Gansert and Sprick 1998), and conifer Pinus pinaster (Puri et al. 2015), but sugar concentrations peaked at least 15 days earlier in xylem than bark. This may be related to the substrate supply for branch growth and osmotic pressure to increase freezing resistance in early spring (Cernusak and Cheesman 2015; Vandegehuchte et al. 2015). However, patterns in twig bark and xylem starch concentrations differed among tree species. For example, seasonal changes in twig bark and xylem starch concentrations for the five broad-leaved species in our study were similar and consistent to the above-mentioned broad-leaved tree species, with starch accumulation occurring soon after complete leaf expansion and continuing until October. This seasonal dynamic is characteristic of temperate deciduous trees except that the highest twig bark and xylem starch concentrations for O. europaea occurred in May (Drossopoulos and Niavis 1988).
Our results suggest synchronicity in bark and xylem NSC dynamics via synthesis and storage of starch for broad-leaved tree plays a vital role in maintaining balance between carbohydrate supply and demand. In contrast, twig bark starch concentrations for conifers Pks and Pkn were highest in spring and also in September, but xylem starch concentrations remained static, that is, the accumulation of starch in xylem was suppressed, and the accumulation of starch in bark was accelerated. This is consistent with bark and xylem starch dynamics of Picea abies stem (Egger et al. 1996) and branch xylem starch for P. abies and Pinus sylvestris (Schӓdel et al. 2009), but different from bark and xylem starch dynamics in P. pinaster, which were highest in the spring (Puri et al. 2015). The starch patterns observed in conifers in our study were generally characteristic of evergreen conifers (Fischer and Höll 1992; Hoch et al. 2003). Most photosynthetic products accumulated in the twigs, especially in bark, which caused twig TNC concentrations to increase significantly, likely to ensure adequate carbohydrate supply to support shoot growth (Fischer and Höll 1992; Schӓdel et al. 2009). Thus, seasonal variation in twig NSC concentrations in seven species followed the corresponding index of bark due to higher seasonal fluctuations of NSC and its components in bark compared with those in the xylem, with the exception of xylem starch in Qm and Am.
Differences between xylem and twig NSC among species and functional groups
Our results revealed obvious seasonal patterns in twig and xylem NSC dynamics between leaf habits and among xylem anatomies, but not any consistent patterns of twig or xylem NSC concentrations among the seven species. We also found that neglecting bark NSC influenced the inter-specific NSC comparison at each sampling time, across sampling periods, and across functional type comparisons. Inter-specific differences in NSC concentrations and distribution in twigs (Barbaroux et al. 2003; Schӓdel et al. 2009), stems (Yu et al. 2011; Zhang et al. 2014), and leaves (Wang et al. 2014) occur in the same habitat at the same period in temperate tree species due to different growth strategies and ecological adaptations. It is generally believed that the proportion of parenchyma (sum of ray parenchyma and axial parenchyma) in the xylem for broad-leaved tree species is higher than that of coniferous trees, leading to higher NSC concentrations in the former (Kramer and Kozlowski 1979), but this is not always consistent or universal.
Our results showed accumulated twig starch in evergreen species at the beginning of the growing season, but not in xylem; however, this did not change the twig and xylem TNC patterns between leaf habits where xylem and twig TNC concentrations followed evergreen > deciduous group at the beginning of the growing season, whereas the opposite trend occurred during the middle and late growing season. The main reason was that the twig and xylem starch concentrations in evergreen species was < 30% of TNC. Our results contradict those of Hoch et al. (2003) in that they observed higher branch xylem TNC in all six deciduous broad-leaved species than that in evergreen conifers during spring but agrees with Schӓdel et al. (2009) who found higher branch xylem TNC concentrations in evergreen Picea than deciduous Carpinus in spring and the opposite pattern after spring.
Differences in xylem anatomy and annual radial stem growth may also influence NSC dynamics. For example, annual radial stem growth occurs mostly before leaf expansion in spring for ring-porous species (Hinckley and Lassoie 1981), but not until after leaf expansion for diffuse-porous species (Zhang et al. 2014), and ring-porous species can exhibit higher NSC concentrations than diffuse-porous species (e.g., Barbaroux et al. 2003; Dietze et al. 2014). For example, in October, Quercus petraea exhibited higher starch, and slightly lower sugar concentrations than F. sylvatica in twigs and xylem, resulting in higher twig and xylem TNC concentrations in the former than the latter based on twig xylem–bark separation analysis (Barbaroux et al. 2003). We also found slightly higher TNC accumulation in twig and xylem for ring-porous xylem anatomy than diffuse-porous xylem anatomy during the middle and late growing season, but no significant differences within above groups. Although twig xylem–bark separation analysis in this study did not change the inference regarding functional groups, we still suggest that variability in concentration of NSC among twig tissues should be considered in such functional group comparisons, especially in spring.
NSC concentrations, content, and distribution within twig tissues
In general, twig sugar, starch, and TNC concentrations for the seven tree species measured in our study were comparable to values measured in new branches and slightly higher than those in old/larger branches (≥ 2-year-old branches and diameter ≤ 3 cm) for temperate tree species (Zhang et al. 2013). The range of NSC concentrations measured in twigs in our study was similar to the range of observations measured by the other authors for twigs in other temperate tree species (Hoch et al. 2003; Yu et al. 2011).
Our data showed that sugar and TNC concentrations for all species, and starch concentrations for all species except Qm and Am, were higher in twig bark than in the xylem. Sugar, starch, and TNC concentrations are usually higher in bark than xylem for branches and twigs (Ashworth et al. 1993; Li et al. 2002; Puri et al. 2015; Yang et al. 2016; Rosell et al. 2021) with the exception that twig xylem sugar and starch concentrations were higher than twig bark for adult F. sylvatica and Q. petraea trees (Barbaroux et al. 2003), and this general pattern was also observed in stem bark and xylem (Egger et al. 1996; Anderegg and Callaway 2012; Gruber et al. 2013; Zhang et al. 2014). Higher NSC concentrations in bark compared to xylem likely result from a larger proportion of ray and axial parenchyma cells and higher metabolic activity in bark (Kramer and Kozlowski 1979). For example, twig bark sugar, starch, and TNC concentrations for P. pinaster were about twice that of xylem (Puri et al. 2015). The twig bark sugar and starch concentrations were higher than those of xylem for 1-year-old Cunninghamia lanceolata in late July, and bark TNC concentrations were nine times higher than that of xylem (Yang et al. 2016). The twig bark sugar concentrations for O. europaea (Drossopoulos and Niavis 1988), M. pumila Mill. var. domestica ‘Jonagold’ (Yoshioka et al. 1988), and F. sylvatica seedlings (Gansert and Sprick 1998) were also higher than in xylem, and the opposite pattern was observed for starch concentrations of these species. Our results provide further evidence that NSC concentrations differ between bark and xylem tissues in the same organ.
Our results indicate that sugar was the main NSC component in twigs for all species except for Qm and Am. The distribution of NSC components in branches varies among species. For example, sugar was the main NSC component in twigs of temperate trees in the middle of the growing season (Barbaroux et al. 2003; Yu et al. 2011; Zhang et al. 2013; Salomón et al. 2016); however, current-year twig NSC of F. sylvatica seedlings (Gansert and Sprick 1998), branch NSC of Q. petraea and F. sylvatica in October (Barbaroux et al. 2003), shoot NSC in Acer rubrum, Q. rubra, and Q. velutina seedlings (Maguire and Kobe 2015), and 1-year-old twig for Q. pyrenaica in April and October (Salomón et al. 2016) were dominated by starch. Critically, these patterns differed significantly when looking at twig NSC concentrations in separate tissues (Barbaroux et al. 2003; Hoch et al. 2003; Schӓdel et al. 2009; Puri et al. 2015).
Our results indicate that sugar was the main NSC component in twig bark for all species, and for twig xylem in most species (i.e., Pks, Pkn, Jm, and Bp); however, in the other species (Qm, Uj, and Am), sugar was the main NSC component in twig xylem in the early growing season, whereas starch became the main NSC component later in the growing season. Previous reports that have quantified branch bark and xylem NSC concentrations have found inconsistent results, though a few papers have explicitly determined both bark and xylem NSC concentrations simultaneously. For example, among evergreen coniferous tree species, branch bark and xylem have consisted mostly of sugar (Puri et al. 2015), whereas branch xylem has consisted mainly of starch (Hoch et al. 2003; Schӓdel et al. 2009) or sugar (Takahashi and Furuhata 2016). For deciduous broad-leaved tree species, other studies have observed that branch bark and xylem consisted mainly of starch for Q. petraea, whereas bark consisted mainly of sugar and xylem consisted mainly of starch for F. sylvatica in October (Barbaroux et al. 2003). Similarly, branch xylem consisted mainly of starch in F. sylvatica from March to July during bud break (Schӓdel et al. 2009), and in Q. petraea, Acer canpestre and F. sylvatica from March to October (Hoch et al. 2003). Our results, combined with those of past research, indicate that the distribution of sugar and starch between twig bark and xylem differ among species, especially during key phenological periods, and further highlight the importance of looking at both starch and sugar separately in bark and xylem for understanding the multiple roles of NSC for different tissues (Maguire and Kobe 2015).
We observed that bark sugar, starch, and TNC content accounted for 66%, 54%, and 61% of the twig, respectively. The NSC content distribution between bark and xylem disproportionately corresponded to bark biomass and ranged from 63 to 70% for coniferous evergreens, and 43 to 57% for broad-leaved species. In addition, the seasonal variation in xylem NSC content ranged from 12 to 36%, though the contribution of xylem NSC to twig NSC content was lower than bark. Bark exhibits high NSC concentrations that translate into high content, despite bark biomass frequently being a smaller proportion of stem biomass, but not for twig or small diameter branch which the proportion of bark decreased rapidly with increasing branch diameter, then bark proportion stays at relatively constant value for certain branch diameter due to the allometric relationship between phloem and xylem (Kenney et al. 1990; Senelwa and Sims 1999; Adler et al. 2005; Rosell et al. 2017). Bark NSC reserves can account for 30% of total stem NSC reserves in large tree stems (Oren et al. 1988; Zhang et al. 2014). Twig bark of the 1-year-old Abies balsamea accounted for 91% and 80% of branch starch and sugar content, respectively (Little 1970). However, twig bark is often removed when estimating canopy NSC reserves, such that only xylem NSC concentrations are considered (Gruber et al. 2011; Hoch et al. 2003), likely underestimating actual canopy reserves. Moreover, the sugar and starch distribution in twig bark and xylem (Barbaroux et al. 2003) and seasonal variations in NSC concentrations (Zhang et al. 2013) have a large impact on NSC content estimates. Our results, along with those of past studies, caution that ignoring twig bark NSC content or seasonal variations in the relative contribution of bark and xylem NSC to total twig contents will bias the estimates of NSC reserves and limit the usefulness of NSC as an indicator of tree carbon status.
Conclusion
Differences in the physiological functions of bark and xylem result in differences in the NSC concentrations in each tissue. We observed seasonal NSC dynamics in twig bark and xylem in seven temperate tree species exhibiting different xylem anatomies and leaf habits across key phenological periods associated with varying carbohydrate supply and demand. Our results indicate that bark was larger NSC pool than xylem in twigs across a variety of species exhibiting different xylem anatomies and leaf habits. The inter-specific and functional group comparison of NSC components between xylem and twig among species varied significantly when only xylem—and not bark—was considered. Therefore, our results improve our understanding of NSC dynamics at the twig level and, importantly, resolve how the dynamics of bark and xylem contribute to twig NSC balance. These findings point to the important role of bark in determining twig NSC dynamics. Future research should therefore increasingly follow a bark–xylem approach for more species in the key phenological phase for a better understanding of NSC distribution and its function in the twig.
Author contribution statement
The co-authors jointly developed the idea and designed the experiment. The field work was carried out by LYG and XCW. LYG, DPA, XCW, and HZS analyzed the data and wrote the manuscript. DPA revised the manuscript.
References
Adler A, Verwijst T, Aronsson P (2005) Estimation and relevance of bark proportion in a willow stand. Biomass Bioenergy 29:102–113. https://doi.org/10.1016/j.biombioe.2005.04.003
Anderegg WRL, Callaway ES (2012) Infestation and hydraulic consequences of induced carbon starvation. Plant Physiol 159:1866–1874. https://doi.org/10.1104/pp.112.198424
Ashworth EN, Stirm VE, Volenec JJ (1993) Seasonal variations in soluble sugars and starch within woody stems of Corms sericea L. Tree Physiol 13:379–388. https://doi.org/10.1093/treephys/13.4.379
Aubrey DP, Mortazavi B, O’Brien JJ, JMcGee D, Hendricks JJ, Kuehn KA, Teskey RO, Mitchell RJ, (2012) Influence of repeated canopy scorching on soil CO2 efflux. For Ecol Manag 282:142–148. https://doi.org/10.1016/j.foreco.2012.06.041
Aubrey DP, Teskey RO (2018) Stored root carbohydrates can maintain root respiration for extended periods. New Phytol 218:142–152. https://doi.org/10.1111/nph.14972
Barbaroux C, Bréda N (2002) Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol 22:1201–1210. https://doi.org/10.1093/treephys/22.17.1201
Barbaroux C, Bréda N, Dufrêne E (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157:605–615. https://doi.org/10.1046/j.1469-8137.2003.00681.x
Buysse J, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44:1627–1629. https://doi.org/10.1093/jxb/44.10.1627
Cernusak LA, Cheesman AW (2015) The benefits of recycling: how photosynthetic bark can increase drought tolerance. New Phytol 208:995–997. https://doi.org/10.1111/nph.13723
Chapin F, Schulze ED, Mooney H (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447. https://doi.org/10.1146/annurev.ecolsys.21.1.423
Cheng FY, Wang CK (2015) Estimating nonstructural carbon content of tree crown considering its spatial variability: A case study on Juglans mandshurica and Ulmus japonica. Chin J Appl Ecol 26: 2253–2264. https://doi.org/10.13287/j.1001-9332.20150604.009
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65:667–687. https://doi.org/10.1146/annurev-arplant-050213-040054
Drossopoulos JB, Niavis CA (1988) Seasonal changes of the metabolites in the leaves, bark and xylem tissues of olive tree (Olea europaea L) II. Carbohydrates. Ann Bot 62:321–327. https://doi.org/10.1093/oxfordjournals.aob.a087664
Egger B, Einig W, Schlereth A, Wallenda T, Magel E, Loewe A, Hampp R (1996) Carbohydrate metabolism in one- and two-year-old spruce needles, and stem carbohydrates from three months before until three months after bud break. Physiol Plantarum 96:91–100. https://doi.org/10.1111/j.1399-3054.1996.tb00188.x
Fischer C, Höll W (1992) Food reserves of scots pine (Pinus sylvestris L.) II. Seasonal changes and radial distribution of carbohydrate and fat reserves in pine wood. Trees 6:147–155. https://doi.org/10.1007/bf00227524
Furze ME, Huggett BA, Aubrecht DM, Stolz CD, Carbone MS, Richardson AD (2019) Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol 221:1466–1477. https://doi.org/10.1111/nph.15462
Gansert D, Sprick W (1998) Storage and mobilization of nonstructural carbohydrates and biomass development of beech seedlings (Fagus sylvatica L.) under different light regimes. Trees 12:247–257. https://doi.org/10.1007/PL00009715
Gholz HL, Cropper WP (1991) Carbohydrate dynamics in mature Pinus elliottii var. elliottii trees. Can J Res 21:1742–1747. https://doi.org/10.1139/x91-240
Gruber A, Pirkebner D, Oberhuber W (2013) Seasonal dynamics of mobile carbohydrate pools in phloem and xylem of two alpine timberline conifers. Tree Physiol 33:1076–1083. https://doi.org/10.1093/treephys/tpt088
Gruber A, Pirkebner D, Oberhuber W, Wieser G (2011) Spatial and seasonal variations in mobile carbohydrates in Pinus cembra in the timberline ecotone of the Central Austrian Alps. Eur J For Res 130: 173–179. https://doi.org/10.1078/0944-7113-00019
Hartmann H, Moura CF, Anderegg WRL, Ruehr NK, Salmon Y, Allen CD, Arndt SK, Breshears DD, Davi H, Galbraith D, Ruthrof KX, Wunder J, Adams HD, Bloemen J, Cailleret M, Cobb R, Gessler A, Grams TEE, Jansen S, Kautz M, Lloret F, O’Brien M (2018) Research frontiers for improving our understanding of drought induced tree and forest mortality. New Phytol 218:15–28. https://doi.org/10.1111/nph.15048
Hartmann H, Trumbore S (2016) Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol 211:386–403. https://doi.org/10.1111/nph.13955
Hinckley TM, Lassoie JP (1981) Radial growth in conifers and deciduous trees a comparison. Mitteilungen-Vienna, Forstliche Bundesversuchanstalt 142:17–56
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081. https://doi.org/10.1046/j.0016-8025.2003.01032.x
Kenney WA, Sennerby-Forsse L, Layton P (1990) A review of biomass quality research relevant to the use of poplar and willow for energy conversion. Biomass 21:163–188. https://doi.org/10.1016/0144-4565(90)90063-P
Kozlowski TT (1992) Carbohydrate sources and sinks in woody plants. Bot Rev 58:107–222. https://doi.org/10.1007/BF02858600
Kramer PJ, Kozlowski TT (1979) Physiology of woody plants. Academic Press, Orlando
Li MH, Hoch G, Körner C (2002) Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees 16:331–337. https://doi.org/10.1007/s00468-002-0172-8
Little CHA (1970) Derivation of the springtime starch increase in balsam fir (Abies balsamea). Can J Bot 48:1995–1999. https://doi.org/10.1139/b70-291
Maguire AJ, Kobe RK (2015) Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol Evol 5:5711–5721. https://doi.org/10.1093/treephys/tpaa080
Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecol Monogr 86:495–516. https://doi.org/10.1002/ecm.1231
McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011) The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol 26:523–532. https://doi.org/10.1016/j.tree.2011.06.003
Mei L, Xiong YM, Gu JC, Wang ZQ, Guo DL (2015) Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 177:333–344. https://doi.org/10.1007/s00442-014-3186-1
Mims JT, O’Brien JJ, Aubrey DP (2018) Belowground carbohydrate reserves of mature southern pines reflect seedling strategy to evolutionary history of disturbance. Forests 9:653. https://doi.org/10.3390/f9100653
Morin C, Bélanger G, Tremblay GF, Bertrand A, Castonguay Y, Drapeau R, Michaud R, Berthiaume R, Allard G (2011) Diurnal variations of nonstructural carbohydrates and nutritive value in Alfalfa. Crop Sci 51:1297–1306. https://doi.org/10.2135/cropsci2010.07.0406
Morris H, Plavcová L, Cvecko P, Fichtler E, Gillingham MAF, Martínez-Cabrera HI, McGlinn DJ, Wheeler E, Zheng J, Ziemińska K (2016) A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytol 209:1553–1565. https://doi.org/10.1111/nph.13737
Oren R, Schulze ED, Werk KS, Meyer J, Schneider BU, Heilmeier H (1988) Performance of two Picea abies (L.) Karst. stands at different stages of decline: I. Carbon relations and stand growth. Oecologia 75:25–37. https://doi.org/10.1007/BF00378810
Oswald SW, Aubrey DP (2020) Xeric tree populations exhibit delayed summer depletion of root starch relative to mesic counterparts. Forests 11:1026. https://doi.org/10.3390/f11101026
Pallardy SG (2008) Physiology of woody plants, 3rd edn. Academic Press, Burlington
Piper FI, Vinegla B, Linares JC, Camarero JJ, Cavieres LA, Fajardo A (2016) Mediterranean and temperate treelines are controlled by different environmental drivers. J Ecol 104:691–702. https://doi.org/10.1111/1365-2745.12555
Puri E, Hoch G, Körner C (2015) Defoliation reduces growth but not carbon reserves in Mediterranean Pinus pinaster trees. Trees 29:1187–1196. https://doi.org/10.1007/s00468-015-1199-y
Richardson AD, Carbone MS, Huggett BA, Furze ME, Czimczik CI, Walker JC, Xu X, Schaberg PG, Murakami P (2015) Distribution and mixing of old and new nonstructural carbon in two temperate trees. New Phytol 206:590–597. https://doi.org/10.1111/nph.13273
Rosell JA, Wehenkel C, Pérez-Martínez A, Palacios JAA, García-Jácome SP, Olguín M (2017) Updating bark proportions for the estimation of tropical timber volumes by indigenous community-based forest enterprises in Quintana Roo, Mexico. Forests 8:338. https://doi.org/10.3390/f8090338
Rosell JA (2019) Bark in woody plants: understanding the diversity of multifunctional structure. Integr Comp Biol 59:535–547. https://doi.org/10.1093/icb/icz057
Rosell JA, Piper FI, Jiménez-Vera C, Vergílio PCB, Marcati CR, Castorena M, Olson ME (2021) Inner bark as a crucial tissue for non-structural carbohydrate storage across three tropical woody plant communities. Plant Cell Environ 44:156–170. https://doi.org/10.1111/pce.13903
Ruswick SK, O’Brien JJ, Aubrey DP (2021) Carbon starvation is absent regardless of season of burn in Liquidambar styraciflua L. For Ecol Manag 479:118588. https://doi.org/10.1016/j.foreco.2020.118588
Salomón R, Rodríguez-Calcerrada J, Zafra E, Morales-Molino C, Rodríguez-García A, González-Doncel I, Oleksyn J, Zytkowiak R, López R, Miranda JC, Gil L, Valbuena-Carabaña M (2016) Unearthing the roots of degradation of Quercus pyrenaica coppices: a root-to-shoot imbalance caused by historical management? For Ecol Manag 363:200–211. https://doi.org/10.1016/j.foreco.2015.12.040
Santos M, Barros V, Lima L, Frosi G, Santos MG (2021) Whole plant water status and non-structural carbohydrates under progressive drought in a Caatinga deciduous woody species. Trees 35:1257–1266. https://doi.org/10.1007/s00468-021-02113-y
Schӓdel C, Blochl A, Richter A, Hoch G (2009) Short-term dynamics of nonstructural carbohydrates and hemicelluloses in young branches of temperate forest trees during bud break. Tree Physiol 29:901–911. https://doi.org/10.1093/treephys/tpp034
Senelwa K, Sims REH (1999) Fuel characteristics of short rotation forest biomass. Biomass Bioenergy 17:127–140. https://doi.org/10.1016/S0961-9534(99)00035-5
Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT (2014) How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ 37:153–161. https://doi.org/10.1111/pce.12141
Shi PL, Körner C, Hoch G (2006) End of season carbon supply status of woody species near the treeline in western China. Basic Appl Ecol 7:370–377. https://doi.org/10.1016/j.baae.2005.06.005
Takahashi K, Furuhata K (2016) Shoot growth and seasonal changes of nonstructural carbohydrate concentrations at the upper and lower distribution limits of three conifers. Landsc Ecol Eng 12:239–245. https://doi.org/10.1007/s11355-016-0294-6
Tixier A, Gambetta GA, Godfrey J, Orozco J, Zwieniecki MA (2019) Non-structural carbohydrates in dormant woody perennials; the tale of winter survival and spring arrival. Front Glob Chang 2:18. https://doi.org/10.3389/ffgc.2019.00018
Tixier A, Orozco J, Amico Roxas A, Earles JM, Zwieniecki MA (2018) Diurnal variation in non-structural carbohydrate storage in trees: remobilization and vertical mixing. Plant Physiol 178:1602–1613. https://doi.org/10.1104/pp.18.00923
Vandegehuchte MW, Bloemen J, Vergeynst LL, Steppe K (2015) Woody tissue photosynthesis in trees: salve on the wounds of drought? New Phytol 208:998–1002. https://doi.org/10.1111/nph.13599
Wang QW, Qi L, Zhou W, Liu CG, Yu DP, Dai LM (2018) Carbon dynamics in the deciduous broadleaf tree Erman’s birch (Betula ermanii) at the subalpine treeline on Changbai Mountain, Northeast China. Am J Bot 105:42–49. https://doi.org/10.1002/ajb2.1006
Wang WN, Li JN, Wang HR, Gu JC (2014) Seasonal dynamics of leaf nonstructural carbohydrate content in four temperate tree species. J Northeast For Univ 42:46–49
Wang XC, Liu F, Wang CK (2019) Towards a standardized protocol for measuring leaf area index in deciduous forests with litterfall collection. For Ecol Manag 447:87–94. https://doi.org/10.1016/j.foreco.2019.05.050
Yang QP, Zhang WD, Li RS, Xu M, Wang SL (2016) Different responses of non-structural carbohydrates in above-ground tissues/organs and root to extreme drought and re-watering in Chinese fir (Cunninghamia lanceolata) saplings. Trees 30:1863–1871. https://doi.org/10.1007/s00468-016-1419-0
Yoshioka H, Nagai K, Aoba K, Fukumoto M (1988) Seasonal changes of carbohydrates metabolism in apple trees. Sci Hortic-Amsterdam 36:219–227. https://doi.org/10.1016/0304-4238(88)90056-8
Yu LM, Wang CK, Wang XC (2011) Distribution of non-structural carbohydrates in three temperate tree species. Chin J Plant Ecol 35:1245–1255. https://doi.org/10.3724/SP.J.1258.2011.01245
Zar JH (1996) Biostatistical analysis. Upper Saddle River, NJ, Prentice Hall.
Zhang HY, Wang CK, Wang XC (2013) Comparison of non-structural carbohydrate concentrations in new and old branches of twelve temperate tree species. Acta Ecol Sin 33:5675–5685. https://doi.org/10.5846/stxb201304210762
Zhang HY, Wang CK, Wang XC (2015) Spatial variability of nonstructural carbohydrate concentrations in canopy branches and leaves of five temperate tree species. Acta Ecol Sin 35:6496–6506. https://doi.org/10.5846/stxb201402080223
Zhang HY, Wang CK, Wang XC (2014) Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees 28:77–89. https://doi.org/10.1007/s00468-013-0931-8
Acknowledgements
The author gratefully acknowledges the support of the Fundamental Research Funds for the Central Universities (No. 2572019CP14 and 2572018BA05), and Scientific Research Foundation for Returned Overseas Chinese Scholars. This work was also supported by the USDA National Institute of Food and Agriculture through McIntire Stennis project 1023985 and was based on work supported by the Department of Energy to the University of Georgia Research Foundation through Grant Number DE-EM0004391.
Funding
This article was funded by Fundamental Research Funds for the Central Universities (Grant Nos. 2572019CP14, 2572018BA05), Scientific Research Foundation for Returned Scholars of Ministry of Education, the USDA National Institute of Food and Agriculture through McIntire Stennis project (Grant No. 1023985), and the Department of Energy to the University of Georgia Research Foundation (Grant No. DE-EM0004391).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Communicated by Victor Resco de Dios.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, L., Aubrey, D.P., Wang, X. et al. Seasonal non-structural carbohydrate dynamics differ between twig bark and xylem tissues. Trees 36, 1231–1245 (2022). https://doi.org/10.1007/s00468-022-02284-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-022-02284-2