Abstract
Key message
Our analysis of short-term growth responses to weather conditions provided evidence that the non-native tree species Ailanthus altissima may have a high growth potential in a warmer and drier environment, as predicted by global warming scenarios.
Abstract
Non-native tree species may alter ecosystem services in colonized forest communities. These novel forests represent a challenge for management, which is further accentuated by anthropogenic global change. Knowledge on the intra-annual growth performance and tree water relations of established and novel tree species is required to elucidate the drivers underlying forest dynamics. Here, we analysed intra-annual radial growth of the introduced tree species Ailanthus altissima (Mill.) Swingle and co-occurring Castanea sativa Mill. in novel forest communities in southern Switzerland. We used four years of continuously measured growth data in two sites to identify weather conditions in which the species achieved highest hourly growth and conditions in which largest proportions of seasonal growth occurred. While both species reached the largest proportion of seasonal growth and achieved the highest hourly growth under the most frequent weather conditions, A. altissima featured the highest growth rates in a wider range of weather conditions than C. sativa, particularly at high temperature and high vapour pressure deficit. We conclude that A. altissima has a greater potential than C. sativa to sustain growth in warm-dry conditions. Importantly, the advantages of A. altissima may be more accentuated in forest stands with low interspecific competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of non-native tree species can result in novel forest communities, thus potentially altering the provisioning of ecosystem services (Charles and Dukes 2007). Depending on expected benefits and/or disadvantages, new species may require different management strategies based on species traits and forest dynamics (Krumm and Vítková 2016; Richardson et al. 2014), particularly under projected climate change.
The analysis of tree growth offers the possibility to reduce existing uncertainties in future forest dynamics, since different growth responses of co-occurring tree species may lead to changes in competitive interactions and ultimately in species composition (Bolte et al. 2009). To develop scenarios of tree growth in a changing climate, knowledge about the influence of environmental factors on growth is important. Tree species sensitivity to environmental conditions is traditionally assessed using dendroecological approaches by correlating annual tree growth with mean monthly temperature, precipitation sums, or drought periods (Eilmann and Rigling 2012; Huang et al. 2010; Schuster and Oberhuber 2013). While such studies are suitable for identifying environmental drivers affecting seasonal to annual growth, the influence of short-term weather conditions remains spurious. The analysis of intra-annual growth dynamics is needed to identify the specific weather conditions that are driving cambial activity (Deslauriers et al. 2003; Köcher et al. 2012).
In this study, we analyse hourly resolved growth patterns of tree of heaven, Ailanthus altissima (Mill.) Swingle, and the co-occurring sweet chestnut, Castanea sativa Mill., in Swiss forests. A. altissima is an early successional tree native to China. Since the 1950s, it spreads particularly in forests of southern Switzerland (Gurtner et al. 2015; Knüsel et al. 2015). Its expansion coincided with the abandonment of chestnut forests that had been cultivated in monocultures as orchards or coppices for more than 2000 years (Conedera et al. 2004; Tinner et al. 1999). The formation of novel forest communities with admixed A. altissima has led to uncertainties regarding their management, accentuated by recent climate change (Wunder et al. 2016).
Both species are ring-porous, but they differ in drought tolerance (Kowarik and Säumel 2007; Trifilo et al. 2004). While C. sativa experienced significant reductions in annual radial growth in severe drought years, A. altissima did not, as shown in recent dendroecological analyses in the same region (Knüsel et al. 2015). However, apart from the different responses in severe drought years, knowledge on the environmental drivers of stem growth of the two species is limited. Thus, a better understanding of the impacts of short-term weather conditions on stem growth of A. altissima and C. sativa is crucial for a more reliable estimation of future growth patterns under a changing climate.
We analyse growth data resolved to an hourly resolution by applying the zero growth (ZG) concept by Zweifel et al. (2016), which assumes no growth in periods of stem shrinkage. This approach enabled us to identify the weather conditions that drive or limit stem growth of A. altissima and C. sativa and to derive implications for expected future climatic conditions.
We propose that a species, which is able to grow at its highest growth rates over a wide range of environmental conditions, has a competitive advantage over a species with a limited range. We further propose that a species that achieves large amounts of its growth in conditions in which it exhibits highest growth rates, has a competitive advantage compared to a species that achieves large amounts of its growth in conditions in which it does not reach highest growth rates. Our specific objectives were (1) to quantify the range of environmental conditions during periods of maximum growth and (2) to determine whether environmental conditions during maximum growth were similar to conditions responsible for the largest fractions of growth.
Materials and methods
Study sites
Location
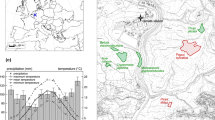
The study was conducted in two lowland forests near the village of Avegno (AV) in southern Switzerland that used to be managed as orchards of C. sativa. The two sites, AV_west and AV_east, are situated on opposite slopes in the lower part of the Maggia valley (46°12′49″N, 8°44′37″E; Fig. 1), about 500 m from each other. Both sites are located on rock debris below a rock face at elevations of 300–370 m a.s.l. with inclinations between 25° and 33°. Soils in the region are classified as haplic podzol on crystalline bedrock (Blaser et al. 2005).

(adapted from Knüsel et al. 2015). Map data: DTM-AV DOM-AV © 2018 swisstopo (DV033594)
Study sites AV_west and AV_east in the lower Maggia valley in Ticino, southern Switzerland
Four dominant or co-dominant A. altissima and C. sativa trees with similar diameter at breast height (DBH) were selected for the study in each site. On all 16 investigated trees, stem radial variation was monitored with high precision for 4 years in AV_west (2013–2016) and 3 years in AV_east (2014–2016), with a common period from 2014 to 2016. The investigated trees in AV_west were older than in AV_east (Table 1).
Environmental conditions
The climate in the study area is characterized by a mean annual precipitation of 1862 mm and a mean annual temperature of 12.7 °C (MeteoSwiss climate station Locarno-Monti, Fig. 2). About half of the annual precipitation falls during the vegetation period (992 mm, May–Sep) in short, heavy spells, whereas winters are usually dry and mild.
Comparison of a mean monthly temperature (°C) and b monthly precipitation sum (mm) during the growing season (May–Sep) for individual years of the study period 2014–2016 (colour) and the reference period 1981–2010 (grey). Violin and box plots are used to characterize the quantiles and density distributions of the climate during the reference period. Climate data are from the nearest climate station (6 km from the study sites; MeteoSwiss station Locarno-Monti)
In the common study period (2014–2016), the growing season (May–Sep) of 2015 was exceptionally warm relative to the mean of the reference period 1981–2010 (Fig. 2a; 2014: − 0.23 °C, 2015: + 1.49 °C, 2016: + 0.90 °C), and all three growing seasons experienced below-average precipitation (Fig. 2b; 2014: − 104 mm, 2015: − 309 mm, 2016: − 270 mm).
Forest history and composition
Both sites had been managed as orchards of C. sativa and were abandoned towards the end of the 1950s. In the following decades, they were increasingly colonized by native (Fraxinus excelsior L., Tilia spp., Prunus avium (L.) L.) and non-native tree species (A. altissima, Robinia pseudoacacia L., Paulownia tomentosa (Thunb.) Steud.). Currently, the canopy trees in both sites are around 30–70 years, with C. sativa and A. altissima being the main species. In AV_west, the share of C. sativa is higher than of A. altissima (60% vs. 25%), while it is reverse in AV_east (30% vs. 60%, Knüsel et al. 2015). Since 2011, the C. sativa trees of the study area have been attacked by a gall wasp (Dryocosmus kuriphilus Yasumatsu). The infestation led to a progressive reduction of leaf area. Starting in 2012, the arrival and subsequently increasing presence of a parasitoid (Torymus sinensis Kamijo) allowed for a progressive recovery of the C. sativa trees (Gehring et al. 2017).
Inter- and intraspecific competition differs strongly between the two sites (Fig. 3). AV_west features a basal area that is twice as high as in AV_east (48.3 vs. 22.9 m2 ha−1), likely owed to the higher age of the trees in AV_west (Knüsel et al. 2015). In addition, the investigated trees in AV_west are mainly surrounded by C. sativa, while in AV_east, they are mainly surrounded by A. altissima (Fig. 3).
Basal area proportion of Ailanthus altissima and Castanea sativa growing in a circle of 10 m radius around the trees equipped with a dendrometer (other tree species not shown). Each point represents an investigated tree. The sizes of the circles and triangles are proportional to the sum of the basal area of surrounding trees. Only trees > 10 cm DBH (diameter at breast height) were considered
Meteorological measurements
Air temperature and relative humidity were measured at 10 min intervals in both study sites within the forest stands 2 m above ground (sensors from Sensirion SHT, Sensirion, Stäfa, Switzerland). In addition, soil water potential (Ψsoil) was measured at the same position 5–10 cm below ground (Decagon MPS-2, Decagon, Pullmann WA, USA). However, Ψsoil measurements in AV_east were erroneous, and therefore, the Ψsoil data from this site were not used. Vapour pressure deficit (VPD) was calculated from temperature and relative humidity (WMO 2008). For all analyses, forest meteorological data were aggregated to hourly values.
The two sites were quite similar in terms of mean monthly temperatures (maximum difference: 0.29 °C), but AV_west showed a higher variation between maximum and minimum temperatures. The amplitudes of VPD varied in the short term, but generally, mean monthly VPD was similar between the sites (maximum difference: 0.12 kPa).
Dendrometer measurements and data processing
Data collection
Stem radial changes were monitored with high precision point dendrometers (ZN11-T-WP, Natkon, Oetwil am See, Switzerland). The point dendrometers consist of a potentiometer fixed on a carbon fibre frame and anchored with stainless steel rods in the heartwood of the stem at breast height. The moving rod of the potentiometer is softly pressed against the stem surface with a spring to smoothly follow the stem radius changes. The sensors were mounted parallel to the slope line to avoid tension wood. The temperature sensitivity of the dendrometers was < 0.3 µm °C−1 and was not further corrected. Measurements were taken every 30 s, averaged and stored every 7 min with a DecentLab data logger (DecentNode, DecentLab, Dübendorf, Switzerland), and finally averaged to hourly values.
Data cleaning
The raw stem radius data were cleaned using the automated procedure described by Zweifel et al. (2016) to remove the spikes and jumps that may occur due to electronic failures of sensors and loggers or mechanical disturbances of the dendrometers. After this automated pre-processing, the data were manually checked to identify and eliminate remaining outliers.
Extraction of the growth signal from the radial changes
Radial changes in the dendrometer signal integrate several reversible and irreversible processes. The largest amount of reversible radial change is linked to the tree water status and mainly reflects changes in the water content of elastic, living tissues in the bark, cambium, and xylem (Sevanto et al. 2011; Zweifel and Häsler 2001; Zweifel et al. 2000). Irreversible growth can be divided into three main processes that may vary in their contribution to radial increment: (1) cell division in the cambium; (2) cell enlargement in the xylem and phloem; and (3) lignification and cell-wall thickening in the xylem. Cell enlargement makes up the largest part of increment, followed by cell division. Lignification and cell-wall thickening lead to marginal radial changes only (Cuny et al. 2015). Therefore, growth considered in this study mainly represents cell enlargement in the xylem and phloem as well as cell division in the cambium.
To extract irreversible growth from dendrometer data, we applied the zero growth (ZG) concept by Zweifel et al. (2016) to separate water-related and growth-related changes in stem radius (Methods S1). The ZG concept assumes that growth starts as soon as the previous stem diameter maximum is exceeded and ends when the stem starts shrinking again (Fig. 4c, d). Consequently, it is assumed that there is no growth in periods of stem shrinkage.
Stem radial change over the whole measurement period (a, b) and stem radial change and growth for the growing season 2015 (c, d) of Ailanthus altissima and Castanea sativa. Each line represents one tree. Growth was calculated according to the zero growth concept (Zweifel et al. 2016). Growth is only displayed from the point, where the stem radius exceeds the maximum of the previous year. Vertical dashed lines represent the common growing season (May–Sep) used for most analyses. In 2015, one dendrometer per site and species had to be excluded due to malfunction
Growth along climate gradients
G frac
To quantify the weather conditions in which growth occurred, we determined the fraction of seasonal growth (Gfrac) that occurred in a given range of temperature (bins of 1 °C), VPD (0.1 kPa), and Ψsoil (10 kPa). For this, we first standardized hourly growth in the common growing seasons (May–Sep in the years 2014–2016) for each year and each tree by dividing hourly growth by the sum of total growth during the respective growing season. The standardized hourly growth rates were then summed up for the given ranges of temperature (e.g., 20–21 °C), VPD (e.g., 0.2–0.3 kPa), and Ψsoil (e.g., − 10 to − 20 kPa) for calculating the percentage of seasonal growth achieved in a given range of environmental conditions. Finally, to compare the resulting Gfrac between the two species for each site, data were smoothed by species and site using a loess smoother in the R package ggplot2 (Wickham 2009). In addition, the shortest range of temperature, VPD and Ψsoil in which 50% of seasonal growth occurred (G50frac), was determined based on the smoothed curves using an estimate of the area under the curve calculated with the auc function in the R package MESS (Venables and Ripley 2002).
G max
We additionally quantified the weather conditions in which the species reached highest growth rates (Gmax). We defined Gmax as the 90% quantile of hourly growth for each tree and year. To obtain weather conditions allowing for Gmax, we extracted temperature, VPD and Ψsoil values for all hours in which a tree reached Gmax. This resulted in different frequencies of Gmax for different environmental conditions. These frequency distributions of Gmax along the three environmental variables (temperature, VPD, Ψsoil) were then pooled by site and species. To be able to statistically compare the distributions between species, we used the function qcomhd of the R package WRS2 (Wilcox et al. 2013), which compares user-specified quantiles of two independent distributions using a percentile bootstrap to calculate confidence intervals. Furthermore, we quantified the smallest range of temperature, VPD and Ψsoil that contained 50% of all hours with maximum growth (G50max) using the hdr function in the R package hdrcde (Hyndman 2018). As for growth proportions, the analyses were restricted to the three common growing seasons (2014–2016).
Ranges of weather conditions with growth
To quantify the ranges of environmental conditions in which growth (all hours with growth > 0.01 µm) and Gmax occurred, we pooled all temperature, VPD and Ψsoil values that were measured in hours with growth or hours with Gmax. From these values, we extracted the maximum and minimum temperature, VPD and Ψsoil for each investigated tree. Differences in the maxima and minima of the growth ranges between species in the same site were statistically compared with two-sided Mann–Whitney tests.
Growth rates
Hourly growth (dendrometer data)
Average hourly growth was compared between species in each year and over the whole period as well as between the two sites with two-sided Mann–Whitney tests.
Annual growth (increment core data)
To obtain longer term annual growth data from trees equipped with dendrometers, we collected two increment cores from each tree in November 2017. The first increment core was taken at a distance of 20 cm in an angle of 45° downhill of the dendrometer sensing rod. The second core was taken on the other side of the stem, mirrored at the slope line. The total bark thickness of the increment cores was measured after their extraction. In the lab, thin sections were cut from the increment cores with a core microtome (Gärtner and Nievergelt 2010), and the yearly amounts of xylem growth, i.e., mean tree-ring widths (TRW), were measured using the image analysis program ROXAS (von Arx and Carrer 2014). To compare TRW between trees, the ring widths of the two cores were averaged and tested for significant differences with a two-sided Mann–Whitney test. In addition, we quantified growth with the basal area increment (BAI). BAI was calculated based on TRW data using the bai.out function in the R package dplR (Bunn et al. 2018). Differences in BAI between the species were tested for significance with a two-sided Mann–Whitney test. Moreover, we tested differences in BAI between the study period (2013–2016) and the preceding 20-year period (1993–2012) for C. sativa with two-sided Mann–Whitney tests to check for growth reductions potentially induced by the gall wasp infestation.
A comparison of annual growth derived from increment cores and dendrometer data was not possible due to the small number of trees with continuous dendrometer data over the whole study period.
We finally tested the influence of tree eccentricity on the measured growth at species level by reanalysing the dendrometer data excluding apparently eccentric trees. However, we did not identify changes in the resulting patterns of the analyses. Therefore, the data of all measured trees were used in the analyses. All analyses were performed with the R software (R Core Team 2018).
Results
Stem radial variation
The seasonal stem radius change patterns were similar for both species (Fig. 4). Stem radius started to increase between the end of March and early April and showed a strong increase from May to July. Dendrometer curves flattened out between September and October and decreased over winter. The winter decrease in stem radius was generally more pronounced in C. sativa than in A. altissima. Small differences in the seasonal patterns were also visible between the sites (Fig. 4a, b).
Furthermore, stem radial change was characterized by diurnal cycles with a stem shrinkage during the day and an increase during the night. The amplitude of the diurnal cycles was significantly different between the species (Fig. 4c, d), i.e., it was about five times larger for C. sativa (daily mean 121.6 µm ± 79.4) than for A. altissima (24.9 µm ± 14.5).
Growth along climate gradients
Temperature
Both species reached largest Gfrac (fraction of seasonal growth per 1 °C) under similar temperature conditions, i.e., between 16 and 17 °C (Fig. 5a). However, A. altissima reached considerably higher Gfrac at temperatures above 23 °C in AV_east compared to C. sativa.
Fraction of seasonal growth (Gfrac) and maximum hourly growth (Gmax, 90% quantile) along the measured temperature gradient of Ailanthus altissima and Castanea sativa. a Smoothed Gfrac per 1 °C. Shaded areas around lines represent the 95% confidence intervals of the loess function. Smoothed lines are only shown for temperatures, where growth occurred. Points represent Gfrac of a single tree in one growing season. Shaded rectangles indicate ranges (min and max in brackets) of G50frac (50% of seasonal growth). The number in front of the bracket is the value at the maximum of the smoothed curve, visualized with a dashed line. b Density distributions of Gmax. Shown are aggregated yearly density distributions for each species. Grey shaded areas show densities of measured temperature over three growing seasons (2014–2016). Shaded rectangles indicate ranges (min and max in brackets) of G50max (50% of hours with Gmax). The number in front of the bracket is the value at the maximum of the smoothed curve, visualized with a dashed line. c Decile differences (A. altissima–C. sativa) in density distributions of Gmax (visualized in b). Red points (positive values) indicate a shift towards higher temperatures of A. altissima, respectively, C. sativa (turquoise points, negative values). The error bars show the 95% bootstrap confidence interval. Significant decile differences are indicated by asterisks. The dashed vertical grey line marks the 50% quantile. Data are displayed for the common growing seasons (May–Sep) in the years 2014–2016. Note the different axis limits in b, c
The results looked similar for Gmax (maximum hourly growth, 90% quantile; Fig. 5b). Again, the highest frequency of Gmax occurred at similar temperatures for both species and was also similar to temperatures of largest Gfrac. The largest differences in Gmax between the two species were observed for temperatures above ca. 20 °C (Fig. 5c). Although Gmax of A. altissima was significantly (P < 0.05) shifted towards higher temperatures in the 90% quantile in AV_west, the differences were much more pronounced and significant along the whole temperature gradient in AV_east (Fig. 5c).
Furthermore, the temperature range of Gmax was considerably smaller compared to the range of measured temperatures (Fig. 5b, grey shaded areas) for both species in AV_west and for C. sativa in AV_east. A. altissima in AV_east, in contrast, exhibited Gmax nearly across the entire range of measured temperatures (Fig. 5b).
VPD
Both species reached their largest Gfrac in moist air (0.0-0.1 kPa VPD; Fig. 6a). Likewise, the range of G50frac was bound to the lowest measured VPD (0.0–0.3 kPa; shaded rectangles in Fig. 6a), with wider ranges for A. altissima.
Fraction of seasonal growth (Gfrac) and maximum hourly growth (Gmax, 90% quantile) along the measured vapour pressure deficit (VPD) gradient of Ailanthus altissima and Castanea sativa. a Smoothed Gfrac per 0.1 kPa VPD. Shaded areas around lines represent the 95% confidence intervals of the loess function. Points represent Gfrac of a single tree in one growing season. Shaded rectangles indicate ranges (min and max in brackets) of G50frac (50% of seasonal growth). The number in front of the bracket is the value at the maximum of the smoothed curve, visualized with a dashed line. b Density distributions of Gmax. Shown are aggregated yearly density distributions for each species. Grey shaded areas show densities of measured VPD over three growing seasons (2014–2016). Shaded rectangles indicate ranges (min and max in brackets) of G50max (50% of hours with Gmax). The number in front of the bracket is the value at the maximum of the smoothed curve, visualized with a dashed line. c Decile differences (A. altissima–C. sativa) in density distributions of Gmax (visualized in b). Red points (positive values) indicate a shift towards higher VPD of A. altissima, respectively, C. sativa (turquoise points, negative values). The error bars show the 95% bootstrap confidence interval. Significant decile differences are indicated by asterisks. The dashed vertical grey line marks the 50% quantile. Data are displayed for the common growing seasons (May–Sep) in the years 2014–2016. Note the different axis limits in b, c and the logarithmic x-axis in a, b
A. altissima generally reached Gmax in conditions with higher VPD than C. sativa, with more pronounced differences in AV_east. Although the differences were small in AV_west, Gmax of A. altissima occurred in significantly (P < 0.05) higher VPD in both sites for all deciles (Fig. 6c). Moreover, the differences between the species increased towards higher VPD. As for temperature, Gmax of A. altissima occurred over nearly the entire range of measured VPD in AV_east.
Ψ soil (analysis only for AV_west)
Both species reached more than 50% of seasonal growth in the wettest measured soil conditions (> − 10 kPa Ψsoil; Fig. S1a). Small differences in Gfrac were observed in slightly drier soil (> − 50 kPa), where A. altissima grew more than C. sativa. Likewise, for Gmax, there were only small differences between the species (Fig. S1b). Although Gmax of A. altissima was significantly (P < 0.05) shifted to more negative Ψsoil for several deciles, the differences were very small (< 10 kPa; Fig. S1c).
The proportion of growth reached with Gmax made up between 30% and 40% of total seasonal growth. It was slightly higher for C. sativa (AV_west: 38%, AV_east: 35%) than for A. altissima (33%, 32%).
Ranges of weather conditions with growth
The temperature and VPD ranges in which the trees grew (all hours with growth > 0.01 µm) or in which they exhibited Gmax varied between trees (Fig. S2, grey lines). The smallest differences among trees occurred for temperature (Fig. S2a). While the variation in the ranges of growth and Gmax among trees was generally larger in VPD compared to temperature (Fig. S2b), likewise, larger differences were observed for Gmax than for growth. Similar to the patterns evident from Figs. 5 and 6, the ranges of temperature and VPD in which growth or Gmax occurred were larger for A. altissima than for C. sativa. However, differences in the upper limits of the ranges among the species were significant (P < 0.05) in the site AV_east only. The lower limits of the ranges of growth and Gmax, i.e., in cold and humid conditions, were not significantly different between the species.
Growth rates
Hourly growth (dendrometer data)
Median hourly growth of C. sativa was significantly higher (P < 0.001) over the entire period (Fig. 7, dotted lines; AV_west: 1.82 µm h−1, AV_east: 2.83 µm h−1) compared to A. altissima (1.36 µm h−1, 1.73 µm h−1). Furthermore, hourly growth was significantly higher (P < 0.001) in AV_east than in AV_west for both species. Growth rates of A. altissima differed significantly (P < 0.05) among all years in the same site, except for the years 2015 and 2016 in AV_west. For C. sativa growth rates differed significantly (P < 0.05) between the years 2013 and 2014 in AV_west and between the years 2014 and 2016 in AV_east.
Hourly growth in the common growing season (May–Sep). Asterisks denote significant differences between tree species in a single year at the same site (***P < 0.001). Dotted lines represent the median over the entire period. The upper limit of the y-axis was adjusted for a better visibility of site and species differences. All available growing seasons are included
Annual growth (increment core data)
During the study period (2013–2016), the annual radial xylem increment of the trees equipped with a dendrometer was on average between 1.4 and 2.4 mm (Table 2). In the preceding 20-year period (1993–2012) they grew more, ranging from 1.7 to 4.7 mm. In AV_east, the trees generally grew more than in AV_west, but the difference was only significant (P < 0.05) for C. sativa (Table 2). Differences observed for TRW were similar for BAI with significantly (P < 0.05) higher increments for C. sativa in AV_east compared to AV_west. Furthermore, C. sativa showed a significant (P < 0.05) reduction in BAI during the study period compared to the preceding 20-year period in AV_east, but not in AV_west (Table 2, uppercase letters).
Discussion
Daily cycles
The characteristic diurnal cycles of stem radius changes during the vegetation period (Fig. 4c, d) are mainly related to variations in the stem water potential associated with water storage and transport in the stem (Pfautsch et al. 2015; Zweifel et al. 2000). Thus, the daily amplitude of stem shrinkage in the growing season largely reflects the loss of water stored in elastic stem tissue (i.e., phloem and cambium) due to transpiration. The about five times larger daily amplitudes of C. sativa indicate a larger amount of bark water used for transpiration compared to A. altissima. The higher average bark thickness of C. sativa (9.6 ± 2.2 mm) compared to A. altissima (6.2 ± 1.4 mm) supports this assumption, since a thicker bark is suggested to have a higher water storage capacity (Rosell et al. 2014).
A larger amount of bark water available for daily transpiration may be of importance to reduce short-term water stress (Zweifel and Häsler 2001). However, for the species analysed here, the relative amount of bark water to total transpiration is unknown. In mature Picea abies (L.) H. Karst., around 5% of daily transpiration was contributed by water stored in the bark on sunny days (Zweifel and Häsler 2001), which may increase to 100% on moist and cloudy days when transpiration is lowered. However, for ring-porous species like A. altissima and C. sativa, stem water storage is likely to be considerably smaller (Köcher et al. 2013), and thus, the contribution of water stored in the bark used for transpiration may be of higher importance.
Extraction of growth from dendrometer data
The ZG concept (Zweifel et al. 2016) was used to separate irreversible growth from reversible stem radial changes, assuming no growth in periods of stem shrinkage, i.e., in periods of a stem water deficit. A reduction of cell expansion and later also an inhibition of cell division have been found under non-saturated conditions, i.e., under reduced turgor pressure (Balducci et al. 2013; Hsiao and Acevedo 1974; Lockhart 1965). However, it is questionable whether no cell enlargement or division occurs at all under a mild water deficit. The threshold of water deficit inhibiting growth may also be species-specific (Steppe et al. 2015). Indeed, a comparison of growth rates after short and long periods of stem shrinkage implied small amounts of growth during stem shrinkage (< 4% of seasonal growth for both species, Methods S1), which were not captured with the ZG concept. However, such small amounts are in the range of values found for other species (Zweifel et al. 2016). To correctly associate these small amounts of growth and to formally test the ZG concept, independent growth measurements are needed. However, even promising approaches such as nuclear magnetic resonance (Windt and Blumler 2015) offer a limited precision in separating growth from water-related stem radial changes. Alternatively, mechanistic models (De Swaef et al. 2015) or a combination of mechanistic and empirical approaches (Mencuccini et al. 2017) might help to test the assumptions of the ZG concept. In our approach, we furthermore restricted all growth analyses to a common growing season, which probably excluded early spring radial increments related to the rehydration of the stem and not to actual cell growth, which is difficult to distinguish based on dendrometer data alone (Makinen et al. 2008).
Growth along climate gradients
The weather conditions in which a tree is able to achieve highest growth rates can provide insights on the conditions for which growth is least limited. A. altissima achieved Gmax under higher temperature and higher VPD compared to C. sativa (Figs. 5, 6), which provides strong evidence that stem growth of A. altissima is less limited in warm and dry weather conditions. The broad range of Gmax found for A. altissima in both temperature and VPD further suggests that this species will be able to maintain stem growth at high rates in a changing climate. The narrower range of Gmax found for C. sativa, in contrast, suggests that reductions of stem growth may already occur during an earlier stage of a changing climate.
Apart from the warmest and driest conditions within the study period, stem growth of both species seemed to be well adapted to common weather conditions. First, they both reached the largest Gfrac and highest amounts of Gmax at similar temperatures and VPD (Figs. 5, 6). Thus, the largest Gfrac were achieved in weather conditions frequently allowing for Gmax. Second, maxima of Gfrac and Gmax corresponded well with the most frequent weather conditions (Figs. 5, 6, grey shaded areas), again suggesting a good acclimation to common weather conditions. The generally narrower range of weather conditions in which C. sativa grew (Figs. 5, 6) even points towards a better acclimation of this species to current weather conditions. The higher hourly growth (Fig. 7) and the higher (AV_east) or similar (AV_west) amount of annual xylem growth of C. sativa compared to A. altissima (Table 2) further support this assumption.
The similar or higher annual radial increments of C. sativa compared to A. altissima during the study period (Table 2) were somewhat unexpected, since C. sativa trees in the study region were infested by a gall wasp (D. kuriphilus) since ca. 2011, leading to a reduction in the number of lateral shoots and thus leaf area (Gehring et al. 2017), which may then translate into a reduced tree vigour and wood production (Ugolini et al. 2014). However, only the site AV_east showed a significantly lower basal area increment in the study period compared to the preceding 20-year period (Table 2). Yet, the basal area increment of relatively young C. sativa in AV_east showed a steady decrease over the last 20 years, which may also be attributed to the typical age-trend inherent to tree-ring series (Fritts 1976).
The differences in the distributions of Gmax between the species were always more pronounced in one of the two sites (AV_east; Figs. 5, 6 and S2). We hypothesize that an interaction between (1) a facilitation of A. altissima and (2) different competitive environments may be responsible for these asymmetric responses. (1) The higher fraction of A. altissima around conspecific trees equipped with a dendrometer in AV_east (Fig. 3) enhances the potential for facilitation in this site. Although we do not have direct evidence for intraspecific facilitation in A. altissima, O’Neal and Davis (2015) found root grafts between adult A. altissima in natural forest stands. Root grafts are often beneficial to trees (Lev-Yadun 2011) and may positively affect radial growth, as shown for jack pine in boreal forest (Tarroux and Desrochers 2011). (2) The higher competition in AV_west (Fig. 3) coupled with lower facilitation may additionally explain the lower flexibility of A. altissima in this site. Thinning experiments demonstrated that competition may influence not only radial growth patterns (Fig. 7), but also the climate response of trees (Piutti and Cescatti 1997). Lower competition can, for example, reduce vulnerability to drought (Aldea et al. 2017; D’Amato et al. 2013). Thus, A. altissima may have a larger potential to sustain its growth in changing weather conditions in less competitive forest stands with a high abundance of A. altissima. Moreover, the slightly lower age of A. altissima in AV_east could indicate that the species may be more flexible in adjusting its growth to ambient weather conditions at younger ages. Why C. sativa was not able to benefit from a reduced competition in AV_east is not clear. However, responses to inter- or intraspecific competition may vary between species, as shown, e.g., for Pinus sylvestris L. and Fagus sylvatica L. in northern Spain (González de Andrés et al. 2018).
Implications for future climate conditions
The wider temperature and VPD range of Gmax found for A. altissima may enable this species to sustain its stem growth under warmer and drier weather conditions, as predicted for the future of Central Europe (CH2011; IPCC 2014; Schär et al. 2004). At the same time, stem growth of C. sativa may be reduced in a climate changing towards warmer and drier conditions, which would give rise to a competitive advantage of A. altissima over C. sativa. Even if C. sativa may have some potential to acclimate to changing local climate conditions (Savolainen et al. 2007), our results suggest that A. altissima is better suited to acclimate to the fast climatic changes predicted (Aitken et al. 2008). Moreover, the resilience to sudden extreme events such as heat waves, storms, or pest infestations will certainly be crucial for their competitive ability and persistence in the future (Lindner et al. 2010). The vulnerability to drought, for example, is higher for C. sativa (Knüsel et al. 2015), which experienced severe leaf withering damages with, in some cases, a fatal end to the trees in the elevated summer drought stress in 2003 (Conedera et al. 2010). However, we did not find clear differences in Gmax related to dry soil conditions (Fig. S1b), probably due to the very brief period of drier soil conditions within the study period. Moreover, a high VPD and thus a higher evaporative demand was found to be a stronger driver for daily stem increment than other environmental factors including soil water availability (Köcher et al. 2012; McLaughlin et al. 2003).
Potential limitations of the study
We analysed weather conditions occurring at the time of growth. However, stem growth is influenced not only by direct effects of the environment, but it may also be influenced indirectly through reserves that were produced and stored in previous months or even years (Ogle et al. 2015; Richardson et al. 2013). Thus, the use of large proportions of reserves for growth may diminish the importance of actual weather conditions on observed stem growth rates. Yet, current environmental conditions still have to allow for cell division and enlargement, which directly depend on temperature (Körner 2015; Palacio et al. 2014) and tree water status (Balducci et al. 2013; Hsiao and Acevedo 1974; Lockhart 1965). In ring-porous species (such as the two analysed tree species), it is well documented that the formation of the first earlywood cells depends largely on reserves (Fonti et al. 2007; Sass-Klaassen et al. 2011). However, the restriction of our analyses to the common growing seasons has likely excluded parts of earlywood formation and thus those parts of annual growth that are known to depend mainly on reserves.
Finally, stem growth might also be limited by insufficient carbon availability. Yet, results of CO2 fertilization experiments suggest that stem growth of mature trees is rarely carbon–source limited (Bader et al. 2013; Palacio et al. 2014). Based on these findings and the absence of a severe drought in the study period, we do not expect carbon–source limitations to have had a major influence on stem growth in our study.
Conclusions
Our results indicate that both non-native A. altissima and established C. sativa are well adapted to the range of weather conditions experienced during the study period. Stem growth of A. altissima may be less affected by changing weather conditions than C. sativa, due to a broader range of conditions in which it showed maximum growth. Advantages of A. altissima were particularly pronounced at high temperature and high VPD and under lower interspecific competition. Based on our results, we conclude that A. altissima will be able to maintain its growth in warmer and drier conditions expected for the future, whereas C. sativa could experience growth reductions, which may translate into changes in dominance and species abundance in the long term.
Author contribution statement
JW and MC planned and designed the research. SK and JW conducted fieldwork. SK analysed the data. SK, JW, MC, RZ, HB, and SE wrote the manuscript.
References
Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S (2008) Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl 1:95–111
Aldea J, Bravo F, Bravo-Oviedo A, Ruiz-Peinado R, Rodriguez F, del Rio M (2017) Thinning enhances the species-specific radial increment response to drought in Mediterranean pine-oak stands. Agric For Meteorol 237:371–383
Bader MKF, Leuzinger S, Keel SG, Siegwolf RTW, Hagedorn F, Schleppi P, Körner C, Lee J (2013) Central European hardwood trees in a high-CO2 future: synthesis of an 8-year forest canopy CO2 enrichment project. J Ecol 101:1509–1519
Balducci L, Deslauriers A, Giovannelli A, Rossi S, Rathgeber CB (2013) Effects of temperature and water deficit on cambial activity and woody ring features in Picea mariana saplings. Tree Physiol 33:1006–1017
Blaser P, Zimmermann S, Luster J, Walthert L, Lüscher P (2005) Waldböden der Schweiz. Band 2. Regionen Alpen und Alpensüdseite, Birmensdorf, Eidg. Forschungsanstalt für Wald, Schnee und Landschaft WSL; hep Verlag, Bern
Bolte A, Hilbrig L, Grundmann B, Kampf F, Brunet J, Roloff A (2009) Climate change impacts on stand structure and competitive interactions in a southern Swedish spruce–beech forest. Eur J Forest Res 129:261–276
Bunn A, Korpela M, Biondi F, Campelo F, Mérian P, Qeadan F, Zang C, Pucha-Cofrep D, Wernicker J (2018) dplR: dendrochronology program library in R. R package version 1.6.8
CH2011 (2011) Swiss climate change scenarios CH2011. C2SM, MeteoSwiss, ETH, NCCR Climate, and OcCC, Zurich, Switzerland, p 88
Charles H, Dukes JS (2007) Impacts of invasive species on ecosystem services. In: Nentwig W (ed) Biol. invasions. Springer, Berlin, pp 217–237
Conedera M, Manetti MC, Giudici F, Amorini E (2004) Distribution and economic potential of the Sweet chestnut (Castanea sativa Mill.) in Europe. Ecol Mediterranea 30:15
Conedera M, Barthold F, Torriani D, Pezzatti GB (2010) Drought sensitivity of Castanea sativa: case study of summer 2003 in the Southern Alps. Acta Hortic 866:297–302
Cuny HE, Rathgeber CB, Frank D, Fonti P, Makinen H, Prislan P, Rossi S, Del Castillo EM, Campelo F, Vavrcik H, Camarero JJ, Bryukhanova MV, Jyske T, Gricar J, Gryc V, De Luis M, Vieira J, Cufar K, Kirdyanov AV, Oberhuber W, Treml V, Huang JG, Li X, Swidrak I, Deslauriers A, Liang E, Nojd P, Gruber A, Nabais C, Morin H, Krause C, King G, Fournier M (2015) Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat Plants 1:15160
D’Amato AW, Bradford JB, Fraver S, Palik BJ (2013) Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol Appl 23:1735–1742
De Swaef T, De Schepper V, Vandegehuchte MW, Steppe K (2015) Stem diameter variations as a versatile research tool in ecophysiology. Tree Physiol 35:1047–1061
Deslauriers A, Morin H, Urbinati C, Carrer M (2003) Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 17:477–484
Eilmann B, Rigling A (2012) Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol 32:178–187
Fonti P, Solomonoff N, Garcia-Gonzalez I (2007) Earlywood vessels of Castanea sativa record temperature before their formation. New Phytol 173:562–570
Fritts HC (1976) Tree rings and climate. Academic, London
Gärtner H, Nievergelt D (2010) The core-microtome: a new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 28:85–92
Gehring E, Bellosi B, Quacchia A, Conedera M (2017) Assessing the impact of Dryocosmus kuriphilus on the chestnut tree: branch architecture matters. J Pest Sci 91:189–202
González de Andrés E, Camarero JJ, Blanco JA, Imbert JB, Lo Y-H, Sangüesa-Barreda G, Castillo FJ, Turnbull M (2018) Tree-to-tree competition in mixed European beech-Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J Ecol 106:59–75
Gurtner D, Conedera M, Rigling A, Wunder J (2015) Der Götterbaum dringt in die Wälder nördlich der Alpen vor. Wald Holz 7:22–24
Hsiao TC, Acevedo E (1974) Plant responses to water deficits, water-use efficiency, and drought resistance. Agr Meteorol 14:59–84
Huang JG, Tardif JC, Bergeron Y, Denneler B, Berninger F, Girardin MP (2010) Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Global Change Biol 16:711–731
Hyndman R (2018) hdrcde: highest density regions and conditional density estimation. vol R package version 3.2
IPCC (2014) Climate change 2014: synthesis report. In: Pachauri RK, Meyer LA (eds) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Core writing team, Geneva, p 151
Knüsel S, Conedera M, Rigling A, Fonti P, Wunder J (2015) A tree-ring perspective on the invasion of Ailanthus altissima in protection forests. For Ecol Manage 354:334–343
Köcher P, Horna V, Leuschner C (2012) Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol 32:1021–1032
Köcher P, Horna V, Leuschner C (2013) Stem water storage in five coexisting temperate broad-leaved tree species: significance, temporal dynamics and dependence on tree functional traits. Tree Physiol 33:817–832
Körner C (2015) Paradigm shift in plant growth control. Curr Opin Plant Biol 25:107–114
Kowarik I, Säumel I (2007) Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect Plant Ecol 8:207–237
Krumm F, Vítková L (2016) Introduced tree species in European forests: opportunities and challenges. European Forest Institute, Freiburg
Lev-Yadun S (2011) Why should trees have natural root grafts? Tree Physiol 31:575–578
Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J, Seidl R, Delzon S, Corona P, Kolstrom M, Lexer MJ, Marchetti M (2010) Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manage 259:698–709
Lockhart JA (1965) An analysis of irreversible plant cell elongation. J Theor Biol 8:264–275
Makinen H, Seo JW, Nojd P, Schmitt U, Jalkanen R (2008) Seasonal dynamics of wood formation: a comparison between pinning, microcoring and dendrometer measurements. Eur J Forest Res 127:235–245
McLaughlin SB, Wullschleger SD, Nosal M (2003) Diurnal and seasonal changes in stem increment and water use by yellow poplar trees in response to environmental stress. Tree Physiol 23:1125–1136
Mencuccini M, Salmon Y, Mitchell P, Holtta T, Choat B, Meir P, O’Grady A, Tissue D, Zweifel R, Sevanto S, Pfautsch S (2017) An empirical method that separates irreversible stem radial growth from bark water content changes in trees: theory and case studies. Plant Cell Environ 40:290–303
O’Neal ES, Davis DD (2015) Intraspecific Root Grafts and Clonal Growth Within Ailanthus altissima Stands Influence Verticillium nonalfalfae Transmission. Plant Dis 99:1070–1077
Ogle K, Barber JJ, Barron-Gafford GA, Bentley LP, Young JM, Huxman TE, Loik ME, Tissue DT (2015) Quantifying ecological memory in plant and ecosystem processes. Ecol Lett 18:221–235
Palacio S, Hoch G, Sala A, Körner C, Millard P (2014) Does carbon storage limit tree growth? New Phytol 201:1096–1100
Pfautsch S, Renard J, Tjoelker MG, Salih A (2015) Phloem as capacitor: radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. Plant Physiol 167:963–971
Piutti E, Cescatti A (1997) A quantitative analysis of the interactions between climatic response and intraspecific competition in European beech. Can J For Res 27:277–284
R Core Team (2018) R: a language and environment for statistical computing, vol 3.5.1. R Foundation for Statistical Computing, Vienna
Richardson AD, Carbone MS, Keenan TF, Czimczik CI, Hollinger DY, Murakami P, Schaberg PG, Xu X (2013) Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol 197:850–861
Richardson DM, Hui C, Nunez MA, Pauchard A (2014) Tree invasions: patterns, processes, challenges and opportunities. Biol Invas 16:473–481
Rosell JA, Gleason S, Mendez-Alonzo R, Chang Y, Westoby M (2014) Bark functional ecology: evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytol 201:486–497
Sass-Klaassen U, Sabajo CR, den Ouden J (2011) Vessel formation in relation to leaf phenology in pedunculate oak and European ash. Dendrochronologia 29:171–175
Savolainen O, Pyhajarvi T, Knurr T (2007) Gene flow and local adaptation in trees. Annu Rev Ecol Evol S 38:595–619
Schär C, Vidale PL, Lüthi D, Frei C, Häberli C, Liniger MA, Appenzeller C (2004) The role of increasing temperature variability in European summer heatwaves. Nature 427:332
Schuster R, Oberhuber W (2013) Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees 27:61–69
Sevanto S, Holtta T, Holbrook NM (2011) Effects of the hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant Cell Environ 34:690–703
Steppe K, Sterck F, Deslauriers A (2015) Diel growth dynamics in tree stems: linking anatomy and ecophysiology. Trends Plant Sci 20:335–343
Tarroux E, Desrochers A (2011) Effect of natural root grafting on growth response of jack pine (Pinus banksiana; Pinaceae). Am J Bot 98:967–974
Tinner W, Hubschmid P, Wehrli M, Ammann B, Conedera M (1999) Long-term forest fire ecology and dynamics in southern Switzerland. J Ecol 87:273–289
Trifilo P, Raimondo F, Nardini A, Lo Gullo MA, Salleo S (2004) Drought resistance of Ailanthus altissima: root hydraulics and water relations. Tree Physiol 24:107–114
Ugolini F, Massetti L, Pedrazzoli F, Tognetti R, Vecchione A, Zulini L, Maresi G (2014) Ecophysiological responses and vulnerability to other pathologies in European chestnut coppices, heavily infested by the Asian chestnut gall wasp. For Ecol Manage 314:38–49
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
von Arx G, Carrer M (2014) ROXAS—a new tool to build centuries-long tracheid-lumen chronologies in conifers. Dendrochronologia 32:290–293
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Wilcox RR, Erceg-Hurn DM, Clark F, Carlson M (2013) Comparing two independent groups via the lower and upper quantiles. J Stat Comput Simul 84:1543–1551
Windt CW, Blumler P (2015) A portable NMR sensor to measure dynamic changes in the amount of water in living stems or fruit and its potential to measure sap flow. Tree Physiol 35:366–375
WMO (2008) Guide to meteorological instruments and methods of observation, Appendix 4B. vol WMO-No. 8 (CIMO Guide). World Meteorological Organization, Geneva
Wunder J, Knüsel S, Gurtner D, Conedera M (2016) The spread of tree of heaven in Switzerland. In: Krumm F, Vítková L (eds) Introduced tree species in European forests: opportunities and challenges. European Forest Institute, Joensuu, pp 374–385
Zweifel R, Häsler R (2001) Dynamics of water storage in mature subalpine Picea abies: temporal and spatial patterns of change in stem radius. Tree Physiol 21:561–569
Zweifel R, Item H, Häsler R (2000) Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees 15:50–57
Zweifel R, Haeni M, Buchmann N, Eugster W (2016) Are trees able to grow in periods of stem shrinkage? New Phytol 211:839–849
Acknowledgements
We would like to thank Luca Jelmini, Franco Fibbioli, Ivan Candolfi and Florim Sabani for field and lab assistance. Furthermore, we would like to thank Richard Peters for helpful discussions on the manuscript. This study was funded by the Swiss National Science Foundation (SNF) and the French National Research Agency (ANR) (Project-Nr: 310030L_156661), the Federal Office for the Environment (FOEN) and the Swiss Federal Research Institute for Forest, Snow and Landscape Research (WSL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Piovesan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knüsel, S., Conedera, M., Zweifel, R. et al. High growth potential of Ailanthus altissima in warm and dry weather conditions in novel forests of southern Switzerland. Trees 33, 395–409 (2019). https://doi.org/10.1007/s00468-018-1785-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-018-1785-x