Abstract
Key message
We analyzed the response of Vochysia divergens Pohl trees to hydrological variation over seasons in a Brazilian wetland-based 26 physiological parameters. Dry season conditions had a greater effect on the photosynthetic function than those in the wet season.
Abstract
Plants in seasonal wetlands are subject to large hydrological fluctuations and the physiological trade-offs that occur during these variations are still poorly understood. Within the Brazilian Pantanal, the largest tropical seasonal wetland in the world, the abundance of Vochysia divergens Pohl (Vochysiaceae) has been increasing and it forms mono-specific stands. The physiological performance of V. divergens trees to withstand seasonal variations in flooding and drought was evaluated to understand how the hydrological regime affects the species habitat and encourages new areas to be occupied. Individuals were evaluated for changes in their physiological parameters by means of CO2 response curves, chlorophyll fluorescence, photosynthetic pigment and nitrogen (N) concentrations. Drought conditions caused reductions of 82, 64 and 80% of the maximum rate of CO2-saturated photosynthesis (Amax m), electron transport (Jmax m), and carboxylation (Vcmax m) per unit leaf mass, an increase in leaf fluorescence (F0: 27%) and non-photochemical quenching (ΦNPQ: 18%), and a decrease in photochemical quantum yield of photosystem II (ΦPSII in: 29%). The dry season also caused a significant reduction in leaf photosynthetic pigments and an increase in leaf N concentration, but most of the N was allocated away from carboxylation and electron transport proteins and toward leaf structure. Our data indicate that dry season drought caused a significant decline in biochemical properties associated with leaf gas exchange and an increase in allocation to leaf structure. The ability to rapidly shift to high photosynthesis as soon as water levels rise in the wet season may be critical for the growth and expansion of this species in the Pantanal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Considered the largest floodplain in the world covering 150,000 km2, the Pantanal has been threatened over the last 4–5 decades by the invasion of Vochysia divergens Pohl, a flood-tolerant species native to the Amazon Basin that forms monospecific stands once it becomes established in its naturalized habitat (Nunes da Cunha and Junk 2001, 2004; Ariera and Nunes da Cunha 2006; Junk et al. 2006). Vochysia divergens germinates and becomes established during the dry season after the flood pulse disperses seeds into the floodplain (Nunes da Cunha and Junk 2001, 2004). Once established, the naturalized population expands, causing declines in native plant species diversity (Ariera and Nunes da Cunha 2006), changes in the chemical composition of soils (Vourlitis et al. 2011), and an increase in the rate of evapotranspiration (Sanches et al. 2011). Vochysia divergens is a fast-growing pioneer tree species that can reach a height of 25–28 m (Pott and Pott 1994). The leaves have a life span of 8–9 months, and are produced regularly throughout the year with a brief break during the December–March flooding period (Dalmolin et al. 2015).
While V. divergens appears to be an aggressive invader, populations reportedly decline during the 4–5 months long dry season (Nunes da Cunha and Junk 2004; Junk et al. 2006) presumably because of decline in physiological performance. Seasonal wetlands that have well-marked wet and dry seasons experience extreme hydrological fluctuations, and plants in these systems are exposed to temporal fluctuations in resource availability that alter their leaf gas exchange and population dynamics (Araya et al. 2011; Savage and Cavender-Bars 2011; Lovell and Manges 2013; Wyse et al. 2013). Studies of plant physiology and species distribution patterns in wetland areas are usually focused on the effects of flooding, due to the potential for physiological stress that occurs due to prolonged exposure to anaerobic conditions (Rodriguez-Gonzalez et al. 2010; Correa-Araneda et al. 2012; Dalmagro et al. 2016), and the effects of drought are often neglected even though seasonal drought stress may be just as important as anaerobic stress in limiting plant physiological performance in seasonal wetlands (De Steven et al. 2010; Parolin et al. 2010; Osland et al. 2011).
Flood-tolerant species generally have morphological and anatomical characteristics or alternative metabolic pathways to overcome anoxia (Gibbs and Greenway 2003; Lovell and Menges 2013), such as adventitious root formation, lenticel hypertrophy, and aerenchyma formation in stems and roots (Kozlowski 2002; Mielke et al. 2003, 2005; Steffens et al. 2013), which improve plant–atmosphere gas exchange (Armstrong 1980). Physiological changes can also be observed, for example some plants have the ability to switch between aerobic and anaerobic respiration (Kozlowski 2002), chlorophyll synthesis is inhibited, and many plants exhibit a decline in the permeability and hydraulic conductivity of roots under anaerobic conditions (Gaspar 2011). Declines in root permeability and hydraulic conductivity also cause a decline in water uptake and stomatal closure that is mediated by synthesis and/or accumulation of abscisic acid (Chaves et al. 2009). The decline in stomatal conductance and lower concentration of chlorophyll lead to a concomitant decline in leaf CO2 assimilation (Saibo et al. 2009; Asharaf and Harris 2013). Similar responses are often observed in plants exposed to drought stress, even in plants that are considered drought tolerant (Lovell and Menges 2013). Under low water availability conditions, stomatal closure promoted by high ABA levels is one of the first strategies used by plants to reduce transpiration rate and maintain cell turgor (Melcher et al. 2009), and the reduced stomatal conductance limits the CO2 assimilation (Saibo et al. 2009; Asharaf and Harris 2013). Under severe water stress, dehydration occurs in the mesophyll cells and an inhibition of the biochemical processes related to photosynthesis can occur (Damayanthi et al. 2010; Anjun et al. 2011). For example, severe water stress can result in the down-regulation of ribulose 1,5-bisphosphate regeneration, ATP synthesis, photochemistry of photosynthesis indicated by chlorophyll fluorescence parameters, ribulose 1,5-bisphosphate carboxylase/oxygenase activity, and/or a permanent state of photoinhibition (Flexas and Medrano 2002). Thus, plants that are exposed to frequent water stress caused by both super- and sub-optimal water availability presumably have flexible physiological capacity to withstand large variations in hydrology and water availability.
Given its invasive nature in the Pantanal, the population dynamics and ecophysiology of V. divergens have been studied in an effort to understand why this species is able to expand so rapidly and form extensive mono-specific stands (Nunes da Cunha and Junk 2001, 2004; Ariera and Nunes da Cunha 2006; Junk et al. 2006; Dalmolin et al. 2012, 2013; Dalmagro et al. 2013). However, most of the ecophysiological works have focused on how flooding alters photosynthetic performance (Dalmolin et al. 2012, 2013; Dalmagro et al. 2013, 2014). Therefore, dry season effects on leaf gas exchange and biochemistry are not well studied or understood, and there is interest in knowing how the physiological performance could contribute to the population declines that are often observed during the dry season (Nunes da Cunha and Junk 2004; Junk et al. 2006). Thus, the goal of the present work was to evaluate how drought altered the leaf physiological performance and leaf chemistry of V. divergens. Because V. divergens appears to be well-adapted to flooded conditions (Dalmolin et al. 2012, 2013; Dalmagro et al. 2013), we hypothesized that there would be a decrease in physiological rates, due to the reallocation and altered utilization of foliar resources such as pigments and nitrogen, in response to drought conditions experienced during the dry season.
Materials and methods
Site study
This study was conducted in two field campaigns, March (wet season) and August (dry season) 2012, in the Brazilian Pantanal (16°39′50″S, 56°47′50″W) at a field site located approximately 160 km SSW from Cuiabá, Mato Grosso, at an elevation of 116 m above sea level. The 30 years average annual rainfall is 1420 mm and temperature 26.5 °C, and rainfall is strongly seasonal with the dry season extending from May to September (Vourlitis and da Rocha 2011). The flat topography of the area causes extensive flooding during the wet season (Nunes da Cunha and Junk 2001), and average flood height is typically 1–2 m in depth during the peak wet season (Nunes da Cunha and Junk 2004). The site is within the invasion front of an extensive forest that is dominated almost exclusively by V. divergens (Vourlitis et al. 2011). Vegetation is a mixture of trees and grasses, with tree species including V. divergens and Curatella americana L. (Dilleniaceae) and an understory consisting mainly of the grass Gymnopogon spicatus (Spreng.) Kuntze (Vourlitis et al. 2011).
Gas exchange measurements
We selected five young V. divergens plants that were 3–5 m tall, growing in full sun, and with a minimum distance of 50 m between each individual. Gas exchange measurements were made on the third or fourth leaf from the apex of the branch that was fully expanded, had no obvious disease symptoms, and exposed to full sunlight. The same plants were measured in flooded and dry seasons.
The photosynthetic response to the partial pressure of CO2 at the sites of carboxylation (A/Cc) was measured using a portable photosynthesis system (LI-6400, LI-COR, Lincoln, NE, USA). Measurements were made during the morning when temperatures are cooler and the vapor pressure deficit is lower to avoid the possibility of a midday photosynthetic depression (Dalmolin et al. 2012). Leaves were placed in the fluorometer chamber (LI-6400-40, LI-COR, Lincoln, NE, USA), which was adjusted to provide a constant photosynthetic photon flux density (PPFD) of 1000 µmol (photon) m−2 s−1 to ensure light saturation for photosynthesis (Dalmolin et al. 2013; Dalmagro et al. 2014), a 28 °C block temperature, and a relative humidity of 60%, similar to the ambient conditions (Dalmolin et al. 2012). After 15 or 20 min of acclimation to these conditions, the LI-6400 was adjusted to increase the CO2 supply in 15 steps from 50 to 1200 ppm. The assimilation was recorded only after the coefficient of variation was < 0.3%. Maximum photosynthesis on a leaf area basis (Amax a) was estimated as the mean of the three measurements made at 1200 ppm and saturating light [1000 µmol (photon) m−2 s−1]. Following Dalmagro et al. (2013), A/Cc curves were used instead of A/Ci curves because they rely on mesophyll conductance (gm), thus avoiding the underestimation of Vcmax (Epron et al. 1995; Manter and Kerrigan 2004).
The parameters of the A/Cc curve were calculated using the Farquhar et al. (1980) model, which summarizes the dependence of the net carbon assimilation rate [A (µmol(CO2) m−2 s−1)] on the partial pressure of CO2 at the sites of carboxylation [Cc (µmol(CO2) mol−1)], in which A is modeled as the minimum of Rubisco-limited (Ac), RUBP-limited (Aj) or triose-phosphate utilization TPU (Ap) according to Eqs. 1, 2, 3 and 4,
where Rd is the mitochondrial respiration rate in light, which was estimated from Ac, Vcmax is the maximum rate of Rubisco activity [µmol m−2 s−1], Cc is the molar concentration of CO2 in the chloroplast [Pa], O is the molar concentration of O2 at Rubisco [µmol(O2) mol−1], Γ* is the CO2 compensation point in the absence of mitochondrial respiration [µmol(CO2) mol−1], and Kc and Ko are the Michaelis–Menten coefficients of Rubisco activity for CO2 and O2, respectively [µmol mol−1], which were calculated for the leaf temperature of each curve fallowing the equations derived by Bernacchi et al. (2001).
The potential electron transport rate (J) (Eq. 3) is related to Jmax through an empirical relationship (Farquhar and Wong 1984):
where J is the potential rate of electron transport [µmol m−2 s−1] that is dependent upon photosynthetic photon flux density (PPFD), θ is the curvature of the light response curve, and α is the quantum yield of electron transport. The value of α was fixed at 0.3 [mol (electron) mol (photon)−1], based on an average C3 photosynthetic quantum yield of 0.093 and a leaf absorbance of 0.8 (Long et al. 1993). Mass-based estimates (Amax.m, Vcmax.m and Jmax.m) were obtained by dividing area-based values by leaf dry mass per unit area (MA).
Chlorophyll fluorescence measurements
Light curve responses of the fluorescence parameters were performed with a modulated pulse fluorometer (LI-6400-40, LI-COR, Lincoln, NE, USA) coupled with a photosynthesis measurement portable system (LI-6400, LI- COR, Lincoln, NE, USA). Measurements were made at a block temperature of 28 °C and a relative humidity of 60% to be consistent with the A/Cc curve measurements. Leaves of five plants were exposed to total darkness for 12 h by covering them with aluminum foil. Measurements of the minimum (F0) and maximum (Fm) fluorescence were made between 04:30 and 05:30 hours local time by applying saturating light pulses of 6600 µmol m−2 s−1 PPFD for 0.8 s. When the fluorescence signal approached the steady state (Fs) in actinic light, a similar pulse was applied to obtain the maximum value of the fluorescence light (Fm′), and the minimum fluorescence in the light (F0′) was measured over PPFD values varying from 0 to 1200 µmol photons m−2 s−1 by applying 5 s pulse of far-red light at 3 min intervals.
The fluorescence values were used to calculate photochemical quenching (qL), which indicates the redox state of the primary acceptor electron quinone A (QA) in photosystem II (PSII) according to Kramer et al. (2004) and Maxwell and Johnson (2000):
The yield of the three concurrent processes of energy transfer from chlorophyll in PSII—the photochemical efficiency of PSII (ΦPSII), non-photochemical quenching (ΦNPQ) and extinction by other energy losses (ΦNO) (fluorescence, basal rate of non-radioactive decay and formation of Chl triplets), were calculated according Kramer et al. (2004):
where NPQ is the non-photochemical quenching energy, which is the apparent rate constant for loss of heat from PSII:
\(q{\text{L}}\), \({\Phi _{{\text{PSII}}}}\), \({\Phi _{{\text{NPQ}}}}\), \(~{\Phi _{{\text{NO}}}}\), and NPQ were calculated for 1500 µmol photons m−2 s−1.
Nitrogen concentration and fractioning among leaf proteins
Leaf nitrogen concentration per unit dry mass (Nm) was measured on six leaves per plant using a procedure described by Embrapa (2009). The same leaves used for evaluation of gas exchange and fluorescence were used for nitrogen analysis. Following Niinemets and Tenhunem (1997) and Rodríguez-Calcerrada et al. (2008), the proportion of Nm allocated to Rubisco (Pr) and electron transport chain proteins (Pb) were calculated as:
where Nm is the total N content per leaf mass (g kg−1), SLA is the specific leaf area (m−2 kg−1), 6.25 is the conversion factor for the amount of N in Rubisco (gN Rubisco−1), 20.5 is the specific activity of Rubisco µmol CO2 (g Rubisco)−1 s−1, 8.06 µmol cytochrome f (gN in components of electron transport)−1 accounts for the amount of cytochrome f per unit N that is within the bioenergetics of electron transport, and 156 mol electrons (mol cytochrome f)−1 s−1 is the activity factor of the electron transport per unit of cytochrome f. The proportion of N allocated to light-harvesting components (PI) was calculated as:
where Cm is the concentration of total chlorophyll per unit dry mass, and CB is the weighted average of the number of chlorophyll quantity per amount of nitrogen that is present in the photosystems (PSII and PSI) and the antennae of PSII (LHCII) (Hikosaka and Terashima 1995; Niinemets and Tenhunen 1997; Rodríguez-Calcerrada et al. 2008). The proportion of N allocated to leaf structure (Ps) was calculated as:
The values of leaf chlorophyll concentration per unit dry mass (Cm) was determined following Wellburn (1994) and Porra et al. (1989). Nitrogen-use efficiency (PNUE) was calculated on the basis of concentration N and rate of photosynthesis.
Statistical analysis
A/Cc curves were analyzed using a spreadsheet developed in Matlab (V7.3, Matworks Inc., Natick, MA, USA) and provided by Su et al. (2009). The parameters derived from the A/Cc curves and other variables were analyzed using a paired t test, because the same plants were measured seasonally. Physiological variables were presented on a mass and area basis, because temporal variations in leaf physiology are not independent of temporal variations in leaf anatomy (SLA) (Niinemets and Tenhunen 1997), which change seasonally as a function of water availability for many plants like V. divergens (Dalmagro et al. 2013). Data were tested for normality and heteroscedasticity, and none of the response variables required transformation to fulfill assumptions of normality or homogeneous variance. All statistical tests were conducted using Number Cruncher Statistical Software (NSCC V7).
Results
Microclimate
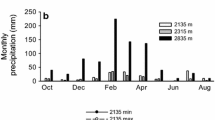
Seasonal climate variations are typical of the Pantanal region (Vourlitis and da Rocha 2011). The average temperature over the study period was 26.1 °C, with the minimum temperature (18.2 °C) observed in July and the maximum temperature (28.7 °C) in November (Fig. 1). The vapor pressure deficit (VPD) varied between a minimum of 0.13 kPa in January and a maximum of 3.02 kPa in August, with an average value of 1.01 kPa for the study period. The total rainfall was 1638 mm, which is about 215 mm above the long-term (30 years) average, with approximately half of the rainfall received between January and May (Fig. 1). Rainfall was low (< 100 mm) between June and September, representing the climatological dry season for the region (Vourlitis and da Rocha 2011), and no measureable rainfall was observed for July and August.
Total monthly precipitation (PPT; grey-bars) and average monthly atmospheric vapor pressure deficit (VPD; white circles, dotted line), temperature (black circles; solid line) and oxygen dissolved in the soil (white squares, dotted line), for the study site between January 2012 and December 2012. The scales for rainfall and VPD are displayed on the left-hand axis and the scales for temperature and oxygen dissolved in the soil on the right-hand axis. Values are means ± SE
During the January–May wet season, overflow of the Cuiabá River caused widespread flooding, which promoted waterlogging and a decrease in the soil O2 concentration (Fig. 1). Soils were essentially anoxic (O2 concentration < 2%) from January to April 2012 and again in December 2012, when the flood pulse of the 2012–2013 wet season arrived. Soil O2 concentrations increased rapidly as soon as flood water receded in May and remained at 20% between July and September (Fig. 1).
Gas exchange parameters
A lower level of the maximum rate of photosynthetic capacity (Amax) was observed during the dry period compared to the flooded period, and limitation by Rubisco activity occurred in Cc values < 20 Pa during the dry season vs. 60 Pa during the wet season (Fig. 2). Amax per unit area (Amax a) and per unit mass (Amax m) declined by 53 and 82%, respectively, during the dry season, while the maximum rate of electron transport (Jmax) per unit area (Jmax a) and mass (Jmax m) declined by 44 and 79%, respectively, during the dry season. However, no significant differences for the maximum rate of carboxylation per unit area (Vcmax a) were observed, although Vcmax per unit mass (Vcmax m) was 62% lower during the dry season (Table 1). The Jmax/Vcmax ratio (on a mass and area basis) also declined significantly during the dry season (Table 1), but no significant differences were observed for the CO2 compensation point (Γ*) or the rate of mitochondrial respiration (Rd) (data not shown).
Mean (+ se: n = 5) leaf photosynthesis response vs. chloroplast CO2 (A/Cc) curves for Vochysia divergens during the flooding (black circles) and dry (white circles) seasons. The solid lines represent the Farquhar et al. (1980) model functions for Rubisco and the dashed lines represent the model functions for RuBP
Chlorophyll fluorescence parameters
Changes in hydrology significantly affected fluorescence from the light curves (Fig. 3; Table 2). There was a 21% increase in the initial fluorescence (F0) in the dry season, but maximum fluorescence (Fm) and the maximum quantum efficiency of PSII photochemistry (Fv/Fm) were not significantly different between the flooded and dry periods (Table 2). Seasonal drought caused a significant decline in the effective photochemical quantum yield of PSII (ΦPSII), which mirrored the dry season decline Amax, and there was an associated increase in non-photochemical quenching (ΦNPQ) throughout the PPFD response curves (Fig. 3) and at saturating PPFD (1500 μmol m−2 s−1; Table 2). Higher values of ΦPSII were accompanied by larger values of JETR in the flooded period; however, light-saturated rates of photochemical quenching (qL) were not statistically different between the flooded and dry periods (Table 2), indicating that the dry season decline in ΦPSII and JETR was due more to a decline in Amax than in light-harvesting ability.
Mean (± se; n = 5) response of photochemical and non-photochemical yields of absorbed energy with photosynthetic photon flux density (PPFD) in leaves of Vochysia divergens in the flooding and dry seasons. Circles represent the photochemical quantum yield of PSII (ΦPSII), squares non-photochemical quenching (ΦNPQ) and triangles photochemical quenching (ΦNO). Black symbols represent the flooding and white symbols the dry season
Nitrogen partitioning
Leaf N concentration per unit leaf mass (Nm) and area (Na) was significantly higher during the dry season, and there were significant seasonal differences in the proportion of N allocated to Rubisco (Pr), electron transport chain proteins (Pb), light-harvesting pigments (Pl), and structure (Ps) (Table 3). For example, Pr and Pb declined by nearly three- and fivefold, respectively, during the dry season, while the dry season decline in Pl was relatively less, of the order of 1.5-fold (Table 2). In contrast, the amount of N allocated to structure increased by nearly 1.3-fold during the dry season. Together, the decline in A and the increase in N during the dry season led to a significant decline in photosynthetic N-use efficiency (PNUE: Table 3).
Photosynthetic pigments
Chlorophyll a and b contents were significantly lower in the dry season, leading to a > twofold reduction in total chlorophyll during the dry season (Table 4). However, the ratio of chlorophyll a:b increased by > twofold during the dry season because the dry season decline in chlorophyll b was larger than for chlorophyll a (ca. 4- vs. 1.7-fold; Table 4). Leaf carotenoid content also exhibited a significant decline (1.8-fold) during the dry season.
Discussion
Our data support the hypothesis that there will be a decrease in physiological rates, photosynthetic pigments, and changes in leaf nitrogen (N) allocation of V. divergens when exposed to dry season drought conditions. The reduction in the biochemical parameters derived from the A/Cc curves, Amax, Jmax, and Vcmax, indicates that drought, which occurs during the dry season, caused a biochemical limitation to leaf photosynthesis. This biochemical limitation appeared to be due to both reductions in carboxylation and electron transport, which in turn led to a switch in the allocation of N from photochemical pools (Rubisco, electron transport and light-harvesting proteins) to leaf structural pools, indicating that seasonal drought had a large effect on leaf photosynthetic potential.
Xu and Baldocchi (2003) have shown that Amax is highly correlated with Vcmax, and even though rates of Vcmax a were not significantly affected by variations in hydrology Vcmax m was significantly lower during the dry season (Table 1). A reduction in Vcmax m is often attributed to a decrease in the amount of Rubisco (Jacob et al. 1995), or its low activation state (Nakano et al. 1997), as leaves increase allocation to mass. The significant dry season reduction in the N allocated to Rubisco (Pr), and increase in allocation to structure (Ps) (Table 3), indicate a decline in the amount and/or activation state of Rubisco (Porra et al. 1989; Wellburn 1994; Niinemets and Tenhunen 1997).
While the dry season decline in Amax may have been driven in part to a decline in Vcmax, the significant reduction in the ratio Jmax/Vcmax during the dry season was mostly due to the reduction in Jmax (around 47.3%). Thus, the decline in Amax, which is measured at high Cc, may be more reflective of limitations in RuBP regeneration by the rate of electron transport (Jmax) than by the rate of carboxylation (Sharkey et al. 2007). It is known that the Rubisco activity and RuBP regeneration have different sensitivities to water stress acclimation (Bernacchi et al. 2001), and that RuBP regeneration capacity is more sensitive than the capacity of carboxylation in situations of low leaf water potential (Medrano et al. 2002). The significant reduction in Jmax observed in the dry season (Table 1) can be attributed to several factors such as a decline in the concentration a lower investment of leaf pigments (Table 4), and a decline in the investment of N to electron transport proteins (Table 3) (Rodríguez-Calcerrada et al. 2008; Asharaf and Harris 2013). The sharp decrease in the content of photosynthetic pigments is commonly observed in plants subjected to drought stress (Din et al. 2011; Asharaf and Harris 2013) and is a good indicator of signs of oxidative stress (Bacelar et al. 2006). This decrease may result from the chlorophyll degradation (Smirnoff 1993) or due to a deficiency in the chlorophyll synthesis in conjunction with changes in the structure of the thylakoid membrane (Brito et al. 2003).
Significant reductions in Jmax and the fluorescence parameters obtained in the dark (F0) and under varying PPFD (JETR, ΦPSII and ΦNPQ) provide evidence that the dry season decline in V. divergens Amax was due in large part to photochemical limitations. The observed increase in Fo can indicate damage to the PSII reaction center or a decrease in the transmission capacity of the excitation energy from the antenna to the reaction center, since F0 is the fluorescence when QA (quinone primary receiving of electrons from PSII) is completely oxidized and the PSII reaction center is open (Baker and Rosenqvst 2004). Although we found that a reduction in the maximum efficiency of PSII, represented by Fv/Fm, was not statistically significant between seasons, there was a significant reduction in the efficiency of photosystem II (ΦPSII) (Table 2), indicating that a smaller proportion of absorbed energy was used in this photochemical pathway due to the reduction in the functioning of this photosystem. Decreases in ΦPSII under water stress may be attributed to damage to the oxygen evolving complex (OEC), which promotes the generation of reactive oxygen species (ROS) that are responsible for photoinhibition and oxidative damage (Anjun et al. 2011). Decrease in ΦPSII can also be ascribed to degradation of the D1 protein constituents of the PSII reaction center (Zlateve and Yordanov 2004).
Declines in ΦPSII and ΦNO led to an increase in ΦNPQ (Table 2), indicating an increase in non-photochemical energy dissipation during the dry season. The increased of ΦNPQ indicates that the rate of electron transport is reduced in comparison to the normal state (Cornic and Briantais 1991), and demonstrate that the pH of the lumen of the thylakoid is acidic and that the transport of H+ by ATP synthase is the limiting ATP synthesis (Lawlor and Tezara 2009). The significant dry season decrease in JETR (Table 2) further implicates photochemical limitations to RuBP regeneration, but despite the lower JETR and ΦPSII, photoinhibition was probably not observed in this study since the values of Fv/Fm were not significantly affected by seasonal drought and were higher than 0.75 (Maxwell and Johnson 2000).
Vochysia divergens plants showed significantly higher Nm and Na values during the dry season (Table 3), which, according to Wright et al. (2001), is a common phenomenon. Wright et al. (2001) suggested that the higher N allowed plants exposed to dry conditions to achieve a higher A per unit area at a lower stomatal conductance, which would act to reduce transpiration rates. Some of this additional leaf N was allocated to structural components (Table 3), which may help to conserve limited water reserves, reduce leaf loss to herbivore damage, and increase leaf longevity (Reich et al. 1995; Hikosaka et al. 1998; Wright et al. 2001; Brennes-Arguedas et al. 2006). In particular, herbivore pressure on V. divergens increases substantially during the dry season (Dalmolin et al. 2015), thus allocating N to structure or secondary metabolites may reduce the impact of herbivore damage.
In conclusion, V. divergens exhibited a reduction in leaf CO2 assimilation during the period of highest water deficit in the Pantanal. This decrease was promoted by biochemical and photochemical changes that were associated with an increase in N allocation to the leaf structure. These coordinated changes in physiology appear to increase the chances of survival of V. divergens seedlings during a time of presumably high water stress. The ability to survive under stressful conditions, coupled with flooding tolerance and a rapid shift to C gain as soil moisture rises during the wet season, may be a key property that allows the successful expansion of V. divergens into new habitats such as the Brazilian Pantanal.
Author contribution statement
AC Dalmolin, FA Lobo, and CER Ortiz, designed the experiment. AC Dalmolin, HJ Dalmagro, and MZ Antunes Junior performed the experiment. AC Dalmolin and FA Lobo performed statistical analyses. AC Dalmolin, GL Vourlitis, and FA Lobo wrote the manuscript.
Abbreviations
- A:
-
Net carbon assimilation rate (µmol CO2 m−2 s−1)
- A c :
-
Rubisco-limited photosynthesis
- A j :
-
RUBP-limited photosynthesis
- A p :
-
Triose-phosphate utilization TPU photosynthesis limited
- A/C c :
-
Photosynthetic response to the partial pressure of CO2 at the sites of carboxylation
- A max a :
-
Maximum photosynthetic rate (per unit leaf area)
- A max m :
-
Maximum photosynthetic rate (per unit leaf mass)
- Ca:
-
Concentration of atmospheric CO2
- Chl a:
-
Chlorophyll a
- Chl b:
-
Chlorophyll b
- Car:
-
Carotenoids
- F 0 :
-
Minimal fluorescence from dark-adapted leaves
- F m :
-
Maximal fluorescence from dark-adapted leaves
- F v/F m :
-
Maximum quantum efficiency of PSII photochemistry
- J max a :
-
Maximum electron transport rate at saturating light (per unit leaf area)
- J max m :
-
Electron transport rate at saturating light (per unit leaf mass)
- J max/V cmax :
-
Ratio of the electron transport rate at saturating light and the maximum Rubisco carboxylation velocity rate at saturating light
- J ETR :
-
Maximum electron transport rate from fluorescence
- Nm :
-
Leaf nitrogen concentration (per unit leaf mass)
- Na :
-
Leaf nitrogen concentration (per unit leaf area)
- q L :
-
Photochemical quenching from a lake model
- PPFD:
-
Photosynthetic photon flux density
- P r :
-
Nitrogen allocated to Rubisco
- P b :
-
Nitrogen allocated to electron transport chain proteins
- P l :
-
Nitrogen allocated to light-harvesting pigments
- P s :
-
Nitrogen allocated to structure
- PNUE:
-
Photosynthetic nitrogen use efficiency
- Rd:
-
Rate of mitochondrial respiration in the light
- Ratio a/b :
-
Ratio between chlorophyll a and b
- SLA:
-
Specific leaf area
- Total Chl:
-
Total chlorophylls (a + b)
- V cmax a :
-
Maximum Rubisco carboxylation velocity (per unit leaf area)
- V cmax m :
-
Maximum Rubisco carboxylation velocity (per unit leaf mass)
- VPD:
-
Vapor pressure deficit
- Γ*:
-
CO2 compensation point in the absence of Rd
- Φ PSII :
-
Effective photochemical quantum yield of PSII
- ΦNO:
-
Photochemical quenching from a lake model
- Φ NPQ :
-
Non-photochemical quenching from a lake model
References
Anjun SA, Xie X, Wang L, Saleem F, Man C, Leil W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agr Res 6:2026–2032
Araya YN, Silvertown J, Gowing DJG, McConway KJ, Linder HP, Midgley G (2011) A fundamental, eco-hydrological basis for niche segregation in plant communities. New Phytol 189:253–258
Ariera J, Nunes da Cunha C (2006) Fitossociologia de uma floresta inundável monodominante de Vochysia divergens Pohl (Vochysiaceae) no Pantanal Norte, MT, Brasil. Acta Bot Bras 20:568–580
Armstrong W (1980) Aeration in higher plants. Adv Bot Res 7:225–332
Asharaf M, Harris PJC (2013) Photosynthesis under stressful environments: An overview. Photosynthetica 51:163–190
Bacelar EA, Santos DL, Moutinho-Pereira JM, Gonçalves BC, Ferreira HF, Correia CM (2006) Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: changes on structure and chemical composition of foliage and oxidative damage. Plant Sci 170:596–605
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluoescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosythesis. Plant Cell Environ 24:253–259
Brennes-Arguedas T, Horton MW, Coley PD, Lokvam J, Waddell RA, Meizosa- O’Meara BE, Cursar TA (2006) Contrasting mechanisms of secondary metabolite accumulation during leaf development in two tropical tree species with different leaf expansion strategies. Oecologia 149:91–100
Brito G, Costa A, Fonseca HMAC., Santos CVV (2003) Response of Olea europaea ssp. maderensis in vitro shoots exposed to osmotic stress. Sci Hort 97:411–417
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Cornic G, Briantais JM (1991) Partitioning of photosynthetic eléctron floe between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183:178–184
Correa-Araneda FJ, Urrutia J, Sotomora Y, Figueroa R, Hauenstein E (2012) Effects of the hydroperiod on the vegetative and community structure of freshwater forested wetlands, Chile. J Freshw Ecol 27:459–470
Dalmagro HJ, Lobo FA, Vourlitis G, Dalmolin AC, Antunes Junior MZ, Ortiz CER, Nogueira JS (2013) Photosynthetic parameters of two invasive tree species of the Brazilian Pantanal in response to seasonal flooding. Photosynthetica 51:281–294
Dalmagro HJ, Lobo FA, Vourlitis GL, Dalmolin AC, Antunes Junior MZ, Ortiz CER, Nogueira JS (2014) The physiological light response of two tree species across a hydrologic gradiente in Brazilian savanna (Cerrado). Photosynthetica 52:22–35
Dalmagro HJ, Lobo F, de A, Vourlitis, Dalmolin GL, Antunes Junior AC, Rodriguez-Ortiz MZ, Nogueira CE JS (2016) Photosynthetic response of a wetland- and an upland-adapted tree species to seasonal variations in hydrology in the Brazilian Cerrado and Pantanal. Acta Physiol Plant 38:107–127
Dalmolin AC, Dalmagro HJ, Lobo FA, Antunes Junior MZ, Ortíz CER, Vourlitis GL (2012) Effects of flooding and shading on growth and gas exchange of Vochysia divergens Pohl (Vochysiaceae) of invasive species in the Brazilian Pantanal. Braz J Plant Physiol 24:75–84
Dalmolin AC, Dalmagro HJ, Lobo FA, Antunes Junior MZ, Ortíz CER, Vourlitis GL (2013) Photosynthetic light and carbon dioxide response of the invasive tree, Vochysia divergens Pohl, to experimental flooding and shading. Photosynthetica 51:379–386
Dalmolin AC, Lobo FA, Vourlitis GL, Silva PR, Dalmagro HJ, Antunes Junior MZ, Ortíz CER (2015) Is the dry season na importante driver of phenology and growth for two Brazilian savana tree species with contrasting leaf habits? Plant Ecol 216:407–417
Damayanthi MMN, Mohotti AJ, Nissanka SP (2010) Comparison of tolerant ability of mature field grown tea (Camellia sinensis L.) cultivars exposed to drought stress in Passara area. Trop Agric Res 22:66–75
De Steven D, Sharitz RR, Barton CD (2010) Ecological outcomes and evaluation of success in passively restored southeastern depressional wetlands. Wetlands 30:1129–1140
Din J, Khan SU, Ali I, Gurmani AR (2011) Physiological and agronomic response of canola varieties to drought stress. J Anim Plant Sci 21:78–82
EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Manual de análises químicas de solos, plantas e fertilizantes. Ed: Fábio Cesar da Silva. 2ª ed. 2009. p 627
Epron D, Goddard D, Cornic G, Gentry B (1995) Limitation of net CO2 assimilation rate by internal resistance to CO2 transfer in the leaves of two tree species (Fagus sylvatica L. and Castanea sativa Mill.). Plant Cell Environ 18:43–51
Farquhar GD, Wong SC (1984) An empirical model of stomatal conductance. Aust J Plant Physiol 11:191–210
Farquhar GD, Caemmerer S von, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Flexas J, Medrano H (2002) Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann Bot 89:183–189
Gaspar M (2011) Aquaporinas: de canais de água a transportadores multifuncionais em plantas. Rev Bras Bot 34:481–491
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618
Hikosaka K, Hanba YT, Hirose T, Terashima I (1998) Photosynthetic nitrogen-use efficiency in leaves of woody and herbaceous species. Funct Ecol 12:896–905
Jacob J, Greitner C, Drake BG (1995) Acclimation of photosynthesis in relation to Rubisco and non-structural carbohydrate contents and in situ carboxylase activity in Scirpus olneyi at elevated CO2 in the field. Plant Cell Environ 18:875–884
Junk WJ, Nunes da Cunha C, Watzen KM, Pettermann P, Trussmann C, Marques MI, Adis J (2006) Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat Sci 8:278–309
Kozlowski TT (2002) Physiological-ecological impacts of flooding on riparian forest ecosystems. Wetlands 22:550–561
Kramer DM, Johanson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of Q A redox state and excitation energy fluxes. Photosynth Res 79:209–218
Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of process. Ann Bot 103:561–579
Long SP, Postl WF, Bolhar Nordenkampf HR (1993) Quantum yields for uptake of carbon dioxide in C3 vascular plants of contrasting habitats and taxonomic groupings. Planta 189:226–234
Lovell JT, Menges E (2013) Dominant species responses to drought in seasonal wetlands: evidence from reciprocal transplants across a moisture gradient. J Torrey Bot Soc 140:157–169
Manter DK, Kerrigan J (2004) A/Ci curve analysis across a range of woody plant species: influence of regression analysis parameters and mesophyll conductance. J Exp Bot 55:2581–2588
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Medrano H, Escalona JM, Bota J, Gulias J, Flexas J (2002) Regulation of photosynthesis of C-3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann Bot 89:895–905
Melcher K, Ng LM, Zhou XE, Soon FF, Xu Y, Suino-Powell KM, Parks S, Weinwe JJ, Fujii H, Chinnusamy V, Kovach A, Li J, Wang Y, Li J, Peterson FC, Jensen DR, Young E, Volkman BF, Cutler SR, Zhu J, Cutler SR, Zhu J, Xu HE (2009) A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature 462:602–608
Mielke MS, Almeida AAF, Gomes FP, Aguilar AG, Mangabeira PAO, (2003) Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ Exp Bot 50:221–231
Mielke MS, Almeida AAF, De Gomes FP, Mangabeira PAO, Costa Silva D, (2005) Effects of soil flooding on leaf gas exchange and growth of two neotropical pioneer tree species. New For 29: 161–168
Nakano H, Makino A, Mae T (1997) The effect of elevated partial pressure of CO2 on the relationship between photosynthetic capacity and N content in the rice leaves. Plant Physiol 115:191–198
Niinemets O, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Nunes da Cunha C, Junk WJ (2001) Distribution of woody plants communities along the flood gradient in the Pantanal of Poconé, Mato Grosso, Brazil. Int J Ecol Environ Sci 27:63–70
Nunes da Cunha C, Junk WJ (2004) Year-to-year changes in water level drive the invasion of Vochysia divergens in Pantanal grasslands. Appl Veg Sci 7:103–110
Osland MJ, Gonzales E, Richardson CJ (2011) Coastal freshwater wetland plant community response to seasonal drought and flooding in northwestern costa rica. Wetlands 31:641–652
Parolin P, Waldhoff D, Piedade MTF (2010) Gas exchange and photosynthesis. In: Junk WJ et al. (eds) Amazonian floodplain forests ecophysiology, biodiversity and sustainable management. Ecological Studies. Springer, New York, pp 203–222
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solventes: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Pott A, Pott VJ (1994) Plantas do Pantanal. Editora Embrapa, Embrapa—CPAP, Corumbá
Reich PB, Kloppel BD, Ellsworth DS, Walters MB (1995) Different photosynthesis-nitrogen relations in decíduos hardwood and evergreen coniferous trees species. Oecologia 104:24–30
Rodríguez-Calcerrada J, Pardos JA, Gil L, Reich PB, Aranda I (2008) Light response in seedlings of a temperate (Quercus petraea) and a sub_Mediterranean species (Quercus pyrenaica): contrasting ecological strategies as potencial keys to regeneration performance in mixed marginal populations. Plant Ecol 195:273–285
Rodriguez-Gonzalez PM, Stella JC, Campelo F, Ferreira MT, Albuquerque A (2010) Subsidy or stress? Tree structure and growth in wetland forests along a hydrological gradient in southern europe. For Ecol Manag 259:2015–2025
Saibo NJM, Lourenço T, Oliveira MM (2009) Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann Bot 103:609–623
Sanches L, Vourlitis GL, Alves MC, Pinto-Junior OB, Nogueira JS (2011) Seasonal patterns of evapotranspiration for a Vochysia divergens forest in the Brazilian Pantanal. Wetlands 31:1215–1225
Savage JA, Cavender-Bares JM (2011) Contrasting drought survival strategies of sympatric willows (genus: Salix): consequences for coexistence and habitat specialization. Tree Physiol 31:604–614
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Steffens B, Steffen-Heins A, Sauter M (2013) Reactive oxygen species mediate growth and death in submerged plants. Front Plant Sci 4:4–179
Su Y, Zhu G, Miao Z, Feng Q, Chang Z (2009) Estimation of parameters of a biochemically based model of photosynthesis using a genetic algorithm. Plant Cell Environ 32:1710–1723
Vourlitis GL, da Rocha (2011) HR flux dynamics in the cerrado and Cerrado-forest transition of Brazil. In: Hill MJ, Hanan NP (eds) Ecosystem function in global Savannas: measurement and modeling at landscape to global scales. CRC, Inc., Boca Raton, pp 97–116
Vourlitis GL, Lobo FA, Biudes MS, Ortíz CER, Nogueira JS (2011) Spatial variations in soil chemistry and organic matter content across a Vochysia divergens invasion front in the Brazilian Pantanal. Soil Sci Soc Am J 75:1553–1560
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids using various solventes with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wright IJ, Reich PB, Westoby M (2001) Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct Ecol 15:423–434
Wyse SV, Macinnis-Ng CMO, Burns BR, Clearwater MJ, Schwendenmann L (2013) Species assemblage patterns around a dominant emergent tree are associated with drought resistance. Tree Physiol 33:1269–1283
Xu L, Baldocchi DD (2003) Seasonal trends in photosynthetic parameters and stomatal conducatance of blue oak (Quercus douglasii) under prolonged summer drought and hight temperature. Tree Physiol 23:865–877
Zlateve ZS, Yordanov IT (2004) Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. plants. BJPP 30:3–18
Acknowledgements
This research was supported in part by Instituto Nacional de Ciência e Tecnologia em Áreas Umidas (INAU), Programa Institutos Nacionais de Ciências e Tecnologia—CNPq/MCT, a U.S. National Science Foundation-Office of International Science and Engineering (NSF-OISE) grant to GLV, and a Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Science Without Borders grant to GLV. Additional logistic support provided by the Universidade Federal de Mato Grosso Programa de Pos-Graduacão em Física Ambiental (UFMTPGFA) is gratefully appreciated. The Brazilian Coordination for the improvement of Higher Education Personnel (CAPES) provided scholarships to ACD, HJD, and MZAJ. The authors thank the SESC reserve—RPPN, particularly to the park-ranger and Renan Gonçalves de Oliveira, for the support field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by C. Lovelock.
Rights and permissions
About this article
Cite this article
Dalmolin, Â.C., de Almeida Lobo, F., Vourlitis, G.L. et al. Physiological adjustments of an invasive tree species to extreme hydrological events in a tropical seasonal wetland. Trees 32, 1365–1375 (2018). https://doi.org/10.1007/s00468-018-1718-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-018-1718-8