Abstract
Key message
In response to irradiance seasonality, saplings under brighter conditions increased leaf biomass with high photosynthesis to realize high growth, whereas understory saplings depend on photosynthesis during canopy leaf fall, and larger root biomass contributed to storage.
Abstract
We studied the changes in photosynthesis, biomass partitioning, and starch content in Abies firma saplings in a mixed deciduous forest in southwest Japan. Saplings were examined under high, intermediate, and low irradiance levels. The relative irradiance at intermediate and low irradiance levels increased significantly during winter and spring due to leaf fall in the deciduous canopy trees. The maximum photosynthetic rates (P max) under low irradiance were lower than those under high irradiance throughout the year. The needle daily carbon balance in summer was negative under low irradiance, whereas the balance became positive in winter and spring due to increases in irradiance. The needle daily carbon balance in intermediate- and high-irradiance saplings remained positive year-round, although the midday photosynthetic depression suppressed the daily needle carbon gain under high irradiance. The starch content in needles, branches, and roots was higher in spring due to high photosynthetic production, and then decreased in summer under all irradiance levels. Root starch under low irradiance levels may be used to maintain carbon balance during summer, thereby contributing to survival. High needle biomass coupled with a high P max facilitated carbon gain under high and intermediate irradiance, whereas high relative root biomass under low irradiance may enhance storage ability. Overall, A. firma saplings seemed to acclimate to different irradiance levels with seasonal changes through changes in photosynthetic traits, starch storage, and biomass partitioning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding survival and acclimation mechanisms of canopy tree saplings under contrasting irradiance has important implications for forest regeneration and management (Mitchell and Arnott 1995; Kimmins 1997; Valladares and Niinemets 2008; Čater et al. 2014). Photosynthesis, carbon allocation, and biomass partitioning play key roles in the survival and adaption of saplings to various irradiance levels (Givnish 1988; Kitajima 1994; Canham et al. 1999; Johnson and Smith 2005). For example, saplings under high irradiance levels exhibit higher photosynthetic capacity to maximize carbon absorption; in contrast, leaves under lower irradiance levels show reduced photosynthetic capacity and decreased dark respiration rates (R d ) to maintain low photosynthetic light compensation points (Koike 1986; Kitajima 1994; Kenzo et al. 2011). Carbon allocation also changes with irradiance levels; carbon is preferentially allocated to growth under high irradiance, whereas allocation to reserves such as root starch increases under low irradiance, particularly within the forest understory (Kozlowski 1992; Kobe 1997; Canham et al. 1999). Under low irradiance, saplings rely on stored carbon to meet their energy demands during periods of negative carbon balance (Myers and Kitajima 2007; Dietze et al. 2014); thus, species with high relative root biomass (high root-to-shoot ratios) exhibit high survival under low irradiance (Kitajima 1994).
The Japanese fir (Abies firma Sieb. et Zucc.) generally forms mixed forests together with various deciduous broad-leaved canopy trees and Tsuga sieboldii Carr. between the warm temperate and cool-temperate zones in southwestern Japan (Yamanaka 1962; Horikawa 1972; Ando et al. 1977). The Japanese fir tree is a key species within the forest canopy, and mature trees can reach 40 m in height and 2 m in diameter (Suzuki 1980; Suzuki and Tsukahara 1987). Fir saplings grow at various irradiance levels, from the forest understory to open sites, exhibiting distinct variation in growth rate, crown shape, and needle photosynthetic traits across irradiance levels (Nakao 1985; Kenzo et al. 2000, 2014). Saplings have high shade tolerance and can survive 20–60 years with very slow growth rates under closed-canopy conditions (Yuruki and Aragami 1973; Suzuki 1980; Aragami 1987). Shaded saplings found in the understory with relative irradiance typically less than 5% alter their crown to an umbrella shape to capture weak irradiance efficiently (Nakao 1985; Kenzo et al. 2014). Needles also display a large plasticity in response to changes in irradiance. The needle photosynthetic rate at light saturation (P max) and needle mass per area (LMA) strongly decreases under low irradiance levels (relative irradiance 5–10%) after changing crown shape (Kenzo et al. 2000). However, in saplings under high irradiance levels, annual height growth can reach 40 cm, and crowns change to a conical shape (Aragami 1987; Kenzo et al. 2014). Furthermore, sun needles develop under high irradiance and exhibit higher P max and a thicker palisade layer in the needle lamina compared with shade needles under lower irradiance (Nakao 1985; Kenzo et al. 2000). Similar light acclimation via changes in morphological and physiological properties of the crown and needles has been reported in many evergreen conifer saplings, including several Abies species such as A. alba Mill., A. balsamea (L.) Mill., and A. mariesii Mast. (Mailly and Kimmins 1997; Kohyama 1980; Duchesneau et al. 2001; Grassi and Bagnaresi 2001; Landhäusser and Lieffers 2001; Robakowski et al. 2003).
Seasonal changes in temperature and irradiance may also substantially affect sapling carbon balance and survival (Kimura 1969; Lassoie et al. 1983; Lei and Koike 1998; Miyazawa and Kikuzawa 2005). Particularly in mixed forests with deciduous canopy trees, irradiance at the forest understory can change drastically with the leaf phenology of the canopy layer. Although trees in boreal forests show low or negligible photosynthetic capacity during very cold winters (Bourdeau 1959; Schaberg et al. 1995, 1998; Verhoeven et al. 1999; Öquist and Huner 2003), the saplings of evergreen tree species in the understory of temperate mixed forests exhibit large carbon gains during canopy leaf fall in the winter and early spring (Hashimoto and Shirahata 1995; Katahata et al. 2005; Miyazawa and Kikuzawa 2005; Hitsuma et al. 2012). The carbon gains during canopy leaf fall (i.e., the dormant season) may be stored in plant structures for later allocation to growth and survival during the growing season, although the proportions of allocation may vary with irradiance level (Kozlowski 1992). Increases in stored carbon such as starch, which is the main storage form for carbohydrates in conifers (Gower et al. 1995), occur in the needles, stems, and roots in several evergreen conifer trees, including Abies species, during the dormant season (Miyake 1902; Kimura 1969; Schaberg et al. 2000). Despite the importance of the seasonal light regime to understand the mechanisms of sapling acclimation to forest environments with different irradiance levels, few studies have examined the dynamics of photosynthesis, carbon balance, and stored carbon throughout an entire year. In this study, we focus on seasonal changes in needle carbon gain and stored starch in A. firma in a mixed deciduous forest. We hypothesized that substantial carbon gain occurs during canopy leaf fall (dormant season) and carbon storage (i.e., starch content) increases in the season; that stored carbon is decreased during summer by growth and the need to maintain the carbon balance; and that biomass partitioning to needle and root components varies with the irradiance level. To test these hypotheses, we measured seasonal changes in needle photosynthesis, daily carbon balance, growth, biomass partitioning, and starch content in A. firma saplings growing under different irradiance levels in a temperate mixed forest in southwestern Japan.
Materials and methods
Study site
The study was conducted in a mixed deciduous-broadleaf and evergreen conifer forest in Komenono, Ehime, Japan (132°54′E, 33°53′N, 700 m a.s.l.). Annual precipitation in the region is approximately 1800 mm, and the mean annual, monthly minimum, and maximum air temperatures are 12.3, − 2.3, and 27.4 °C, respectively (Fig. 1). Soils were mainly classified as brown forest soils based on the Japanese forest soil classification standard, and the parent material was strongly weathered granite (Sanquetta et al. 1994). The canopy layer consisted of A. firma and various deciduous broad-leaved trees such as Carpinus laxiflora (Sieb. et Zucc.) Blume, Castanea crenata Sieb. et Zucc., and Quercus serrata Murray. The canopy comprised approximately 25% A. firma trees and 70% deciduous broad-leaved trees (Sanquetta et al. 1994). Dwarf bamboo [Sasa borealis (Hack.) Makino et Shibata] and some evergreen shrubs such as Camellia japonica L. and Eurya japonica Thunb. occurred at low density in the understory. Leaf fall in the canopy layer begins in late November or early December, and canopy leaf flush occurs during May (Kenzo et al. 2014).
Seasonal changes in monthly average, maximum and minimum temperature (a), monthly precipitation (b), and relative irradiance in saplings for needle photosynthesis measurements for three irradiance levels (c high, intermediate, and low). Different letters associated with relative irradiance indicate significant differences among months at the same irradiance level (Bonferroni test, P < 0.05). ‘ns’ indicates not significant. The results of the statistical analysis are shown in Table 1. Asterisks indicate significant differences among irradiance levels in same the month (ANOVA, *, P < 0.05; **, P < 0.01; ***, P < 0.001)
Plant material and irradiance levels
We randomly selected 36 saplings of A. firma under different irradiance levels for the study. All sapling heights were about 1 m. Irradiance was measured using an illumination meter (Digital Illumination Meter T-1H, Konica-Minolta, Japan) at a height of 150 cm above the ground on a cloudy day, with ten replicates for each sapling. We conducted the measurements from 1100 to 1330. Simultaneously, we measured the irradiance under open conditions and calculated the relative irradiance [relative irradiance = (sapling irradiance/irradiance at open condition) × 100] for each sapling. Relative irradiance was recorded every 2–3 months during 1.5 years. Using the relative irradiance in August, we divided the saplings into three relative irradiance categories: more than 40% irradiance was considered high irradiance; 5–40% was intermediate irradiance; and less than 5% was considered low irradiance. The relative irradiance of the saplings ranged from 0.5 to 92% in August. The relative irradiance increased during canopy leaf fall (December to April), varying from 12 to 98%.
Measurements of needle photosynthetic traits
We measured the needle gas exchange for 9 of the 36 saplings. Measurements were taken for three saplings for each irradiance level. The photosynthetic rate at light saturation (P max) and dark respiration rate (R d ) of current-year needles attached at top of the crown were measured using a portable photosynthesis meter (SPB-H4, ADC, UK) in August and October 1999 and in January, April, May, July, and October 2000. P max was measured at ambient temperature and carbon dioxide (CO2) concentration (ca. 360–390 ppm). Photosynthetic photon flux density (PPFD) was maintained at 1000 μmol m−2 s−1, which was saturated light intensity for this species (Kenzo et al. 2000). All measurements were conducted from 0800 to 1100 to avoid midday depression of photosynthesis (Hodges 1967). We also measured R d after 30–60 min of dark acclimation. To calculate the daily needle carbon balance, we conducted diurnal measurements of the needle gas exchange in March, August, October 1999, and January 2000 for the same saplings used for the above photosynthesis measurements. The gas exchange was measured on a fully expanded current-year needle at the upper crown surface. The rate of gas exchange was recorded every 5–10 min at an interval of about 15–24 h including daylight hours (approximately 0700–1800). The diurnal needle carbon balances (daily carbon balance) were computed from the diurnal courses in net needle CO2 exchange rate [e.g., Total CO2 = (A 1 × t 1) + (A 2 × t 2) + … (A n × t n ), where A is the needle net CO2 assimilation rate, and t is the time to the next measurement point; Kenzo et al. 2003]. We divided the carbon balance during daytime (determined as measurement time greater than PAR > 1 μmol photon m−2 s−1) and nighttime (nighttime carbon loss). When the nighttime respiration rate was not measured throughout the entire night, we calculated it by assuming that the average value of the measured nighttime rate continued until sunrise. All environmental conditions were ambient, and an open-top chamber was used. We conducted measurements on 3 days that had mostly sunny conditions. All needles used for the gas exchange measurements were collected after the measurements to determine the needle area as a basis for calculating the area-based CO2 assimilation rate. The area of fresh needles was calculated using a leaf area meter (Area Meter MK2, Delta Devices).
Measurements of starch content and sapling growth
We collected the fresh roots (about 1 g, 3–5-mm diameter) of 16 saplings from all selected saplings in the morning (at approximately 0700–0900), including those for which photosynthesis measurements were taken, to avoid daily fluctuations in starch content (Kenzo et al. 2013). Measurements were made for five saplings at high irradiance, six saplings at intermediate irradiance, and five saplings at low irradiance; these were conducted in April, August, and October 1999, and March 2000. We also collected 1-year-old needles and branches in April and August. We also measured the height of all sampled saplings in March 1999 and 2000. The relative height growth rates (RGRh) of the saplings were calculated using the formula,
where Ht 1 and Ht 2 are the height (in cm) at the early (t 1) and later inventory (t 2), and t (time) is measured in years.
All samples were stored in a compact freezer immediately after collection. In the laboratory, all samples were dried at 60 °C for 3 days, after which dry mass was measured (Kenzo et al. 2013). Then, the dried samples were ground to a fine powder. Starch concentrations were determined by extracting 0.1 g of aliquots of dry sample in perchloric acid, and then combining the solubilized starch with iodine (Pucher et al. 1948; Pate et al. 1990). We also prepared blanks and varying concentrations of known starch standards with iodine to calculate a calibration curve. The fractional transmittance was read using a UV/VIS spectrophotometer (Shimadzu UV-1400, Kyoto, Japan) at 620 nm. The proportion of starch remaining in roots from April to August was calculated as the starch content in August divided by the starch content in April. Reports on the growth phenology of saplings of A. firma indicated that shoot elongation and the stem increment of most saplings started in late April and stopped by late August at the study site (Kenzo et al. 2014).
Determination of biomass partitioning of saplings
All 36 saplings under different irradiance levels were excavated and divided into needle, stem, branch, and root components in the field. All fresh weights were measured using an electronic balance in the laboratory. All samples were dried in an oven at 105 °C for 80 h until they reached constant mass, after which dry mass was measured. The root-to-shoot ratio (root dry mass/all aboveground dry mass) and the needle mass ratio (needle dry mass/non-needle organs dry mass) were calculated. We also calculated the root starch pool size from the root biomass and root starch content.
Statistical analysis
The effects of the independent values (month and irradiance level) on dependent variables including the relative irradiance, P max, R d , daily carbon balance, nighttime carbon loss, and root starch content were evaluated by fitting generalized linear mixed models (Sokal and Rohlf 1995). The effects of individual saplings were included as random effects. Type III tests of the fixed effects (Wald-type test) were performed. Post hoc multiple comparison testing was performed using the Bonferroni method when the result was significant at P < 0.05. To test the seasonal effect on these dependent variables (e.g., P max) at the same irradiance level, we constructed generalized linear mixed models with the Wald-type test and conducted post hoc multiple comparison testing using the Bonferroni method. We performed ANOVA to test the irradiance effects on the relative growth rate, needle and branch starch contents, proportion of remaining starch, biomass partitioning, and relative irradiance in August. Tukey–Kramer’s or Tukey’s tests were used to compare means when ANOVA indicated significant differences (Sokal and Rohlf 1995). All analyses were conducted using SPSS for Windows (ver. 22; IBM, Armonk, NY, USA).
Results
Seasonal changes in irradiance levels
The irradiance levels experienced by the saplings changed dramatically with leafing phenology of upper deciduous canopy trees (Fig. 1c; Table 1), except for saplings under high irradiance levels. Leaf fall in the canopy layer began in late November to early December, and leaf flush occurred in May. The relative irradiance in saplings increased with canopy leaf fall from 2 to 15% under low irradiance levels and from 10 to 50% under intermediate irradiance levels (Fig. 1c). We also observed direct sunlight on sapling needles during the defoliated season under low and intermediate irradiance levels. By contrast, the relative irradiance did not vary greatly throughout the year in saplings under high irradiance.
Seasonal changes in net CO2 assimilation rate in needles
Clear seasonal changes in P max were observed at all irradiance levels (P < 0.05, Fig. 2; Table 1). P max decreased in summer and winter and increased in spring and autumn. P max was higher under high and intermediate irradiance compared with low irradiance levels throughout the year; values at high irradiance were approximately twice as high as those at low irradiance. R d also changed with the season (P < 0.05, Fig. 2; Table 1), declining in January and increasing in April, July, and August. Similar to P max, R d was higher under high and intermediate irradiance levels than under low irradiance.
Changes in the maximum photosynthetic rate at light saturation (P max, positive values with solid line), and dark respiration rate (R d , negative values with dotted line). Different letters indicate significant differences among months (P < 0.05). For P max, letters in italics, underlined letters, and normal font indicate high, intermediate, and low irradiance levels, respectively. For R d , the statistical results for the three irradiance levels are displayed together because the same statistical result was found for all levels. Bars indicate the standard error. The results of the statistical analysis are shown in Table 1. Asterisks indicate significant differences among irradiance levels in the same month (ANOVA; *, P < 0.05; **, P < 0.01; ns, P > 0.05). Number in the parentheses indicates number of saplings
Seasonal changes in daily needle carbon balance and nighttime carbon loss among irradiance levels
Diurnal changes in the needle gas exchange and daily needle carbon balance showed distinct patterns across seasons and irradiance levels (Figs. 3, S1, S2, S3, and Table 1). Although the seasonal patterns of the daily needle carbon balance differed among irradiance levels, the nighttime needle carbon loss (nighttime respiration) had the same seasonal trends among irradiance levels: it was highest in August and decreased in January and March (Fig. 3; Table 2). Saplings under high irradiance received strong direct sunlight and exhibited high positive net carbon assimilation during the day, although midday photosynthetic depression occurred in August (Fig. S1D). In fact, the daily needle carbon balance in August was lower compared with the other seasons (P < 0.05, Fig. 3). The daily carbon balance for saplings under intermediate irradiance levels remained positive throughout the year (Fig. 3). Especially in March, saplings received strong direct sunlight and had a higher net needle CO2 assimilation rate due to the leaf fall of overstory trees (Fig. S2A, B). By contrast, saplings under low irradiance levels in August and October were strongly shaded with exposure to few sunflecks (Fig. S3C, E); negative net needle CO2 assimilation was even observed during the day (Fig. S3D, F). In comparison, during the leaf fall period, positive net needle CO2 assimilation was recorded throughout the daytime in January and March (Fig. 3, S3B, H). The daily needle carbon balance was also negative in August and October, whereas carbon gain significantly increased in January and March (P < 0.05, Fig. 3). Although daily PPFD did not significantly change in saplings under high irradiance (Table S1), saplings in intermediate and low irradiance received significantly large amounts of PPFD during the leaf fall season in January and March compared to August and October (P < 0.05, Table S1).
Changes in needle daily carbon balance (solid line) and nighttime carbon loss (dotted line). Different letters indicate significant differences among months (P < 0.05). Letters in italics, underlined, and normal font are high, intermediate, and low irradiance levels, respectively. Bars indicate the standard error. The results of the statistical analysis are shown in Table 1. Asterisks indicate significant differences among irradiance levels in the same month (ANOVA; *, P < 0.05; **, P < 0.01; ns, P > 0.05). Numbers in parentheses are the numbers of saplings
Seasonal changes in starch content
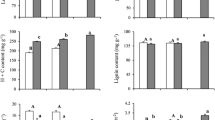
The root starch content per tissue dry weight and starch pool size showed clear seasonality; content and pool size increased in spring, decreased sharply in August, and increased again from October to spring (Fig. 4a, b; Table 1). The needle and branch starch contents in April were also higher than those observed in August, although both starch contents were lower than that of roots in August (Table 2). The starch contents in August were less than 0.4% in needles and 0.6% in branches, whereas the values in roots were 2.0–6.4% (Table 2). The root starch content in April was higher under high and intermediate irradiance levels compared with low irradiance, whereas the content in August was highest under low irradiance (Fig. 4a; Table 2). By contrast, the needle and branch starch contents were similar among the sapling irradiance levels in both April and August (Table 2).
Changes in root starch content (a) and starch pool size (b) among sapling irradiance levels. Different letters indicate significant differences among months (P < 0.05). Letters in italic, underlined, and normal font indicate high, intermediate, and low irradiance, respectively. Bars indicate the standard error. The results of the statistical analysis are shown in Table 1. Asterisks indicate significant differences among irradiance levels in the same month (ANOVA; *, P < 0.05; **, P < 0.01; ns, P > 0.05). Numbers in parentheses are the numbers of saplings
Relative growth rate, remaining starch content, and biomass partitioning among irradiance levels
The relative height growth rate (RGRh) in saplings was significantly higher under intermediate and high irradiance levels compared with under low irradiance (Table 2). The starch remaining in roots was significantly higher under low irradiance (38.2%) compared with intermediate (9.7%) and high irradiance (5.1%), although a similar relationship was not observed for needles or branches (Table 2). Biomass partitioning among the needle, stem (including branch), and root components differed significantly among irradiance levels (P < 0.05, ANOVA, Fig. 5). Saplings under high and intermediate irradiance had larger needle biomass compared with those under low irradiance, whereas saplings under low irradiance had a relatively large root component (Fig. 5). The root-to-shoot ratio was also higher under low irradiance, whereas high needle biomass, as indicated by the needle mass ratio, was observed in saplings under intermediate and high irradiance (Table 3).
Discussion
Seasonal changes in daily carbon balance among irradiance levels
Consistent with our hypothesis, large carbon gains occurred in saplings during the canopy leaf fall period, particularly in the spring, regardless of irradiance level, although similar large carbon gains were also observed in October in saplings under high irradiance. High photosynthetic assimilation due to an improved light environment in January and March caused high daily carbon gain in saplings, particularly under intermediate and low irradiance levels. Although P max in January was suppressed by low temperatures compared to other seasons, the decreased respiration rate at night also contributed to the large carbon gain. The nighttime carbon loss declined by 1/3 to 1/7 compared to summer because nighttime temperatures were 10–20 °C lower in winter and spring, and because Q 10 values (the proportional increase in R d with 10 °C warming) of various plant species usually increase under lower growth temperature such as winter (Amthor 1989). Similarly, high carbon gains during winter have been reported for several evergreen conifer species in both mature and sapling trees in forests with milder winter temperatures (Waring and Franklin 1979; Schaberg et al. 2000); this pattern is particularly strong for saplings growing under deciduous canopy trees (Hashimoto and Shirahata 1995; Hitsuma et al. 2012). For example, in Douglas-fir (Pseudotsuga menziesii) trees in western Oregon where winters are mild and wet, photosynthesis during the dormant season (late October through late April) accounted for as much as 55% of total annual carbon assimilation (Emmingham and Waring 1977). The positive relationship between annual diameter growth and winter temperature in A. firma observed by Takahashi and Okuhara (2012) also supports the importance of winter carbon assimilation by photosynthesis in this species.
By contrast, summer may not be the optimal season for photosynthetic production of saplings, particularly under high and low irradiance, although the mechanism driving photosynthetic limitation differed between high and low irradiance. Under high irradiance, midday depression of photosynthesis and high nighttime respiration suppressed daily carbon gain. The reduction of sapling growth in A. firma under open compared to moderately shaded conditions (Nakao 1985) also indicates limited carbon gain during summer at high irradiance. A similar midday depression of photosynthesis has also been reported in two Abies species in North America; the midday declines occurred under clear skies in open conditions, although the depression was not as obvious on cloudy days and large amounts of carbon gain occurred (Hodges 1967; Hodges and Scott 1968). Drier soil, high temperature, and high vapor pressure deficit (VPD) in summer may induce stomatal closure with high respiration rates, which may then cause midday depression of photosynthesis (Hodges 1967). In this study, the midday depression was also lower on cloudy days (data not shown), and thus the surplus carbon on cloudy days may be important for sapling carbon balance and growth during summer. In contrast to high irradiance levels, reduced light intensity due to elongated leaves in the canopy layer may cause decreased photosynthetic assimilation of saplings under intermediate and low irradiance levels. In addition, increased needle R d caused by high temperatures may also suppress needle daily carbon balance. Particularly, notable was the negative daily carbon balance in saplings under strongly shaded low irradiance levels, which was maintained until at least October. Sunflecks, which can allow relatively high photosynthetic assimilation in understory plants (Pearcy 1983; Ustin et al. 1984; Chazdon 1988) including Abies alba (Robakowski et al. 2004), occurred occasionally in the summer, and thus the contribution to daily needle carbon balance in the saplings was limited under low irradiance levels.
Stored starch, biomass partitioning, and survival strategy among irradiance levels
Saplings of A. firma adapted to different irradiance levels by altering biomass partitioning, such as the root-to-shoot ratio and use of stored carbon, which primarily originated from carbon assimilation during the leaf fall period of the canopy layer. Carbon assimilated during canopy leaf fall was stored in the various structures of saplings, particularly the roots, regardless of irradiance level. Many evergreen conifer species store assimilated carbon as starch during the dormant season (Gower et al. 1995). Schaberg et al. (2000) found that increases of starch and sugars in red spruce seedlings were driven by photosynthetic production during the dormant season. Similar increases in starch and sugar content in needles, branches, and roots just prior to the growing season have been observed in many evergreen conifer species of both mature and sapling trees, including several Abies species (Miyake 1902; Kimura 1969; Little 1970; Höll 1985; Amundson et al. 1992; Webb and Kilpatrick 1993; Schaberg et al. 2000; Hoch et al. 2003). Stored starch under high and intermediate irradiances may be mainly used for growth, whereas saplings under low irradiance may use it to maintain the carbon balance during the summer, when the carbon balance is negative. Further detailed study of the allocation of stored starch to growth versus compensation for negative carbon balance among irradiance levels and seasons will provide a better understanding of the responses and acclimation of fir saplings to the seasonal changes in irradiance in mixed deciduous forest.
Biomass partitioning among needles, stems, and roots changed greatly depending on the irradiance level of the growing saplings. Increases in needle biomass under high and intermediate irradiance levels may have contributed to increases in photosynthetic production, as a thick needle layer with high P max can optimally use high light (Sprugel et al. 1996; Kenzo et al. 2000; Landhäusser and Lieffers 2001; Kato and Yamamoto 2002). In contrast, under low light, small numbers of needles were arranged in an umbrella-shaped crown to avoid self-shading and intercepted weak irradiance at a minimal cost in needle construction and maintenance (Leverenz and Hinckley 1990; Williams et al. 1999). Kenzo et al. (2014) reported that the Leaf Area Index (LAI) under low irradiance levels (relative irradiance in summer less than 5%) of A. firma saplings with an umbrella-shaped crown decreased to approximately 1, whereas the LAI in saplings at intermediate and high irradiance reached 2–3. The relative amount of the root component increased at low irradiance; an increased root-to-shoot ratio may be advantageous for starch storage to maintain carbon balance during summer. In fact, the relative starch pool of the root component at the level reached 88% compared with the needle (1%) and stem (11%) components in summer (data not shown). In addition, higher carbon allocation to storage in saplings within a dark understory may also contribute to their ability to recover from damage to stems and needles by fallen branches, pathogens and herbivore attack, all of which frequently occur in the understory (Myers and Kitajima 2007). High storage capacity for starch and other resources in roots have been reported for many shade-adapted plants such as spring ephemerals, understory trees, and saplings with shade tolerance (Kozlowski 1992; Poorter and Kitajima 2007; Kenzo et al. 2013). Large storage capacity with a high root-to-shoot ratio allows plants to survive longer periods in the shaded understory (Kitajima 1994; Kobe 1997; Myers and Kitajima 2007). Similar increases in the root-to-shoot ratio of A. firma saplings under deeply shaded conditions (relative irradiance 1%) have also been reported in a natural fir-hemlock forest in southern Japan (Yuruki and Aragami 1973). However, previous studies have also observed increases in the relative amount of leaf biomass of tree seedlings rather than increases in root biomass especially in nursery experiments with the same species (Givnish 1988; Geiger and Servaites 1991). Nakao (1985) also reported that A. firma seedlings increased needle biomass rather than root biomass under moderate shade (relative irradiance was controlled to about 10% by shade cloth), although the experiment did not consider seasonal changes in irradiance, and examined only four growing seasons. The conflicting results may have been caused by the relatively short period of shade without significant changes in crown shape. The experiment by Nakao (1985) also did not find any changes in crown shape such as the umbrella form or branch dying up. Results of the present study indicated that the relative root biomass may increase by decreasing the needle and stem (mainly in branches) biomass as a result of living branches’ drying up to construct an umbrella shape crown under long-term suppression of deep shade. Overall, saplings of A. firma under low irradiance may be unable to reach the canopy layer due to limited carbon gains; therefore, long-term survival increases the probability of experiencing canopy gap formation, enabling them to regenerate to the canopy layer. Several demographic studies have also reported that regeneration of A. firma depends on canopy gap formation or large-scale disturbance (Suzuki 1980; Suzuki and Tsukahara 1987; Yuruki and Aragami 1987; Yuruki et al. 1987; Agetsuma 1992; Sanquetta et al. 1994). However, sapling survival under evergreen trees and shrubs may be difficult, as photosynthesis may not occur in winter and spring. In fact, sapling survival of A. firma was inhibited under the shade of evergreen dwarf bamboo (Yuruki and Aragami 1973; Aragami 1987). By contrast, sapling recruitment in deciduous forests occurred frequently compared to evergreen forests, including pure stands of A. firma (Kaji 1975). Therefore, to maintain natural regeneration of A. firma, forests should be managed as mixed forests with deciduous broad-leaved canopy trees.
Conclusions
Saplings of A. firma can establish in a wide range of irradiance levels by having different needle photosynthetic traits, starch storage, and biomass partitioning to roots and needles. Under high and intermediate irradiance levels, saplings allocated carbon to needle biomass with high P max to achieve high assimilation under strong irradiance, although the midday depression of assimilation in summer limited the daily needle carbon gain in saplings under high irradiance. In comparison, saplings under low irradiance levels depend on photosynthetic production during leaf fall under a canopy of deciduous trees, and starch stored during this period may be used to maintain the carbon balance in the summer and may permit long-term sapling survival under shaded conditions.
Author contribution statement
T.K. designed the study, interpreted results and wrote the manuscript. R.Y. and I.N. conducted field measurements and interpreted results.
References
Agetsuma N (1992) Distribution pattern and age structure of Abies firma saplings in a mature mixed forest of A. firma and Fagus japonica. Ecol Res 7:387–389
Amthor JS (1989) Respiration and crop productivity. Springer, New York
Amundson RG, Hadley JL, Incher J, Ellows S, Alscher RG (1992) Comparisons of seasonal changes in photosynthetic capacity, pigments, and carbohydrates of healthy sampling and mature red spruce and of declining and healthy red spruce. Can J For Res 22:1605–1617
Ando T, Chiba K, Nishimura T, Tanimoto T (1977) Temperate fir and hemlock forest in Shikoku. JIBP Synth 16:213–245
Aragami K (1987) Studies on the process of formation of Abies and Tsuga natural forest in the central mountain district of Kyushu. Bull Kyusyu Univ For 57:17–108 (Japanese with English summary)
Bourdeau PF (1959) Seasonal variations of the photosynthetic efficiency of evergreen conifers. Ecology 40:63–67
Canham CD, Kobe RK, Latty EF, Chazdon RL (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121:1–11
Čater M, Diaci J, Roženbergar D (2014) Gap size and position influence variable response of Fagus sylvatica L. and Abies alba Mill. For Ecol Manag 325:128–135
Chazdon RL (1988) Sunflecks and their importance to forest understorey plants. Adv Ecol Res 18:1–63
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Ann Rev Plant Biol 65:667–687
Duchesneau R, Lesage I, Messier C, Morin H (2001) Effects of light and intraspecific competition on growth and crown morphology of two size classes of understory balsam fir saplings. For Ecol Manag 140:215–225
Emmingham WH, Waring RH (1977) An index of photosynthesis for comparing forest sites in western Oregon. Can J For Res 7:165–174
Geiger DR, Servaites JC (1991) Carbon allocation and response to stress. In: Mooney HA, Winner WE, Pell EJ (eds) Response of plants to multiple stresses. Academic Press, New York, pp 103–127
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Funct Plant Biol 15:63–92
Gower ST, Isebrands JG, Sheriff DW (1995) Carbon allocation and accumulation in conifers. In: Smith WK, Hinckley TM (eds) Resource physiology of conifers, acquisition, allocation, and utilization. Academic Press, San Diego, pp 217–254
Grassi G, Bagnaresi U (2001) Foliar morphological and physiological plasticity in Picea abies and Abies alba saplings along a natural light gradient. Tree Physiol 21:959–967
Hashimoto R, Shirahata M (1995) Comparative study of leaf carbon gain in saplings of Thujopsis dolabrata var. hondai and Quercus mongolica var. grosserrata in a cool-temperate deciduous forest. Ecol Res 10:53–64
Hitsuma G, Han Q, Chiba Y (2012) Photosynthesis and growth of Thujopsis dolabrata var. hondai seedlings in the understory of trees with various phenologies. J For Res 17:156–163
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081
Hodges JD (1967) Patterns of photosynthesis under natural environment conditions. Ecology 48:234–242
Hodges JD, Scott DR (1968) Photosynthesis in seedlings of six conifer species under natural environmental conditions. Ecology 49:973–980
Höll W (1985) Seasonal fluctuation of reserve materials in the trunkwood of spruce (Picea abies (L.) Karst.). J Plant Physiol 117:355–362
Horikawa Y (1972) Atlas of the Japanese flora: an introduction to plant sociology of East Asia I. Gakken Co., Tokyo
Johnson DM, Smith WK (2005) Refugial forests of the southern Appalachians: photosynthesis and survival in current-year Abies fraseri seedlings. Tree Physiol 25:1379–1387
Kaji M (1975) Studies on the ecological status of Abies firma forest in Boso Peninsula. Bull Tokyo Univ For 68:1–23 (Japanese with English summary)
Katahata S, Naramoto M, Kakubari Y, Mukai Y (2005) Photosynthetic acclimation to dynamic changes in environmental conditions associated with deciduous overstory phenology in Daphniphyllum humile, an evergreen understory shrub. Tree Physiol 25:437–445
Kato K, Yamamoto SI (2002) Branch growth and allocation patterns of saplings of two Abies species under different canopy conditions in a subalpine old-growth forest in central Japan. Ecoscience 9:98–105
Kenzo T, Yoneda R, Ninomiya I (2000) Changes in morphology and photosynthetic ability of Abies firma seedlings under different light condition. Bull Ehime Univ For 38:11–21 (Japanese with English summary)
Kenzo T, Ichie T, Ninomiya I, Koike T (2003) Photosynthetic activity in seed wings of Dipterocarpaceae in a masting year: does wing photosynthesis contribute to reproduction? Photosynthetica 41:551–557
Kenzo T, Yoneda R, Matsumoto Y, Azani AM, Majid MN (2011) Growth and photosynthetic response of four Malaysian indigenous tree species under different light conditions. J Trop For Sci 23:271–281
Kenzo T, Ichie T, Yoneda R, Tanaka-Oda A, Azani MA, Majid NM (2013) Ontogenetic changes in carbohydrate storage and sprouting ability in pioneer tree species in Peninsular Malaysia. Biotropica 45:427–433
Kenzo T, Yoneda R, Ninomiya I (2014) Changes in crown morphology and shoot phenology under different light condition in sapling of Abies firma. Kanto J For Res 65:327–330 (Japanese with English summary)
Kimmins JP (1997) Forest ecology: a foundation for sustainable management. Prentice Hall, New Jersey
Kimura M (1969) Ecological and physiological studies on the vegetation of Mt. Shimagare. VII. Analysis of production processes of young Abies stand based on the carbohydrate economy. Bot Mag Tokyo 82:6–19
Kitajima K (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428
Kobe RK (1997) Carbohydrate allocation to storage as a basis of interspecific variation in sapling survivorship and growth. Oikos 80:226–233
Kohyama T (1980) Growth pattern of Abies mariessi saplings under conditions of open-growth and suppression. Bot Mag Tokyo 93:13–24
Koike T (1986) Photosynthetic responses to light intensity of deciduous broad-leaved tree seedlings raised under various artificial shade. Environ Control Biol 24:51–58
Kozlowski TT (1992) Carbohydrate sources and sinks in woody plants. Bot Rev 58:107–222
Landhäusser SM, Lieffers VJ (2001) Photosynthesis and carbon allocation of six boreal tree species grown in understory and open conditions. Tree Physiol 21:243–250
Lassoie JP, Dougherty PM, Reich PB, Hinckley TM, Metcalf CM, Dina SJ (1983) Ecophysiological investigations of understory eastern redcedar in central Missouri. Ecology 64:1355–1366
Lei TT, Koike T (1998) Some observations of phenology and ecophysiology of Daphne kamtschatica Maxim. var. jezoensis (Maxim.) Ohwi, a shade deciduous shrub, in the forest of northern Japan. J Plant Res 111:207–212
Leverenz JW, Hinckley TM (1990) Shoot structure, leaf area index and productivity of evergreen conifer stands. Tree Physiol 6:135–149
Little CHA (1970) Derivation of the springtime starch increase in balsam fir (Abies balsamea). Can J Bot 48:1995–1999
Mailly D, Kimmins JP (1997) Growth of Pseudotsuga menziesii and Tsuga heterophylla seedlings along a light gradient: resource allocation and morphological acclimation. Can J Bot 75:1424–1435
Mitchell AK, Arnott JT (1995) Effects of shade on the morphology and physiology of amabilis fir and western hemlock seedlings. New For 10:79–98
Miyake K (1902) On the starch of evergreen leaves and its relation to photosynthesis during the winter. Bot Gazet 33:321–340
Miyazawa Y, Kikuzawa K (2005) Winter photosynthesis by saplings of evergreen broad-leaved trees in a deciduous temperate forest. New Phytol 165:857–866
Myers JA, Kitajima K (2007) Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J Ecol 95:383–395
Nakao T (1985) Ecological studies of Abies and Tsuga forest in Kyushu, Japan. Bull Miyazaki Univ For 11:1–165 (Japanese with English summary)
Öquist G, Huner NP (2003) Photosynthesis of overwintering evergreen plants. Ann Rev Plant Biol 54:329–355
Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J (1990) Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of SW Australia. Ann Bot 65:585–601
Pearcy RW (1983) The light environment and growth of C3 and C4 tree species in the understory of a Hawaii forest. Oecologia 58:19–25
Poorter L, Kitajima K (2007) Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 88:1000–1011
Pucher GW, Leavenworth CS, Vickery HB (1948) Determination of starch in plant tissues. Anal Chem 20:850–853
Robakowski P, Montpied P, Dreyer E (2003) Plasticity of morphological and physiological traits in response to different levels of irradiance in seedlings of silver fir (Abies alba Mill). Trees 17:431–441
Robakowski P, Wyka T, Samardakiewicz S, Kierzkowski D (2004) Growth, photosynthesis, and needle structure of silver fir (Abies alba Mill.) seedlings under different canopies. For Ecol Manag 201:211–227
Sanquetta CR, Ninomiya I, Ogino K (1994) Age structural analysis of the natural regeneration process of a fir-hemlock secondary forest in southwest Japan. J Jap For Soc 76:506–515
Schaberg PG, Wilkinson RC, Shane JB, Donnelly JR, Cali PF (1995) Winter photosynthesis of red spruce from three Vermont seed sources. Tree Physiol 15:345–350
Schaberg PG, Shane JB, Cali PF, Donnelly JR, Strimbeck GR (1998) Photosynthetic capacity of red spruce during winter. Tree Physiol 18:271–276
Schaberg PG, Snyder MC, Shane JB, Donnelly JR (2000) Seasonal patterns of carbohydrate reserves in red spruce seedlings. Tree Physiol 20:549–555
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn. W.H. Freeman, New York
Sprugel DG, Brooks JR, Hinckley TM (1996) Effects of light on shoot geometry and needle morphology in Abies amabilis. Tree Physiol 16:91–98
Suzuki E (1980) Regeneration of Tsuga sieboldii forest. II. Dynamics of development of a mature stand revealed by stem analysis data. Jap J Ecol 30:333–346
Suzuki E, Tsukahara J (1987) Age structure and regeneration of old growth Cryptomeria japonica forests on Yakushima Island. Bot Mag Tokyo 100:223–241
Takahashi K, Okuhara I (2012) Comparison of climatic effects on radial growth of evergreen broad-leaved trees at their northern distribution limit and co-dominating deciduous broad-leaved trees and evergreen conifers. Ecol Res 27:125–132
Ustin SL, Woodward RA, Barbour MG, Hatfield JL (1984) Relationships between sunfleck dynamics and red fir seedling distribution. Ecology 65:1420–1428
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Ann Rev Ecol Evol Syst 39:237–257
Verhoeven AS, Adams WW, Demmig-Adams B (1999) The xanthophyll cycle and acclimation of Pinus ponderosa and Malva neglecta to winter stress. Oecologia 118:277–287
Waring RH, Franklin JF (1979) Evergreen coniferous forests of the Pacific Northwest. Science 204:1380–1386
Webb WL, Kilpatrick KJ (1993) Starch content in Douglas-fir: diurnal and seasonal dynamics. For Sci 39:359–367
Williams H, Messier C, Kneeshaw DD (1999) Effect of light availability and sapling size on the growth and crown morphology of understory Douglas-far and Lodgepole pine. Can J For Res 29:221–231
Yamanaka T (1962) Abies firma and Tsuga sieboldii forests in Shikoku. Res Rep Kochi Univ Nat Sci I 10:19–32
Yuruki T, Aragami K (1973) Studies on natural regeneration of Momi (Abies firma S. et Z., Japanese fir) and Tsuga (Tsuga Sieboldii Carr., Japanese hemlock). Bull Kyusyu Univ For 47:77–124 (Japanese with English summary)
Yuruki T, Aragami K (1987) The development of Momi (Abies firma) and Tsuga (Tsuga sieboldii) forests on the basis of diameter growth. J Jap For Soc 69:478–481
Yuruki T, Aragami K, Marutani T (1987) The development of a small group of Momi (Abies firma) and Tsuga (Tsuga sieboldii) on the basis of diameter and height growth. J Fac Agric Kyushu Univ 31:411–415
Acknowledgements
We thank T. Azechi, S. Kashimura, T. Hirom, Y. Tokuoka, C. Ikeda, A. Tanaka-Oda and S. Tamura for their help in the field. We also thank D. Kabeya for his kind suggestion on statistical analysis. This work was partly supported by JSPS KAKENHI Grant (No. 16K07795) from the Ministry of Education, Science, and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by I. Porth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kenzo, T., Yoneda, R. & Ninomiya, I. Seasonal changes in photosynthesis and starch content in Japanese fir (Abies firma Sieb. et Zucc.) saplings under different levels of irradiance. Trees 32, 429–439 (2018). https://doi.org/10.1007/s00468-017-1640-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1640-5