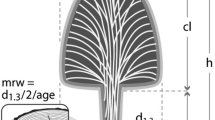
Abstract
Key message
Sapwood area and the radial growth rate of the trunk follow the same pattern at breast height, with an initial increase and subsequent constant value, resulting from the increasing growth allocation toward the crown rather than tree decline. Heartwood area and heartwood volume in the trunk increase more rapidly after this shift occurs.
Abstract
Sapwood (SW) and heartwood (HW) are two functionally distinct classifications of wood in perennial stems for which quantities can vary greatly in tropical trees. Numerous positive correlations have been found between the radial growth rate (RGR) and SW quantity; however, variations in the SW/HW quantities have not been studied in light of the ontogenetic variation of RGR. Wood core sampling, intensive measurements of tree structure (number of branches, stem volumes), and radial growth monitoring were performed on an abundant and highly exploited tree species in French Guiana (Dicorynia guianensis) to investigate the relationship between RGR, SW/HW quantity, tree structure, and their variations on the course of a tree’s ontogeny. SW area and RGR followed the same pattern of variation throughout tree development, both increasing first and reaching a steady state after 50 cm DBH (diameter at breast height). After this value, we observed a strong increase in both the HW area and HW volume increment, concomitant with a more rapid increase in crown volume. The stabilization of RGR for trees with DBH > 50 cm was related not to a tree’s decline but rather to an increasing wood allocation to the crown, confirming that RGR at breast height is a poor indicator of whole-tree growth for bigger individuals. We also confirmed that HW formation is an ontogenetic process managing SW quantity that is continuously and increasingly produced within the crown as the tree grows. This study highlights the effect of growth-mediated ontogenetic changes on the localization of water and carbohydrate storage within a tree, resulting from SW and HW dynamics throughout tree ontogeny.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sapwood (SW) and heartwood (HW) are two functionally distinct classifications of wood in perennial stems. SW conducts water (Gartner 1995) and stores both carbohydrates and water (Hillis 1987), whereas SW is a sink tissue that consumes sugars for its daily metabolism through respiration (Bamber 1976). Therefore, by managing the SW quantity in the stem as a tree grows (Bamber 1976), HW formation maintains tree energetic balance. HW also protects the central part of the stem by its impregnation of secondary compounds during its formation (Hillis 1987). This last characteristic makes HW the most valuable part of the tree in the timber industry. However, the quantities of SW/HW in tree species of commercial importance that grow in natural forests can vary greatly (Cirad 2011). A better understanding of SW/HW dynamics and the variation of their quantities during tree development could enable the timber industry to more accurately select trees for commercial exploitation, increasing profitability and reducing the impact on ecosystems.
Due to its hydraulic function, SW quantity is used as a predictor of tree foliar area (Morataya et al. 1999; White 1993; Whitehead et al. 1984). The ratio of foliar area to SW area is also a common measure of the balance between transpiration and stem water supply (McDowell et al. 2008; Pérez-Harguindeguy et al. 2013) and an important trait contributing to plants’ water-use strategies (Wright et al. 2010). However, SW area (SWa) is not based solely on water conduction, especially in angiosperm species (Spicer and Holbrook 2005). Inner, non-conductive SW can remain alive to store carbohydrates, water, and nutrients resorbed during HW formation (Bamber and Fukazawa 1985; Goldstein et al. 1998). HW is the inner part of the stem that no longer contains living cells. Carbohydrates stored in the SW are exported to another part of the plant body or converted into chemicals that impregnate the cell wall during HW formation (IAWA 1964). HW formation has been hypothesized as an ageing process (Frey-Wyssling and Bosshard 1959; Yang 1990; Zimmermann 1983) and a developmental process, which maintains SW at optimum physiological levels (Bamber 1976; Bamber and Fukazawa 1985); A recent review of HW formation (Kampe and Magel 2013) and a study on plant physiology (Spicer and Holbrook 2005) confirmed the second hypothesis, and HW formation is now widely considered to be an active process regulating tissue quantity dedicated to carbohydrates and water storage (Bamber 1976).
Non-structural carbohydrates (e.g., starch) are preferentially stored in roots, branch stumps, crown branches, twigs, and leaves (Barbaroux et al. 2003; Bazot et al. 2013). Recent studies have revealed that carbohydrates are stored according to both ‘young’ (outermost wood rings) and ‘old’ (innermost) pools (Richardson et al. 2013, 2015), which are respectively used for growth and daily metabolism (Carbone et al. 2013) and for regeneration of stems and roots (Carbone et al. 2013; Vargas et al. 2009). Trees store water in the embolized vessels of the trunk, intracellular space, fibers (Meinzer et al. 2008; Tyree and Yang 1990), as well as axial parenchyma and rays in the SW (Holbrook 1995; Meinzer et al. 2003; Scholz et al. 2007). As trees grow, their daily reliance on stored water in the stem increases to meet the high evaporative demand for a longer fraction of the day than smaller trees and to prevent or repair embolism formation (Goldstein et al. 1998; Meinzer et al. 2004). Several studies have shown that more stored water is drawn from the crown than the trunk (Cermak et al. 2007; Schulze et al. 1985). The crown appears to be very important as a storage compartment.
Several factors (e.g., tree age, site conditions, genetics, diseases, and silvicultural treatments) influence SW and HW quantity in trees (reviewed in Taylor et al. (2002)). Such a diversity of factors may also impact the tree radial growth rate (RGR in cm/year) and could, in turn, explain numerous positive correlations between RGR and SW area or thickness in conifers and angiosperms tree species (Bamber 1976; Busgen and Munch 1929; Carrodus 1972; van der Sande et al. 2015). Variations in SW quantity may be seen as driver of tree RGR rather than simply a consequence of RGR variations by managing wood volume that stores water, thereby allowing more persistent water supply to the crown during the course of the day (Galván et al. 2012; van der Sande et al. 2015). However, there are also examples of negative correlations between RGR and SW quantity (reviewed in (Hillis 1987)), suggesting that the occurrence of both negative and positive correlations may be due to a poor understanding of growth allocation hierarchies (Taylor et al. 2002).
The relationship between RGR and tree size is generally modeled as a “hump-back” curve, suggesting that RGR increases until the tree reaches an optimal size and then declines (Canham et al. 2004; Hérault et al. 2011). Efforts have recently been made to understand RGR at the species level (Hérault et al. 2011; Poorter and Bongers 2006; Wright et al. 2010) using climatic factors or functional traits averaged to particular species. However, very few studies have explained RGR by allocation strategies independent of abiotic factors (temperature, light, water availability). For example, it has been observed that RGR declines with age or tree size, while crown growth rate continues to increase (Sillett et al. 2010; Van Pelt and Sillett 2008). These findings could explain why the accumulation rate of carbon increases continuously with tree size (Stephenson et al. 2014). We then hypothesized that the decrease of RGR in older/larger trees (Meinzer et al. 2011; Wirth et al. 2009), may partially result from a shift in biomass allocation during tree ontogeny rather than from tree decline.
Variations in SW/HW quantities have not been studied throughout a tree’s ontogeny, especially in light of the ontogenetic variation of RGR.
Because SW quantities are often positively correlated with RGR (Bamber 1976; Busgen and Munch 1929; Carrodus 1972; van der Sande et al. 2015), we expect that (1) both follow the same pattern of variation according to tree size and (2) if a shift between trunk and crown growth is observed, it will result in the accumulation of newly formed energy consuming SW in the crown, thus triggering the accumulation of HW. Moreover, as HW forms from the ground to the tree top (Climent et al. 2003; Hatsch 1997; Pinto et al. 2004), we also expect that the main proportion of SW moves from the trunk to the crown as the tree grows.
Measuring tree growth and size at breast height is not an accurate measure as it fails to take into account stem volume or biomass growth of the whole tree (Sillett et al. 2010; Van Pelt and Sillett 2008). Therefore, understanding variations of RGR and SW/HW quantities requires an individual-based approach (Clark et al. 2011) coupled with intensive measurement of tree structure (Sillett et al. 2010). Here, we examine the relationships among the crown/trunk dimensions, RGR, and SW/HW quantities, in different dimensions (area and volume in trunk), in individual trees at different developmental stages.
Our objectives were (1) to investigate the relationship between RGR at breast height and whole-tree structure, (2) to identify structural tree characteristics that indicate the moment at which growth at breast height ceases to be indicative of real growth, (3) to relate these results to SW/HW quantity variations and their locations on the course of a tree’s ontogeny, and finally (4) to discuss the effects of ontogenetic variations of SW/HW locations on tree function.
For age-known trees (e.g., plantation growing trees), RGR is easily estimated. However, it is difficult to estimate RGR for trees growing in natural stands (i.e., in which tree ages are unknown) without census protocol or dendrochronological assessment. Therefore, we decided to work within monitored forest plots to obtain estimates of tree RGR.
For this study, we chose Dicorynia guianensis (Fabaceae; trade name: Basralocus), an endemic species of the Guiana shield (Koeppen 1967), representing more than 50 % of the timber production in French Guiana (Brunaux et al. 2009), which also plays an important role in carbon sequestration and in the production of biomass in the Amazonian forest ecosystem (Fauset et al. 2015; Ter Steege et al. 2013);
Within this species, SW thickness varies between 2 and 10 cm (Cirad 2011). Because SW conducts less water with age in angiosperm species (Spicer and Gartner 2001) and water conductive SW is reduced to the first 2–4 cm in some representative Legumes (Reyes-García et al. 2012), this species is expected to conserve a considerable SW quantity dedicated to water, carbohydrates, or precursors of extractive storage (Taylor et al. 2008). The expected trade-off between storage and conduction of SW, its abundance, and its local commercial importance make this tree species an ideal candidate for the ontogenetic study of HW/SW dynamics.
The data were collected using intensive measurements (Sillett et al. 2010; Van Pelt and Sillett 2008) of tree structures and by collecting wood cores. We also used radial growth measurements taken over a 30-year period. From these data, we were able to calculate stem traits (RGR, SW and HW area at breast height, and cross-sectional area at breast height (CSA)) and structural tree traits (height, stem volume of trunk and crown, and number of branches). The results are discussed with a whole-tree perspective, taking into account the multi-functional aspects of SW and HW, their variations in different parts of the tree, and their links with RGR on the course of a tree’s ontogeny. After estimating the average allometric trajectories of the different tree compartments (crown/trunk, SW and HW), we will discuss the dynamics of SW and HW increment with regards to the dynamics of tree volume increment.
Materials and methods
Study species
Dicorynia guianensis Amsh. (Fabaceae) is one of the area’s most abundant species (Gourlet-Fleury et al. 2004). This emergent semi-tolerant tree can establish in the understory as well as in clearings (Favrichon 1994). It can reach 50 m in height and a diameter at breast height (DBH) of 120 cm. The tall juvenile individual growing in the forest canopy mainly bears plagiotropic sequential axes and two or more vertical reiterated axes in the submittal part of the trunk. These last branches may be considered as the future scaffold branches of the adult tree crown (Fig. 1) (see Drénou (1988) for a detailed description). In cross-section, the brown HW is clearly demarcated from the much lighter SW. The HW is mainly used for carpentry, flooring, and interior paneling, and it has convenient technological properties, such as relatively low volumetric shrinkage (5.1 %), relatively high modulus of rupture (121 MPa) (Cirad 2011) and good but variable natural durability (Amusant et al. 2014).
The architectural stages of development studied for Dicorynia guianensis. A tall juvenile tree (a) bearing plagiotropic axes and two vertical reiterated axes that are considered the future scaffold branches (arrow) and an adult canopy tree (b) compounded by a green crown in its peripheral part, made from living reiterated branches (LRB, dashed lines) borne by a crown composed of leafless branches and shed branch indicators (stumps). The trunk is the stem portion between the ground and the first living branch that supports the whole crown (crown and green crown). Adapted from Roggy et al. (2005) and Drénou (1988) with permission from the authors
Study site
The Paracou experimental site (5°18′N, 52°53′W) is a “terra firme” rain forest belonging to the Caesalpiniaceae facies (Sabatier and Prévost 1990), a typical forest type of French Guiana (Ter Steege et al. 2006). The site has a tropical climate. The dry seasons are mid-August to mid-November and March–April. The mean annual rainfall is 3041 mm. A complete and regular inventory of the DBH of all trees ≥10 cm was taken in permanent plots (125 ha in total).
Sampling trees
In total, 88 canopy trees (crown position index = 3–5; Dawkins 1958) with a wide range of DBH (0.2–1 m) were sampled; 71 were monitored trees from permanent plots at Paracou, and 17 were from a free-sampling area in the same station (Table 1).
Sapwood–Heartwood measurements
Using a manual increments borer, we collected 2 diametrically opposed wood cores per tree. In the case of leaning trees, cores were collected perpendicular to the leaning axis. For each core, we measured bark (Bt1 and Bt2 in mm converted to cm) and SW thickness (SWt1 and SWt2 in mm converted to cm). For small trees (DBH < 25 cm), we collected only one wood core which did not extend through the entire tree. The following variables were calculated (see Table 2, for a list of all abbreviations used in this study):
Cross-sectional area (CSA), in cm2:
Heartwood (HWd) diameter, in cm:
Sapwood (SWt) thickness:
Then, we used these variables to calculate the following variables:
Heartwood area (HWa), in cm2:
Sapwood area (SWa), in cm2:
Sapwood area to heartwood area ratio (SWa:HWa), in cm2/cm2:
Analysis of Heartwood–Sapwood tapering and heartwood volume in trunk
We felled 11 of the 17 trees from the free-sampling area to study the variation of HW and SW volume, in the trunk (HWvol and SWvol, respectively) and the SWa to HWa (SWa:HWa) ratio along the tree. For each tree, we collected wood discs from the trunk at breast height (130 cm), at 3 m, and at every additional 3 m until the main fork. We also collected wood discs from the crown: 1 or 2 from smaller trees or 3 or 4 from larger trees. We recorded the position and height of each disc within the crown structure.
In the laboratory, each disk was planed using a surface planer, and digital photographs were taken of each disk with a scale for reference. ImageJ (Rasband 2012) software processing was used to analyze digital photographs and estimate the mean HW, SW, and bark thickness of each disc. These measurements enabled us to calculate the HWa, SWa, and bark area of each disc; the HW, SW, and bark volume within the trunk (Appendix A); and to graphically represent the SWa:HWa along the tree. From the six unfelled trees, three had been topped for another experiment; thus, from these trees, one wood disc just below the fork and two cores at breast height were collected to calculate HWvol, SWvol, and bark volumes.
Radial growth rate calculation
RGR was calculated as the slope coefficient extracted from regression analysis of CSA against 30 years of measurements for each tree (cm2/year). All regressions were visually inspected to detect potential non-linearity of the relationship between CSA and years of measurement that could lead to error of the RGR estimation. For large trees, the height at which the diameter was measured was occasionally raised to avoid swollen trunk bases. If the height at which the DBH was measured had been raised during the last 5 years, the RGR was calculated from measurements taken prior to rising. If the height at which the DBH was measured was raised during the 5 first years of measurements, the RGR was calculated from measurements taken after rising.
Tree crown assessment
We identified possible declining trees using a previously published crown fragmentation assessment method (Rutishauser et al. 2011). Because liana infestation (LI) has no significant effect on D. guianensis tree growth (Rutishauser et al. 2011), we did not score it; thus, the assessment was restricted to three indexes: crown position (CP), main branch mortality (MB; “scaffold branch mortality”) and secondary branch mortality (SB) (see Rutishauser et al. (2011), for a detailed description).
Measuring the structure of large trees
Quantitative and topological descriptions were used to classify tree parts for measurement and to record the relative positions of stems that compound each part. We divided the tree structure into three parts: trunk, crown, and green crown (Fig. 1b). The green crown was defined as all living reiterated branches of 10 cm basal diameter. Professional tree climbers identified and visually assessed the length of each living reiterated branch of 10 cm and recorded their total number. For all trees, assessment of living reiterated branches of 10 cm basal diameter captured almost the whole green crown. However, branches with a basal diameter between 8 and 10 cm can be of varying importance in the green crown regardless of the size of the tree.
To rationally include these smaller branches, an ‘equivalent number of LRB’ per tree (eqnLRB) was calculated by dividing the sum of their sectional area by the sectional area of a stem of 10 cm of diameter. Finally, the number of LRB was added to the eqnLRB to calculate the total number of living reiterated branches per tree per green crown (nLRB). When the tree climbers completed the LRB assessment, they broke down the crown into stem units. A stem unit was defined as a linear stem portion delimited by a basal and distal branching event. The tree climbers measured the length, basal, and distal circumference of each stem unit as well as the trunk. The circumference at the trunk top (i.e., under the fork) was used to compute the cross-sectional area under the fork (CSAUF). For each described tree, we obtained a topological description allowing for calculation of crown stems (VolC) and trunk volume (VolT). Five of the felled trees (DBH > 25 cm) were described by tree climbers before felling, and the 6 remaining were described on the ground after felling.
Tree crown structure was also described according to processes of branch mortality in the growing crown. We developed a new method to measure crown branch stumps to quantify the number of branches shed during tree development. Each stump diameter was measured by a professional climber. These measurements were used in a series of equations (Appendix B), involving diameter of dead branches inside the stump, bark thickness, and the relationship between the number of LRB of 10 cm of diameter and the diameter of the bearing stem, allowing us to estimate the number of dead reiterated branches (nDRB) per tree. This gave the absolute number of reiterated branches (nARB = nLRB + nDRB) and the proportion of dead reiterated branches (pDRB) compared to nARB.
Branch stumps in individual trees were classified according to two states of healing: ‘healed,’ defined as completely covered with a layer of healing wood; or ‘not-healed,’ defined as partly or not at all covered with a layer of healing wood. The state of healing provides time indices for branches shed; therefore, we could use proportions of healing states within individual trees to indicate branch mortality dynamics.
Data compilation and variable extraction
We encoded tree structure data in a spreadsheet using the Multiscale Tree Graph format (MTG) (Godin and Caraglio 1998). We calculated variables (i.e., VolC, VolT, nARB, nLRB, nARB, nDRB, and pDRB) (Table 2) with AMAPmod software (Godin et al. 1997) in Python 2.7 programming language.
Analysis
R software (R-Core-Team 2013) was used for the statistical analysis. Our main purpose was to give a precise description of the relationships between variables when they are non-linearly related. We avoided the use of log-transformed predictors and/or response variables, generally used in allometric studies (Warton et al. 2006), that linearize relationships between variables and hide the point (e.g., peculiar tree size in this case) for which a gradual shift can occur. However, for some relationships, log–log linear models provided the best fits (Appendix C). In that case, the results were plotted on arithmetic scales. Thus, we proceeded to both linear and non-linear fittings with lm() and nls() functions in the ‘base’ package, using classical linear and non-linear (power or exponential function) models, or commonly-used growth models as Canham, Gompertz, Weibull, or Logistic (see Supplementary Information 1 in Hérault et al. (2011)). For each fitted model we assumed that residual error distribution followed a standard linear model hypothesis. For some fitted models, we calculated confidence and prediction bands by bootstrap re-sampling using 1000 iterations. For each model, we assessed variance homoskedasticity and normality of residuals using diagnostic plots and appropriate statistical tests. Parameter values and their confidence intervals as well as the significance of all fitted models are available (Appendix C).
Estimating increment dynamics of the different tree compartments according to different dimensions
Having identified the mean trajectories of the different tree compartments, we were able to separately compute CSA and HWa annual increments as well as the annual increments of VolTot, VolT, VolC, and HWvol throughout a tree’s ontogeny. These calculations allowed the translation of a set of static observations in a dynamic vision of tree increments with regard to heartwood formation.
Practically, we first set a vector of ages, \(\overrightarrow {\text{Age}} \in {\mathbb{N}}^{n}\), as \(\overrightarrow {\text{Age}} = ({\text{Age}}_{1} ,{\text{Age}}_{2} , \ldots ,{\text{Age}}_{n - 1} ,{\text{Age}}_{n} )\), with n = 200 years.
We then assumed that at Age 1, the tree has a CSA = 314 cm2 (i.e., the area corresponding to DBH = 20 cm) to fit with the range of sizes sampled in this study. Knowing the mean trajectory of RGR according to CSA, we computed a vector of n CSA values \(\overrightarrow {\text{CSA}} \in {\mathbb{R}}^{n}\), with components named c, as
with \({\text{RGR}}_{{{\text{CSA}}_{c - 1} }}\) equal to the annual increment produced by a tree of CSA c−1.
Then, we used each component of \(\overrightarrow {\text{CSA}}\) to compute different vectors of length n corresponding to the different variables of interest by using the different equations developed in this study. The annual increment of each variable of interest was computed by subtracting each component to the previous component within each vector. As the increment of SW is the result of the joint effects of HW formation and tree growth, we computed the evolution of SW quantities according to age as the difference between stem size (i.e., CSA or VolT) and HW amount (i.e., HWa or HWvol). SW annual increment was calculated in the same way as the other variables. The same procedure was also applied to estimate the annual increment of DBH, HWd, and SWt. In this way, we obtained a precise picture of the increment dynamics of the whole-tree, SW, and HW through different tree dimensions (i.e., diameter, area, and volume).
Results
Heartwood, sapwood area, and CSA
There was a clear positive and non-linear relationship between both HWa and SWa and CSA (Fig. 2a, b). As best fitted by a power function, HWa increased monotonically as CSA increased (Fig. 2a), whereas the Gompertz function suggests that SWa increased first and then reached an asymptotic value of 1195 cm2 (Fig. 2b).
Heartwood area (HWa) (a), Sapwood area (SWa) (b), and SWa:HWa ratio (c) as functions of CSA. Red solid lines represent the observed fit, red dashed lines represent confidence, and black dashed lines represent prediction band. HWa against CSA (a) was fitted with a power function, SWa against CSA (b) was fitted with the Gompertz growth function, and the SWa:HWa ratio fitted with exponential decrease function against CSA (c); the gray horizontal line represents SWa:HWa = 1 (N = 88) (color figure online)
The SWa:HWa ratio was higher in small trees and decreased exponentially with CSA (Fig. 2c). HWa and SWa were similar for DBH, approximately 52 cm (CSA = 2137 cm2). SWa:HWa still decreased after this point but rapidly reached an asymptotic value (Fig. 2c). This observation is consistent with the linear increase of HWa with CSA for trees with DBH > 50 cm (CSA > 2000 cm2) (HWa = −829.3 + 0.85 * CSA pv = 0.001, R 2 = 0.95) (Fig. 2a) and the asymptotic behavior of SWa for the same CSA range.
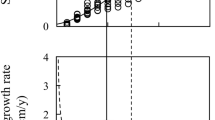
RGR and SW/HW CSA
As SWa, RGR against CSA was best fitted by the Gompertz model (Fig. 3). RGR increased first and then reached an asymptotic value of 31 cm2/year for trees with DBH > 56 cm (CSA > 2500 cm2). The same pattern of variation of both SWa and RGR with CSA was also supported by the observation that high SWa corresponded to high RGR (Fig. 4).
Tree structure and volumes
D. guianensis showed a classic height-CSA relationship explained by a linear model taking log(CSA) as a predictor, suggesting a strong increase in height until DBH 30 cm. After this, height growth continued at a slower rate (Appendix C and D).
The increase in the total number of reiterated branches (nARB) and living reiterated branches (nLRB) in relation to CSA was non-linear (Fig. 5a). The best regression lines for both of these variables were obtained by fitting log–log linear models with CSA, suggesting that the increase of both numbers of branches over the course of tree development was not linear. The first branch mortality event was recorded for trees having DBH = 30 cm (CSA = 700 cm2) (i.e., separation between curves) (Fig. 5a).
Number of reiterated branches (a) and stem volume (b) as a function of CSA. The number of absolute branches (nARB) (a, closed circles) and the number of living branches (nLRB) (a, opened circles) were fitted by a log–log linear relationship transformed to fit the arithmetic scale. Total (VolTot) (b, closed circles) and crown volume (VolC) (b, open circles) were fitted by a power function, whereas trunk volume (VolT) was fitted by a linear function (N = 59)
Trunk volume (VolT) increased linearly in relation to CSA, whereas crown stem volume (VolC) increased according to a power function (Fig. 5b). This suggests that the increase of VolC is more important as trees become bigger. Thus, for trees with DBH < 35 cm (CSA < 1000 cm2), VolC compared to VolT is negligible. The combination of both VolT and VolC also results in an increasing VolTot according to a power function (Fig. 5b).
Heartwood volume and DBH
Trees from the free sampling area exhibited the same linear increase of trunk volume with CSA (Fig. 6) than trees from monitored plots (Fig. 5b). However, the relationship between HWvol and CSA was not linear and fitted by a power function (Fig. 6a). These two different trends resulted in a nonlinear decrease in SWvol:HWvol with CSA. HWvol and SWvol were similar for DBH = 59 cm (CSA = 2730 cm2).
Trunk volume (VolT) and heartwood volume in the trunk (HWvol) (a) and the SWvol:HWvol ratio (b) as a function of CSA. VolT against CSA (a, opened circles) was fitted by a linear function, HWvol was fitted by a power function (a, closed circles), and the SWvol:HWvol ratio (b) was fitted by a log–log linear function and transformed to fit the arithmetic scale; the gray horizontal line represents SWvol:HWvol = 1 (N = 14)
Longitudinal profile of the SWa:HWa ratio
Visualization of the SWa:HWa with height and within trees from the free-sampling area showed that this ratio was always higher than 1 and decreased with height in small trees (DBH < 40 cm), whereas SWa:HWa was close to 1 and constant along the trunk in bigger trees (DBH > 40 cm) (Appendix E). Thus, for small trees, SW is the main component of the trunk, whereas SW and HW are almost equivalent for large trees. Another common characteristic, but especially visible in large or intermediate-sized trees, was the increased amount of SW just below the fork. We did not observe HW just below and within the crown in small trees, whereas HW was present in bigger trees (Appendix E).
Increment dynamics of tree compartments according to different dimensions
Dynamics of DBH increments followed the classical ‘hump-back’ pattern, with a first increase until 100 years followed by a decrease as the tree aged. This pattern was also observed for the HWd increment (Fig. 7a). However, the highest value of HWd increment was observed at 120 years. After this point, HWd increments remained higher than DBH increments, resulting in a negative increment of SWt after 120 years.
While CSA increment dynamics might also be divided into two phases, we did not observe any decrease of either CSA or HWa increments (Fig. 7b), compared to DBH (Fig. 7a). For more than 100 years, the increase in the CSA increment was curvilinear and then slowed to reach a steady state between 150 and 200 years. The increase in the HWa increment was also curvilinear and then slowed, but it did not reach a steady state. This difference between CSA and HWa increment resulted in SWa increments that increased until 100 years and then slowed down.
Dynamics of the VolT increment (Fig. 7c) were very similar to the CSA increment (Fig. 7b), with a first curvilinear increase until 120 years, a slowdown, and then reaching a steady state between 150 and 200 years.
VolC increment dynamics differed from VolT by a lower rate and by the fact that it did not slow down as the tree aged. VolC increments increased at higher rate after 75 years. The combination of VolC and VolT dynamics resulted in a VolTot increment that increased first curvilinearly and then slowed down without reaching a steady state or decreasing (Fig. 7c). Dynamics of the HWvol increment were similar to VolC. As for VolC increments, HWvol increments increased strongly after 75 years. The delayed increase of HWvol increment compared to VolT increment made SWvol increments increase slightly until 100 years and then decrease. Both SWvol and SWa increment dynamics were very similar (Fig. 7b, c).
Discussion
To our knowledge, this is the first time that a tropical tree has been both completely described and monitored for growth over 30 years. Even if qualitative crown assessment proves to be efficient in predicting RGR and mortality of trees (Rutishauser et al. 2011), measurements of tree structure are rarely performed (Sillett et al. 2010; Van Pelt and Sillett 2008) due to methodological issues caused by tree size and stand density. Our non-destructive method, which uses the skills of tree climbers, applied on monitored trees allowed us (1) to depict the ontogenetic variations of structural parameters and (2) to relate them to the ontogenetic variations of both RGR and SW/HW quantity.
Relationship between RGR at breast height and whole-tree structure
Despite strong individual variability in RGR (cm2/year) at breast height for a given diameter class, we clearly showed that this variable follows a mean ontogenetic trajectory (Figs. 4, 7). However, the pattern described here differs significantly from the common “hump-back” curve, which suggests that RGR increases until the tree reaches an optimal size and then declines in response to senescence (Canham et al. 2004; Hérault et al. 2011). Indeed, the Gompertz model is suited to describe the ontogenetic variations of RGR and suggests that, for trees with DBH > 50–60 cm, RGR first increases but reaches an asymptotic value and does not declines. This questions the representativeness of senescent trees in our sampling.
Rutishauser et al. (2011) showed that a high degree of branch mortality, reflecting a high degree of crown fragmentation, leads to declining RGR and in turn to tree death. Trees considered as senescent are not able to replace dead reiterated branches that were previously self-pruned or broken, leading to empty spaces within the crown (i.e., fragmentation). In our study, variables involving dead reiterated branches (i.e., pDRB, nDRB) were neither related to Main Branch mortality indexes (MB) (Kruskal–Wallis test, χ = 2.87, df = 3, pv = 0.41 and χ = 4.718, df = 3, pv = 0.19) nor to Secondary Branches mortality indexes (SB) (Kruskal–Wallis test, χ = 1.57, df = 3, pv = 0.67 and χ = 4.98, df = 3, pv = 0.17). The branch mortality phenomenon highlighted here is interpreted as a result of crown expansion rather than an indicator of tree senescence. Indeed, the distinction between ‘healed’ and ‘not-healed’ branch stumps shows that only 6 trees (30 > DBH > 60 cm) had 100 % not-healed branch stumps (data not shown). For larger trees (DBH > 60 cm), a wide range of both states of stump was represented (20–75 % healed stumps), indicating both ancient and recent branch mortality events within the tree crown. Branch mortality starting near the first fork and continuing toward the upper crown makes this phenomenon a gradual process resulting from the self-thinning of the lower branches by the upper ones. This mode of crown expansion is consistent with the suggested preferential resource allocation to branches that are in more favorable positions (Henriksson 2001). From this point of view, senescent trees were not detected in our samples.
The asymptotic distribution of RGR according to CSA may have resulted from the nature of RGR itself. Whereas RGR is generally computed as the annual increment of tree DBH (i.e., in cm/year) (Canham et al. 2004; Fortunel et al. 2016; Hérault et al. 2011), RGR in this study refers to the annual increment of tree CSA (i.e., in cm2/year). We believe that measuring RGR in this way is more relevant as it integrates both dimensions of transverse cambial activity (i.e., radial and tangential divisions) and that the area increment is a better indicator of tree growth than the diameter increment. A careful analysis of RGR measured in cm/year and in cm2/year on a dataset including all Dicorynia guianensis trees of the Paracou field stations showed that Canham’s model produces the expected ‘hump-back’ curve for RGR in cm/year but not for RGR in cm2/year (Appendix F). This suggests that trunk growth rate does not decrease after the tree reaches an optimal DBH or CSA; rather, it remains constant. This result on a larger dataset (N = 844) is consistent with the differences between DBH and CSA increments of the stem as a function of age observed in our smaller dataset (Fig. 7a, b). It is very likely that senescent trees were present in this wider dataset as well as in datasets from the previous study (Hérault et al. 2011). However, Rutishauser et al. (2011) crown fragmentation assessment performed on 237 individuals identified only 13 % senescent trees (Nicolini, unpublished data). Such a small proportion of declining trees may have had little or no effect on the relationship between RGR and CSA observed here.
Structural tree characteristics for which RGR at breast height ceases to be indicative of whole-tree growth
The asymptotical value of RGR was reached for trees of 50–60 cm of DBH. At this stage, trees have reached the canopy, almost completed their growth in height (32–34 m), and crown expansion has started. Indeed, for these trees, the crown represents 25 % of the whole-tree volume, and branch mortality events are already detected, suggesting that self-shading of lower branches is already occurring.
The stage for which RGR reaches an asymptotic value is also the stage for which VolC increment increases strongly (Fig. 7c). The better correlation observed between VolC and CSAUF (Spearman correlation = 0.96, pv < 0.001) than with CSA (Spearman correlation = 0.90, pv < 0.001) suggests that CSAUF is a better indicator of crown size. Moreover, the ratio between CSA and CSAUF indicates that the trunk tends toward a cylindrical shape (Ratio tend to 1, data not shown) as crown size increases, which is supported by a significant negative correlation between this ratio and VolC (Spearman correlation = −0.62, pv < 0.001). As RGR at the trunk base is constant in large trees, the establishment of a cylindrical trunk requires greater growth in the higher parts of the trunk (i.e., under the fork). Therefore, the stabilization of CSA increments (or the decrease of DBH increments) at the trunk base is not related to tree senescence but rather to a change in growth allocation toward the growing crown and the higher part of the trunk. The monotonic increase of both absolute and living reiterated branches (nARB and nLRB) (Fig. 5) supports this statement. This result for a tropical tree species is in complete agreement with previous studies for temperate tree species (Sillett et al. 2010; Van Pelt and Sillett 2008). RGR measured at breast height is a poor indicator of whole-tree growth because the continuous increasing whole-tree increment (Fig. 7c) does not support the RGR stabilization at breast height (Fig. 7b). Measuring tree growth in terms of DBH increment is even more misleading because it indicates a decrease observed in large trees that actually reflects an increase of whole-tree growth. As shown in 403 tree species by Stephenson et al. (2014), the whole-tree increment of the species under study increases continuously with tree size.
SW/HW quantity variations according to location within trees during tree ontogeny
We found a significant positive relationship between SWa and RGR (Fig. 4). This result supports the positive relationships between RGR and SW area or thickness identified in temperate and tropical species (Bamber 1976; Busgen and Munch 1929; Carrodus 1972; van der Sande et al. 2015). It is likely that the more vigorous the tree, the more photosynthates are created that need to be stored in SW or are sufficient to assume SW respirations costs, thus delaying HW formation. However, the correlation highlighted here does not directly support this causality effect. As RGR varies according to tree ontogeny, linking tree vigor to SW area requires identification of ontogenetic stages of the considered trees and comparison of both variables for individuals that reached the same ontogenetic stages.
The positive link between SWa and RGR on a wide range of tree sizes emphasizes the ontogenetic dependence between SWa and RGR. Indeed, both SWa and RGR follow the same pattern of variations at the trunk base on the course of a tree’s ontogeny (Fig. 2b and 3): SWa and RGR increase until the tree reaches 50–60 cm of DBH; after this point, both variables reach a steady state.
It has been hypothesized that HW increments are constant during tree life and that variations of RGR regulate SW quantity (Långström and Hellqvist 1991; Margolis et al. 1988). However, our analysis of HW increment dynamics shows that the HW increment is not constant (Fig. 7) but rather increases when VolC increment increases, giving strong support to the ontogenetic nature of HW formation (Bamber 1976, 1987). The same authors hypothesized that HW formation manages the amount of energy consuming SW to an optimal level. It is likely that a high production of SW in the crown may increase maintenance costs as a tree grows. From this point of view, HW formation from tree base to treetop limits the accumulation of SW.
The effects of SW/HW quantity variations on tree function as tree grows
Trees must maintain sufficient SW to assure hydraulic functions because this function is vital for the organism. In our sampling, the minimum SW thickness was 2 cm, which corresponds to a range of conductive SW thickness recorded in others Legumes species from Mexico (Reyes-García et al. 2012). However, storage in the trunk is not vital for the organism; thus, this function could be preferentially located in another part of the plant.
Our analysis on the relative amounts of SW and HW at the trunk base or within the trunk (i.e.SWa:HWa and SWvol:HWvol) (Figs. 3, 6, Appendix E) shows that SWa and volume in trunk are higher than HW for trees with DBH < 50 cm and then gradually become lower for trees with DBH > 50 cm. This ontogenetic change in SW and HW relative quantities is in agreement with the pattern observed in a temperate oak species, Quercus petraea (Hatsch 1997). We also observed a sharp increase of SW relative quantity at the crown base compared to the trunk top and generally very few HW within the crown (Appendix E). Therefore, for small trees (DBH < 50 cm), SW is spread throughout the structure of the tree, whereas SW relative amounts are only higher than HW within the crown for larger trees (DBH > 50 cm). Because (1) the SW is a main compartment storing carbohydrates, water, and nutrients (Hillis 1971) and (2) the participation of the crown to the whole-tree volume is very weak in small trees (DBH < 50 cm), it is likely that trunk contains the major part of above-ground tree reserves. Conversely, the relative quantity of SW is low in the trunk, and the crown becomes increasingly important in bigger trees (DBH > 50 cm). Thus, we expected that the major parts of tree reserves concentrate in the crown SW as a tree grows. We suppose that this amount of crown SW is sufficient to store reserves, in turn triggering the acceleration of HW formation in the trunk. Our findings confirm the importance of the crown as a reserve for carbohydrates (Barbaroux et al. 2003) and water (Cermak et al. 2007; Schulze et al. 1985).
A parallel study on D. guianensis showed that the natural durability of trunk SW is higher in bigger trees than in small trees. Therefore, it is likely that trunk SW carbohydrate content decreases with increasing size (Lehnebach 2015), and that carbohydrates might be used for growth, daily metabolism or translocate to crown or roots.
However, this scheme of SW concentration toward the crown as a tree grows results from the way that HW spreads within the tree: from the center to the stem peripheral part and in the same time from the trunk base to the treetop. This questions why HW does not form early during tree life all along the tree structure at the center of the stem and spread radially later?
This question might be answered through tree economics. It would be more economical for the tree to store reserves in the crown, thus lowering the cost of transport of newly formed carbohydrates from leaves to SW than to store reserves in the whole-tree structure. Similarly, if the plant needs to mobilize its reserves to replace shed branches during crown expansion, the cost of transport will be lower if they are stored within the crown.
Whatever the tree size, a considerable quantity of SW is present just under the first fork. The tree may conserve these wood volumes as SW to keep reserves available for re-growth ‘die-back’ (Kile et al. 1981), and the development of epicormic shoots (Nicolini et al. 2003) following accidental damage caused by the fall of neighboring trees or branches.
On the possible deviance from the mean ontogenetic trajectory and its effect on absolute growth rate and sapwood amount
We highlighted a mean growth scenario based on mean stem and tree characteristics and their variations during ontogeny. However, we expect that this mean growth scenario may be altered by different factors.
A shift in growth pattern might be strongly linked to tree crown establishment and the appearance of first reiterated branches. If the reiteration process begins earlier in a tree’s life, we would expect that the investment in crown growth would also begin earlier. Well-developed trees in our sampling with the shortest trunks also have a greater Volc and a lower RGR and SWa than other trees from the same CSA range. In these individuals, investment in crown growth might have started earlier. Indeed, if the canopy height at a particular site is lower than that at another site, we would expect a shorter phase of height growth, a lower trunk height, and an earlier investment in crown growth. In this way, the neighborhood of a tree may impact its allometrical trajectory.
Other important factors are species life history traits. D. guianensis is a semi-shade tolerant species, capable of growing slowly in the understory or faster in clearings (Favrichon 1994). We might expect that trees growing slowly in the understory would not be able to produce and store many carbohydrates due to low levels of photosynthesis, and for the same reason, would not be able to meet the high respiratory demands of the maintenance of a considerable volume of SW, leading to a faster shift from SW to HW. This last consideration could explain differences in SW and HW quantities of two trees exhibiting the same structural traits values but having grown in environments with contrasting light availability, and it is consistent with the dependence of SW production on allocation principles (Schippers et al. 2015).
Deviance from the mean growth scenario and tree growth history could explain, in addition to other factors affecting tree growth (e.g., soil fertility or genetic variability), the high variability observed for both RGR and SWa.
Improvements of forestry practices
In natural forests from French Guiana, Dicorynia guianensis (Basralocus) trees are harvested for their timber between 50 and 90 cm of DBH (Cirad 2011). Our analysis showed that the minimal exploitation diameter (i.e., 50 cm) corresponds to the DBH value for which the SWa is equal to the HWa at breast height; however, this consideration differs when accounting for the relative volume of both SW and HW along the bole.
Indeed, the analysis of HW and SW tapering within felled trees showed that SWvol:HWvol ratio is constant along the bole for trees having reached 60 cm of DBH. This suggests to select trees with DBH ≥ 60 cm in order to optimize the quantity of HW along the bole.
However, this study also highlighted the positive relation between crown volume or its increment and heartwood volume or its increment within trunk. Whereas, estimating the trunk volume in the forest is feasible, the estimation of crown stem volume is more complicated. Nevertheless, due to the strong correlation between CSAUF and crown volume, we expect that CSAUF may be used as a predictor of crown volume in allometrical equations. From this point of view, measuring DBH and diameter under fork (converted to CSA and CSAUF, respectively) may allow foresters to calculate the relative contribution of the trunk and the crown to the tree volume, and in turn optimize the HW volume of the bole by selecting trees with a relative contribution of crown volume to the whole-tree volume >25 % (i.e., trees for which both crown and HW increment increase at higher rate).
Conclusion
This study highlights the importance of accounting for ontogenetic changes in tree growth by the careful descriptive analysis of tree structure. Variation in RGR is a common characteristic of tropical trees; thus, we believe that the shift highlighted here is observable in other species. Accounting for ontogenetic changes in tree growth and understanding the relationship between growth observable at breast height and the whole-tree increment should enable better estimation of carbon dynamics to the stand scale.
This study also highlights the impact of growth ontogenetic changes on SW and HW, which in turn affect the localization of water and carbohydrate storage in trees. Moreover, knowing the mean trajectory of HW increment within trees could lead to a better estimation of forest tree profitability by selecting trees that contain a relatively high amount of HW at the trunk base and along the trunk to optimize the production of homogenous and high quality timber.
Author contribution statement
RL performed the field work, analyzed the data and wrote the manuscript. HM, JB, and EN performed field work and manuscript revision. GLM contributed with statistical assistance and manuscript revision. NA and JB contributed with manuscript revisions.
References
Amusant N, Nigg M, Thibaut B, Beauchene J (2014) Diversity of decay resistance strategies of durable tropical woods species: Bocoa prouacencsis Aublet, Vouacapoua americana Aublet, Inga alba (Sw.) Wild. Int Biodeter Biodegr 94:103–108. doi:10.1016/j.ibiod.2014.06.012
Bamber RK (1976) Heartwood, its function and formation. Wood Sci Technol 10:1–8. doi:10.1007/BF00376379
Bamber RK (1987) Sapwood and heartwood. Technical publication (Forestry Commission of New South Wales Wood Technology and Forest Research Division)
Bamber RK, Fukazawa K (1985) Sapwood and heartwood: a review. For Abstr 46:567–580
Barbaroux C, Bréda N, Dufrêne E (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157:605–615. doi:10.1046/j.1469-8137.2003.00681.x
Bazot S, Barthes L, Blanot D, Fresneau C (2013) Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees-Struct Funct 27:1023–1034. doi:10.1007/s00468-013-0853-5
Brunaux O, Demenois J, Lecoeur N, Guitet S (2009) Directive Regionale d’Aménagement Nord Guyane. Office National des Forêts, Cayenne
Busgen M, Munch E (eds) (1929) The Structure and Life of Forest Trees. Chapman & Hall, UK
Canham CD, LePage PT, Coates KD (2004) A neighborhood analysis of canopy tree competition: effects of shading versus crowding. Can J For Res 34:778–787. doi:10.1139/x03-232
Carbone MS et al (2013) Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phytol 200:1145–1155. doi:10.1111/nph.12448
Carrodus BB (1972) Variability in the proportion of heartwood formed in woody stems. New Phytol 71:713–718. doi:10.1111/j.1469-8137.1972.tb01283.x
Cermak J, Kucera J, Bauerle WL, Phillips N, Hinckley TM (2007) Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol 27:181–198
Cirad (2011) Tropix 7, Caractéristiques technologiques de 245 essences tropicales et tempérées, v.7.2.0 edn
Clark JS et al (2011) Individual-scale variation, species-scale differences: inference needed to understand diversity. Ecol Lett 14:1273–1287. doi:10.1111/j.1461-0248.2011.01685.x
Climent J, Chambel MR, Gil L, Pardos JA (2003) Vertical heartwood variation patterns and prediction of heartwood volume in Pinus canariensis Sm. For Ecol Manag 174:203–211. doi:10.1016/S0378-1127(02)00023-3
Drénou C (1988) Etude de l’architecture d’un arbre guyanais: l’angélique, dicorynia guianensis amshoff caesalpiniaceae. Master Thesis, Université de Montpellier II
Fauset S et al (2015) Hyperdominance in Amazonian forest carbon cycling. Nat Commun. doi:10.1038/ncomms7857
Favrichon V (1994) Classification des espèces arborées en groupes fonctionnels en vue de la réalisation d’un modèle de dynamique de peuplement en forêt guyanaise. Rev Ecol-Terre Vie 49:379–403
Fortunel C, Valencia R, Wright SJ, Garwood NC, Kraft NJB (2016) Functional trait differences influence neighbourhood interactions in a hyperdiverse Amazonian forest. Ecol Lett. doi:10.1111/ele.12642
Frey-Wyssling A, Bosshard AA (1959) Cytology of the ray cells in sapwood and heartwood. Holzforschung 13:129–137. doi:10.1515/hfsg.1959.13.5.129
Galván JD, Camarero JJ, Sangüesa-Barreda G, Alla AQ, Gutiérrez E (2012) Sapwood area drives growth in mountain conifer forests. J Ecol 100:1233–1244. doi:10.1111/j.1365-2745.2012.01983.x
Gartner BL (1995) Patterns of Xylem Variation within a Tree and Their Hydraulic and Mechanical Consequences. In: Gartner BL (ed.) Plant Stems. Academic Press, San Diego, p 125–149. doi:http://dx.doi.org/10.1016/B978-012276460-8/50008-4
Godin C, Caraglio Y (1998) A Multiscale Model of Plant Topological Structures. J Theor Biol 191:1–46. doi:10.1006/jtbi.1997.0561
Godin C, Costes E, Caraglio Y (1997) Exploring plant topology structure with the AMAPmod software : an outline. Silva Fenn 31:355–366
Goldstein G, Andrade JL, Meinzer FC, Holbrook NM, Cavelier J, Jackson P, Celis A (1998) Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant Cell Environ 21:397–406. doi:10.1046/j.1365-3040.1998.00273.x
Gourlet-Fleury S, Guehl JM, Laroussinie O (eds.) (2004) Ecology & management of a neotropical rainforest. Lessons drawn from Paracou, a long-term experimental research site in French Guiana. Elsevier, Paris
Hatsch E (1997) Répartition de l’Aubier et acquisition de la forme de la tige chez le chêne sessile (Quercus petraea (Matt) Liebl): Analyse, modélisation et relation avec le développement du houppier
Henriksson J (2001) Differential shading of branches or whole trees: survival, growth, and reproduction. Oecologia 126:482–486. doi:10.1007/s004420000547
Hérault B et al (2011) Functional traits shape ontogenetic growth trajectories of rain forest tree species. J Ecol 99:1431–1440. doi:10.1111/j.1365-2745.2011.01883.x
Hillis WE (1971) Distribution, properties and formation of some wood extractives. Wood Sci Technol 5:272–289. doi:10.1007/BF00365060
Hillis WE (1987) Heartwood and tree exudates. Springer-Verlag,Berlin
Holbrook NM (1995) Stem water storage. In: Gartner BL (ed) Plant stems: physiology and functional morphology. Academic Press, San Diego, pp 151–174
IAWA (1964) Multilingual glossary of terms used in wood anatomy. Verlagsanstalt Buchdruckerei Konkordia, Winterthur
Kampe A, Magel E (2013) New Insights into Heartwood and Heartwood Formation. In: Fromm J (ed.) Cellular Aspects of Wood Formation, vol 20. Plant Cell Monographs. Springer Berlin Heidelberg, p 71–95. doi:10.1007/978-3-642-36491-4_3
Kile G, Tumbull C, Podger F (1981) Effect of `Regrowth Dieback’ on some properties of Eucalyptus obliqua trees. Aust For Res 11:55–62
Koeppen RC (1967) Revision of Dicorynia (Cassieae, Caesalpiniaceae). Brittonia 19:42–61
Långström B, Hellqvist C (1991) Effects of different pruning regimes on growth and sapwood area of Scots pine. For Ecol Manag 44:239–254. doi:10.1016/0378-1127(91)90011-J
Lehnebach R (2015) Variabilité ontogénique du profil ligneux chez les Légumineuses de Guyane Française., Université de Montpellier
Margolis HA, Gagnon RR, Pothier D, Pineau M (1988) The adjustment of growth, sapwood area, heartwood area, and sapwood saturated permeability of balsam fir after different intensities of pruning. Can J For Res 18:723–727. doi:10.1139/x88-110
McDowell N et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. doi:10.1111/j.1469-8137.2008.02436.x
Meinzer FC, James SA, Goldstein G, Woodruff D (2003) Whole-tree water transport scales with sapwood capacitance in tropical forest canopy trees. Plant Cell Environ 26:1147–1155. doi:10.1046/j.1365-3040.2003.01039.x
Meinzer FC, James SA, Goldstein G (2004) Dynamics of transpiration, sap flow and use of stored water in tropical forest canopy trees. Tree Physiol 24:901–909. doi:10.1093/treephys/24.8.901
Meinzer FC, Woodruff DR, Domec JC, Goldstein G, Campanello PI, Gatti MG, Villalobos-Vega R (2008) Coordination of leaf and stem water transport properties in tropical forest trees. Oecologia 156:31–41. doi:10.1007/s00442-008-0974-5
Meinzer FC, Lachenbruch B, Dawson TE (2011) Size- and age-related changes in tree structure and function. Springer, Netherlands
Morataya R, Galloway G, Berninger F, Kanninen M (1999) Foliage biomass–sapwood (area and volume) relationships of Tectona grandis L.F. and Gmelina arborea Roxb.: silvicultural implications. For Ecol Manag 113:231–239. doi:10.1016/S0378-1127(98)00429-0
Nicolini E, Caraglio Y, Pélissier R, Leroy C, Roggy JC (2003) Epicormic BRANCHES: A GROWTH INDICATOR FOR THE TROPICAL FOREST TRee, Dicorynia guianensis Amshoff (Caesalpiniaceae). Ann Bot 92:97–105. doi:10.1093/aob/mcg119
Pérez-Harguindeguy N et al (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234. doi:10.1071/BT12225
Pinto I, Pereira H, Usenius A (2004) Heartwood and sapwood development within maritime pine (Pinus pinaster Ait.) stems. Trees-Struct Funct 18:284–294. doi:10.1007/s00468-003-0305-8
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743
Rasband WS (2012) ImageJ. US National Institutes of Health, Bethesda
R-Core-Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reyes-García C, Andrade J, Simá JL, Us-Santamaría R, Jackson P (2012) Sapwood to heartwood ratio affects whole-tree water use in dry forest legume and non-legume trees. Trees 26:1317–1330. doi:10.1007/s00468-012-0708-5
Richardson AD et al (2013) Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol 197:850–861. doi:10.1111/nph.12042
Richardson AD et al (2015) Distribution and mixing of old and new nonstructural carbon in two temperate trees. New Phytol 206:590–597. doi:10.1111/nph.13273
Rutishauser E, Barthélémy D, Blanc L, Nicolini E-A (2011) Crown fragmentation assessment in tropical trees: method, insights and perspectives. For Ecol Manag 261:400–407. doi:10.1016/j.foreco.2010.10.025
Sabatier D, Prévost MF (1990) Quelques données sur la composition floristique, et la diversite des peuplements forestiers de guyane francaise. Bois et Forêts des Tropiques 219
Schippers P, Vlam M, Zuidema PA, Sterck F (2015) Sapwood allocation in tropical trees: a test of hypotheses. Funct Plant Biol 42:697–709. doi:10.1071/FP14127
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant Cell Environ 30:236–248. doi:10.1111/j.1365-3040.2006.01623.x
Schulze ED et al (1985) Canopy transpiration and water fluxes in the xylem of the trunk of Larix and Picea trees–a comparison of xylem flow, porometer and cuvette measurements. Oecologia 66:475–483. doi:10.1007/BF00379337
Sillett SC, Van Pelt R, Koch GW, Ambrose AR, Carroll AL, Antoine ME, Mifsud BM (2010) Increasing wood production through old age in tall trees. For Ecol Manag 259:976–994. doi:10.1016/j.foreco.2009.12.003
Spicer R, Gartner BL (2001) The effects of cambial age and position within the stem on specific conductivity in Douglas-fir (Pseudotsuga menziesii) sapwood. Trees 15:222–229. doi:10.1007/s004680100093
Spicer R, Holbrook NM (2005) Within-stem oxygen concentration and sap flow in four temperate tree species: does long-lived xylem parenchyma experience hypoxia? Plant Cell Environ 28:192–201. doi:10.1111/j.1365-3040.2004.01262.x
Stephenson NL, Das AJ, Condit R, Russo SE, Baker PJ, Beckman NG, Coomes DA, Lines ER, Morris WK, Ruger N, Alvarez E, Blundo C, Bunyavejchewin S, Chuyong G, Davies SJ, Duque A, Ewango CN, Flores O, Franklin JF, Grau HR, Hao Z, Harmon ME, Hubbell SP, Kenfack D, Lin Y, Makana JR, Malizia A, Malizia LR, Pabst RJ, Pongpattananurak N, Su SH, Sun IF, Tan S, Thomas D, van Mantgem PJ, Wang X, Wiser SK, Zavala MA (2014) Rate of tree carbon accumulation increases continuously with tree size. Nature 507:90–93
Taylor AM, Gartner BL, Morrell JJ (2002) Heartwood formation and natural durability–a review. Wood Fiber Sci 34:587–611
Taylor A, Freitag C, Cadot E, Morrell J (2008) Potential of near infrared spectroscopy to assess hot-water-soluble extractive content and decay resistance of a tropical hardwood. Holz Roh Werkst 66:107–111. doi:10.1007/s00107-007-0214-4
Ter Steege H et al (2006) Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443:444–447 doi:http://www.nature.com/nature/journal/v443/n7110/suppinfo/nature05134_S1.html
Ter Steege H et al (2013) Hyperdominance in the amazonian tree flora. Science. doi:10.1126/science.1243092
Tyree M, Yang S (1990) Water-storage capacity of Thuja, Tsuga and Acer stems measured by dehydration isotherms. Planta 182:420–426. doi:10.1007/BF02411394
van der Sande M, Zuidema P, Sterck F (2015) Explaining biomass growth of tropical canopy trees: the importance of sapwood. Oecologia 177:1145–1155. doi:10.1007/s00442-015-3220-y
Van Pelt R, Sillett SC (2008) Crown development of coastal Pseudotuga menziesii, includinf a conceptual model for tall conifers. Ecol Monogr 78:283–311. doi:10.1890/07-0158.1
Vargas R, Trumbore SE, Allen MF (2009) Evidence of old carbon used to grow new fine roots in a tropical forest. New Phytol 182:710–718. doi:10.1111/j.1469-8137.2009.02789.x
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81:259–291. doi:10.1017/S1464793106007007
White DA (1993) Relationships between foliar number and the cross-sectional areas of sapwood and annual rings in red oak (Quercusrubra) crowns. Can J For Res 23:1245–1251. doi:10.1139/x93-159
Whitehead D, Edwards WRN, Jarvis PG (1984) Conducting sapwood area, foliage area, and permeability in mature trees of Piceasitchensis and Pinuscontorta. Can J For Res 14:940–947. doi:10.1139/x84-166
Wirth C, Gleixner G, Heimann M (2009) Old-growth forests: function. Fate and Value, Springer
Wright SJ et al (2010) Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91:3664–3674. doi:10.1890/09-2335.1
Yang KC (1990) The ageing process of sapwood ray parenchyma cells in four woody species. University of British Columbia
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer Series in Wood Science. Springer-Verlag, Berlin. doi:10.1007/978-3-662-22627-8
Acknowledgments
We would like to thank all the members of the professional tree climbers team from the Centre de Formation Professionnelle et de Promotion Agricoles (CFPPA) of Matititi, French Guiana. We also thank logistic and scientific managers of the Paracou field station for their advice and support as well as an anonymous reviewer for his constructive and helpful comments. This work was supported by a grant from the French Agricultural Research and International Cooperation Organization (CIRAD) and funding from the European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Communicated by M. Zwieniecki.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lehnebach, R., Morel, H., Bossu, J. et al. Heartwood/sapwood profile and the tradeoff between trunk and crown increment in a natural forest: the case study of a tropical tree (Dicorynia guianensis Amsh., Fabaceae). Trees 31, 199–214 (2017). https://doi.org/10.1007/s00468-016-1473-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1473-7