Abstract
Key message
We demonstrate that tropical trees growing in wet climates can have a marked seasonality in cambium activity and stem growth associated with high temperature and day length of summer.
Abstract
Monitoring the rhythm of tree growth associated with the growth rings can contribute substantially to understanding forest dynamics and the management of tropical forests. In this study, we monitored the girth increment rhythm and described the wood characteristics (anatomy of growth rings, wood specific gravity) in 10 tropical tree species (103 individuals) naturally occurring in a wet and weakly seasonal region of Atlantic Forest in southern Brazil. We aimed to verify whether tree growth dynamics are associated with climate and woody anatomy in tropical trees with contrasting ecological characteristics. We installed permanent dendrometer bands and monthly assessed the girth increment for 22 months. We collected wood samples (non-destructive method), measured wood specific gravity and prepared permanent slides to characterize the growth ring markers. We found growth rings in all species (distinct in six species); deciduous species produced more distinguishable tree rings compared with semi-deciduous and evergreen tree species. Species varied in their accumulated girth growth (in average, from 1.83 to 62.64 mm), growth rates (1–15 %), and annual radial increment (0.16–5.44 mm). Girth increment was positively related to temperature and day length in five out of ten tree species, indicating the possible effects of these climatic variables in triggering cambial activity in these species. The growth pattern varied among species and was marginally associated to the tree deciduousness. We concluded that even in wet and less seasonal climates, there can be an association in the cambium activity and stem growth with the hotter and longer days of summer months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determining the occurrence of annual growth rings and the factors affecting the rhythms of stem growth in tropical trees has been an intriguing question for botanists (Worbes 2002; Lüttge and Hertel 2009). Since the nineteenth century occurrence of growth rings was detected in tropical trees (Worbes 2002). However, at the beginning of twentieth century controversies about the causes of growth rhythms emerged and a series of anatomical and ecological studies have attempted to describe the differences between species and to understand the whole process of periodicity in tropical trees (Mariaux 1995; Worbes 2002; Lüttge and Hertel 2009). Understanding these rhythms is important to predict forest dynamics and their possible effects on food production, conservation and management of tropical forests and predictions in climate change scenarios (Jacoby 1989; Roig 2000; Worbes 2002). Additionally, studies on the ecology, growth dynamics and carbon sequestration of tropical forests are often hindered by insufficient information on the structure and periodicity of tree rings (Dié et al. 2012; Shimamoto et al. 2014).
Tree growth is determined by cambium activity during a specific period of the year resulting in the formation of growth rings. In general, tree rings in woody plants are induced by seasonally alternating favorable and unfavorable growth conditions (Worbes 1995). For trees growing in temperate forests earlywood and latewood are easily identified, and the growth rings usually represent their annual increment, which allows the precise dating of the tree age (Mozeto et al. 1988). However, in tropical trees, the growth rings are usually less evident because the wood anatomy is much more complex and variable (Stahle 1999; Détienne 1989). In tropical trees growth rings are apparently restricted to some species growing in seasonal climates (Worbes 1995, 1999; Botosso and Tomazello-Filho 2001; Callado et al. 2001, 2013). Thus, it is expected that growth ring markers in tropical trees rarely represent annual rhythms.
Anatomical features that clearly denote growth rings in temperate trees, such as ring-porosity of some angiosperms and the pronounced changes in cell wall thickness and cell dimensions of earlywood and latewood in confers, are uncommon in tropical species (Gourlay 1995). However, relatively little is known about the prevalence of growth rings, and even less is known about the factors triggering growth ring formation in tropical plants (Baas and Vetter 1989; Jacoby 1989; Priya and Bhat 1999). Despite the high diversity in ecosystems, there are comparatively few studies on growth rhythm and periodicity in the tropics (Worbes 1989; Vetter and Botosso 1989; Botosso and Vetter 1991; Roig 2000; Lisi et al. 2008).
The wood specific gravity may play an important role in determining the growth rate (Suzuki 1999). In general, high growth rates in the tropical trees are associated with low wood specific gravity (Suzuki 1999; Muller-Landau 2004), and low growth rates are associated with high wood specific gravity (Parolin et al. 1998; van Gelder et al. 2006). This relationship occurs because there is a trade-off between constructing high-density woody (and reducing the probability of physical damage) and tree growth (Zimmerman et al. 1994; van Gelder et al. 2006). Several ecological studies emphasize the role of wood density as an important life-history trait, showing much wider variation in tropical lowland communities than in communities at higher latitudes and altitudes (Baas and Wheeler 2011).
The cambium activity and stem growth are potentially affected by temperature, photoperiod and precipitation (Fahn et al. 1981; Botosso and Tomazello-Filho 2001; Callado et al. 2001, 2013). Climate affects the endogenous metabolism of plants (Lüttge and Hertel 2009) and causes inter-annual variations in limiting resources such as water, light and nutrients, affecting plant growth (Billings 1952; Campos 1970; Lamprecht 1990; Worbes et al. 2003). For example, when the dry season reaches precipitation with less than 60 mm per month the soil water availability decreases, affecting the wood growth, the cambial dormancy, consequently leading to the formation of boundaries in the wood (Worbes 1995, 1999). Despite consequences of low precipitation are suggested as a strong limiting factor for cambium activity and stem growth (Worbes 1999), low temperature and day length, considered important constraining climatic factors in temperate climates (Cunningham and Read 2003), may also affect stem growth in tropics.
In this study, we assessed the anatomical structure of growth rings and monitored the girth growth (for 22 months) of 10 tree species with contrasting leaf phenology (deciduous, semi-deciduous and evergreen) in a rainforest of southern Brazil. We aimed to verify whether tree growth dynamics are associated with climate and woody anatomy in tropical trees with contrasting ecological characteristics. Specifically, we asked the following questions: (1) do the species differ in growth rings distinctiveness and in stem growth rhythm? (2) Is the girth growth rhythm related to the current and historical climate (i.e., temperature, precipitation and day length)? (3) Are the girth growth patterns related to wood anatomical features (i.e., latewood fiber wall thickness, growth rings and porosity), wood specific gravity and species deciduousness?
Methods
Study site
The study was conducted in the Rio Cachoeira Reserve (25°19′15″S and 45°42′24′′W; total area of 8600 ha) and in the Morro da Mina Reserve (25°21′16′′S and 48°46′17′′W; total area of 3300 ha) in the municipality of Antonina, Paraná State, southern Brazil. These reserves are located in a large protected environmental area (PEA Guaraqueçaba) that comprises preserved areas (68 % of the total) of one of the largest fragments of Atlantic Forest in Brazil (Kauano et al. 2012). The soils in the region are Neosols, Gleysols, Cambisols and Argisols (Ferretti and Britez 2006). The climate is humid subtropical (Cfa in the Köppen climate classification), with an average annual temperature of 21.4 °C, an average annual rainfall of 2778 mm, no dry season and rare occurrence of frosts (Fig. 1a). For the study period, 2011 was wetter (3319 mm) and 2012 was drier (2567 mm) than historical records, but average temperatures were similar (20.8 and 20.4 °C, respectively) (Fig. 1c). For this region, differences in day length are ~3 h along the year (Fig. 1b). Considering that monthly rainfall is always higher than 60 mm, even during the winter, the region can be considered aseasonal with regard to water availability (Walter 1983). However, it is possible to recognize climatic differences between the wetter and hotter spring and summer seasons (October to March; averages 297 mm and 23 °C) and the less wet and colder autumn and winter seasons (April–September; averages 134 mm, 18 °C).
Climatic diagram for the period of 1978 to 1999 (a), day length (b), and climate for the studied period from January 2011 to October 2012 (c). Source: a IAPAR (http://www.iapar.br/arquivos/Image/monitoramento/Medias_Historicas/Antonina); b US Naval Observatory (http://aa.usno.navy.mil); c SIMEPAR (http://www.simepar.br/)
The reserves comprise primary and secondary forests distributed in lowland and montane areas, characterized by high species richness, including a total of 306 tree species (Liebsch et al. 2008; Borgo et al. 2011). In the lowland areas, the forest was logged over 30 years and converted into pasture areas for livestock. After pasture abandonment (10 years before starting the experiment), the forests were restored through direct seedling planting and assisted natural regeneration (Ferretti and Britez 2006; Bruel et al. 2010).
Studied species
Ten tree species naturally occurring in the lowland and slope successional areas (20–60 years old) of the Atlantic forest were selected based on their high frequency in the region (Liebsch et al. 2007; Borgo et al. 2011). We selected species representing different leaf phenology: six semi-deciduous, three deciduous and one evergreen (Table 1). We labeled 6–19 individuals of each species (total 103 individuals, Table 1) to ensure the inclusion of individuals belonging to different diameter classes. In general, an effort was made to select adult trees with straight-boled stems, no bifurcations and no apparent trunk deformities. For some species, it was not possible to collect a more representative number of specimens due to the varying number of individuals with the appropriate characteristics at each growth condition.
Growth ring markers and wood specific gravity
To characterize growth ring markers and describe the growth layer boundary, small wood samples 5 mm in diameter containing the tree ring time series (radial increment from pith to bark) were collected at breast height through a non-destructive method (Pressler’s increment borer). The number of sampled radii varied among species (Table 1). To recognize growth rings we used the characteristics of the increment zones by inspection of the anatomical features of the wood. We only identified if growth rings were distinguishable or not in the studied region, independently of their frequency (annual or not).
Small wood samples were embedded in Historesin® (Leica, Heidelberg), and transverse sections were performed on a rotation microtome with a thickness of 7 μm. The sections were stained with toluidine blue O (O’Brien et al. 1965), permanently mounted and thereafter observed under an optical microscope and photographed with a digital camera. For macroscopic wood observations, small wood samples were superficially sanded with micro-abrasive paper. The macro- and microscopic features descriptions of the tree rings structures were made following the terminology proposed by IAWA (1989). We considered growth ring boundaries according to family descriptions present in IAWA (1989), Metcalfe and Chalk (1950) and in the Insidewood web resource (Wheeler 2011). For some species, atypical wood anatomical patterns differing from the typical growth ring boundaries (e.g., intra-ring density fluctuation, fiber density fluctuation, among others; Copenheaver et al. 2006; Speer 2010), were considered as “false” ring.
Additionally, these same wood samples were also used to determine the wood specific gravity of studied species. For this, small wood samples (5 mm in diameter) were collected from 61 individual trees (8–48 samples per species, Table 2) and applied the maximum moisture content method (Smith 1954). First, the weight of water-saturated samples and the weight of the oven-dried samples (105 ± 2 °C for 48 h) are taken. Then, by dividing the weight of oven-dried samples (g) by the green volume of samples (cm3), the wood specific gravity is calculated by the formula proposed by Smith (1954), and using the density of wood substance constant (1.53 g cm3). We did not collect wood samples from Inga edulis to avoid injuries to small trees. Thus, the wood specific gravity of this species was used as reported by Zanne et al. (2013). Wood samples and permanent slides are housed in the wood collection of Embrapa Forestry.
Tree growth monitoring
The girth growth increment for each of the 103 trees was recorded at DBH (diameter at breast height, 1.3 m above ground level) by using steel permanent dendrometer bands with a precision of 0.2 mm (Mariaux 1977; Botosso and Tomazello-Filho 2001; Cardoso et al. 2012). We recorded data monthly, for 22 months (from January 2011). All readings were performed in the early morning when stem size is at a maximum, thus minimizing possible effects of water movement in the trunk (Chitra-Tarak et al. 2015). One month after installation, we adjusted the band dendrometer to the stem in order to minimize eventual measurement errors. This procedure is necessary because band dendrometers have a tendency to underestimate growth or not measure any growth at all, for a period after installation (Keeland and Sharitz 1993; O’Brien et al. 2008; David and Downes 2009). Thus, the first data were taken 2 months after band dendrometer installation. Despite all these methodological control, water-induced fluctuations can potentially affect dendrometer readings, and all girth growth results have to be interpreted cautiously.
Data analysis
The monthly girth increment for individuals of each species was used to calculate the species month average (with sample size varying between six and 19 individuals). From the accumulate girth increment (total in 22 months) we also calculated the girth growth rate (% of girth growth in the studied period) and the annual radial increment (by interpolating for a period of 12 months), in order to facilitate future comparisons with other studies. We tested for differences among species in accumulate total girth growth for the studied period (22 months; January 2011–October 2012) by a Kruskal–Wallis test. We tested for the relationship between girth current (monthly) increment and climate using a Spearman correlation test (Zar 1999). We correlated the average monthly girth increment of each species with monthly day length (standard measure for region, Fig. 1b), monthly rainfall and mean temperature for the studied (2011–2012, Fig. 1c) and historical period (1978–1999, Fig. 1a). The correlations considering current (annual) and historical climates were performed in order to test if trees respond differently to this two different trigging. Also, considering that plants can show a delayed response to climate variation (Marques and Oliveira 2004), we also correlated girth increment with the lagged rainfall (current and historical), temperature (current and historical) and day length, from 1 to 3 months. In all tests we assumed α = 0.05.
Results
Anatomical structure
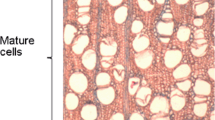
The 10 studied species showed different wood anatomical patterns (Fig. 2; Table 2) and wood specific gravity, which, on average, varied from 0.32 g cm−3 (in Schizolobium parahyba) to 0.70 g cm−3 (Handroanthus serratifolius) (Table 2). Nine species showed diffuse-porous and Citharexylum myrianthum presented semi-ring porous. Five of the species showed distinct growth rings: C. Myrianthum, S. Parahyba, Senna multijuga, Virola bicuhyba, and H. serratifolius. In C. myrianthum, growth rings were marked by gradual differences in vessel diameter between latewood and earlywood, associated with marginal parenchyma bands as well as radially flattened and thick-walled latewood fibers (Fig. 2a, b). S. parahyba had distinct growth rings marked by marginal parenchyma band and by thickening of fiber walls in latewood (Fig. 2c, d). In S. multijuga, the growth rings were characterized by thickening fiber walls and radially flattened latewood fiber, sometimes combined with marginal parenchyma bands (Fig. 2e, f). V. bicuhyba showed distinct growth rings marked by slight and gradual increases in fiber wall thickness (Fig. 2g, h). In H. serratifolius the growth rings were marked by thick-walled latewood fiber, associated with marginal parenchyma bands (Fig. 2i, j). Four species presented scarcely distinct growth rings. In C. canjerana, when visible, these growth rings were characterized by one or more of the following features: differences in fiber wall thickness, changes in vessel diameter and/or fibrous zone (Fig. 2k, l). C. estrellensis, had growth rings with different levels of distinction: distinct and scarcely distinct. Distinct growth rings were marked by gradual fiber wall thickness and decreasing frequency of parenchyma bands towards the latewood, associated with marginal parenchyma bands and fibrous zones (Fig. 2m, n). In some areas these anatomical features were scarcely distinct. Inga edulis, I. marginata and Myrsine coriacea had marked changes in fiber wall thickness, fiber radial size, vessel diameter and/or fibrous zone (Fig. 2o–t). In these species we also observed “false” rings (sometimes forming diffuse ring boundaries), characterized by intra-growth ring density fluctuations, fiber density fluctuations and/or irregular tangential bands of vessels.
Macroscopic (left) and microscopic (right) wood transverse sections tree species. a, b Citharexylum myrianthum; c, d Schizolobium parahyba; e, f Senna multijuga; g, h Virola bicuhyba; i–j Handroanthus serratifolius; k–l Cabralea canjerana; m–n Cariniana estrellensis; o–p Inga edulis; q–r Inga marginata; s–t Myrsine coriacea. Arrows indicate the growth ring boundaries. Scale bar 2500 and 200 μm for macroscopic and microscopic views, respectively
Cumulative and current girth increment
The 10 species showed different patterns of cumulative girth increment along the 22 studied months (Fig. 3). Four species (C. myrianthum, S. parahyba, S. multijuga, and C. estrellensis) exhibited seasonal stem growth, characterized by a period of girth increment and a period with no observed growth (Figs. 3, 4a–c, g). All of these species showed clear growth peaks and the higher increment rates generally during the wettest and hottest period of the year (October–March) and no girth growth during the end of the less wet season and the beginning of the wettest season (June to November) (Fig. 4a–c, g). V. bicuhyba showed a seasonal growth pattern (Fig. 3), with a reduced growth in the winter months (Fig. 3b, d). Three species (M. coriacea, I. marginata and I. edulis) had a continued growth pattern (Fig. 3a), and more than one girth increment events were observed during the year (Fig. 4j, i, h). C. canjerana and H. serratifolius had several small growth peaks during the year (Figs. 3, 4f, e).
Girth current increment (mean ± SD) of 10 tree species over 22 months (from January 2011 to October 2012), in the Atlantic Forest, southern Brazil. Species with distinct growth rings: a Citharexylum myrianthum, b Schizolobium parahyba, c Senna multijuga, d Virola bicuhyba, e Handroanthus serratifolius; species with scarcely distinct (or distinct/scarcely*) growth rings: f Cabralea canjerana, g Cariniana estrellensis, h Inga edulis, i Inga marginata, j Myrsine coriacea. Black and grey bars represent the rainiest (October to March) and the less rainy (April to September) seasons, respectively. Dotted lines represent the period with lowest growth rates and/or dormant period for each species. Note the difference in the y-axis scales. Negative error bars were omitted
Tree species differed in their accumulated girth growth average (H = 46.28; P < 0.0001; Table 3). For example, the girth increment (or annual radial increment) of H. serratifolius was approximately 35 times lower than I. edulis, the species with the highest increment. Higher girth growth were found in I. edulis, C. myrianthum, I. marginata, S. parahyba and M. coriacea and lower girth increment were observed for V. bicuhyba, S. multijuga, C. canjerana, C. estrellensis and H. serratifolius (Table 3). During the observed period, species varied in their growth rates from 1 ± 0.5 % (H. serratifolius) to 15 ± 4.0 % (I. edulis) (Table 3).
Girth increment and climate
The girth increment of the 10 species were, in general, weakly or not related to rainfall (current or historical), but strongly and positively related to temperature (current and historical) and day length for most of species (Table 4). The relationship between growth and climate differed among species: the girth increment of C. myrianthum and S. multijuga and V. bicuhyba was more strongly related to mean temperature and day length (and weakly with rainfall); the increment of I. edulis and S. parahyba were more related to temperature, and the increment of H. serratifolius was related only to rainfall (Table 4). For four species (I. marginata, M. coriacea, C. canjerana and C. estrellensis), no significant correlation was observed (P > 0.05). In general, the girth increment was strongly correlated with the temperature and day length of the same or previous month, suggesting a small delay in growth response (Table 4).
Discussion
Distinctiveness of tree rings varied among the studied species, but was sufficiently clear to mark individual rings in most of species. These rings were delimited by a growth period and non-growth (or less expressive) growth periods. Growth rings were observed in the secondary xylem of reveling anatomical growth marker in all species, as previously seen in other tropical tree species (Alves and Angyalossy-Alfonso 2000). The presence of thick-walled and radially flattened latewood fibers versus thin-walled earlywood was the most common growth ring boundary, commonly found in a great number of tropical species (Worbes 1989; Callado et al. 2001; Lisi et al. 2008). “False” rings as observed in some studied species (especially Inga edulis, I. marginata and Myrsine coriacea) are a ring anomaly relatively frequent in species growing in weakly seasonal tropical regions (Heinrich and Banks 2005). They are explained by temporary growth limitation in a stressful period caused, for example, by low temperatures, flooding (Speer 2010), drought stress (Priya and Bhat 1999; Wimmer et al. 2000), and by abrupt variation from dry to wet period (Masiokas and Villalba 2004). False growth rings are also common in young individuals of pioneer species (Wimmer et al. 2000; Vogel et al. 2001; Lopez et al. 2012) which is the case of I. marginata, M. coriacea and I. edulis (Shimamoto et al. 2014).
Growth ring boundaries can be marked by one or more structural changes or by a combination of characteristics (IAWA 1989; Worbes 1989). This association of two or more wood anatomical features is useful for identifying tree-ring boundaries in tropical species (Stahle 1999; Roig 2000; Vetter 2000). C. myrianthum is a semi-porous species demarcated by marginal parenchyma; this parenchyma was also verified in other tropical trees (Callado et al. 2001). S. multijuga, S. parahyba and H. serratifolius have a combination of latewood thick-walled fibers and marginal parenchyma, previously described for other tropical species (Tomazello-Filho et al. 2004; Marcati et al. 2008; Lisi et al. 2008). C. estrellensis has a decreasing frequency of parenchyma bands towards the latewood resulting, sometimes, in distinct fiber zones (Lisi et al. 2008; Tomazello-Filho et al. 2004). Finally, M. coriacea and V. bicuhyba have latewood fibers with thick walls that are associated or not associated with a fiber zone.
Marginal parenchyma was observed in C. myrianthum, C. estrellensis, S. parahyba and S. multijuga. These cells are related with storage of carbohydrates and transport of nutrients and are necessary for the fast reactivation of cambial cell divisions after a cambial dormancy (Kozlowski et al. 1991; Larson 1995; Dünisch et al. 2002). The presence of marginal parenchyma band in these species could be explained, in some way, by the fast re-growth observed in months with increased temperature and day length. Furthermore, characterizing the nature of the marginal parenchyma is important to accurately determine the growth ring boundary, increasing the confidence of correlations with inductive or inhibiting growth factors (Callado et al. 2013).
The ring-porous structure is frequently associated to autumnal deciduously in trees growing in temperate climates (Boura and De Franceschi 2007). For the studied species we found only one deciduous species presenting semi-ring porous (C. myrianthum), whereas in the two other deciduous species (S. parahyba and H. serratifolia) and in the semi-deciduous species (I. marginata, M. coriaceae, V. bicuyba, S. multijuga, C. canjerana, C. estrellensis) we found diffuse-porous. Thus, as previously reported in other studies (Wheeler and Baas 1993; Poole and van Bergen 2006; Boura and De Franceschi 2007), the relation between deciduously and porosity is possibly much less clear for tropical trees.
The studied species also varied in the timing and magnitude of the girth growth. For example, C. myrianthum, S. parahyba and S. multijuga (and less intensively V. bicuhyba) were characterized by an intense girth growth in the hotter and longer days of the summer and a reduction during the colder and shorter days of the winter. Longer lasting period over 3–5 months was especially observed in both deciduous species (C. myrianthum and S. parahyba), whereas the semi-deciduous and evergreen species presented, in general, a more continuous growth with short interruptions. A similar pattern was previously observed for tropical trees growing in seasonal climates (Mariaux 1969, 1970; Worbes 1995,1999; Botosso et al. 2000) and were associated to a long period of cambial dormancy in deciduous species, and to a short interruption of cambial activity in evergreen species (Alves and Angyalossy-Alfonso 2000; Lisi et al. 2008). However, considering our results, this pattern apparently occurs even in wet and less seasonal climates.
Species with higher accumulated growth, such as I. edulis (evergreen), I. marginata and M. coriacea (semi-deciduous species), were characterized by a continued growing strategy, even in the winter. Meanwhile, a contrasting pattern was observed for both C. myrianthum and S. parahyba (deciduous trees), which also accumulated a large trunk increment, but with growth restricted to spring and summer months. Considering the deciduousness of these five species presenting higher accumulated girth increment (Table 3), it is possible to suggest that evergreen and some semi-deciduous species growing in aseasonal conditions probably photosynthesize during a longer period, including the unfavorable winter season, as recognized previously for other species (Monk 1966, Chabot and Hicks 1982; Escudero and Del Arco 1987; Jurik et al. 1988; Givnish 2002). On the other hand, individuals of deciduous species such as C. myrianthum and S. parahyba presented growth rates similar to growth rates of evergreen and semi-deciduous species. It is possible that these species have a higher rate of photosynthesis per unit leaf mass compared to evergreen species, reducing their transpiration rate during the unfavorable season (Chabot and Hicks 1982; Givnish 2002).
Despite showing a relatively high girth increment in our study (17.43 mm in 22 months), trees of S. multijuga growing in experimental plots of restoration areas showed a much higher growth (36.5 mm in 12 month, Cardoso et al. 2012). It is possible that these differences are imposed by small variations in soil characteristics that can severely limit the tree’s growth (Cardoso et al. 2012). All other species (H. serratifolius, C. estrellensis, C. canjerana and V. bicuhyba) grew very slowly during the year, resulting in a lower accumulated growth after 22 month (<19 mm). Specifically H. serratifolius trees grew much less than previously reported for this species in Seasonal Semi-Deciduous Forest (Lisi et al. 2008; Maria 2002), demonstrating a large spatial variation in tree growth for a given species. Furthermore, variations in tree dimension among species, not considered in analyses, may also explain some differences among species.
The correlation between girth increment and climatic variables varied among species, indicating that species have different sensitivities to climate and day length. For example, whereas the growth of trees of I. edulis, C. myrianthum, S. parahyba, V. bicuhyba, S. multijuga were seasonal and more strongly associated to the temperature and day length, the growth of other species growing seasonally (C. canjerana, C. estrellensis) and species with intermittent growth (I. marginata, M. coriacea, H. serratifolius) did not relate to climate variables in this study. In general, the trees’ growth correlated more tightly with temperature (current or lagged) and day length than to rainfall. This result contrasts previous studies in which cambial growth of tropical trees was associated with the beginning of the wet period (Worbes 1999, 2002). On the other hand, it was already demonstrated that in plants growing in wet tropical climates, small differences in temperature and day length during the year are sufficient to trigger reproductive and vegetative phenological phases (Marques and Oliveira 2008). Thus, it is possible that in tropical areas where there is no dry season, girth increment can potentially occur throughout the year, but the increase in temperature and photoperiod promotes, immediately or lately, a stimulus for the cambium activity for some species.
The growth strategy of the studied species was partially associated with different anatomical and ecological characteristics. C. myrianthum and S. parahyba are species with moderate accumulate growth concentrated in the summer and deciduous leaves, resulting in clear growth ring distinction. Considering that these species lose their leaves in the winter (personal observation), this growth pattern possibly results from a rapid remobilization of carbohydrates to the stems during the winter (Lüttge and Hertel 2009) following the immediate optimization of the stem growth in hotter and longer days of the summer. The girth growth of I. marginata, I. edulis and M. coriacea is high and distributed through the year (not limited by the climate) and is not related with any anatomical characteristics or deciduousness pattern. This strategy seems to result from a low sensitivity of these species to climate limitations, resulting in rapid growth. The species H. serratifolius and C. estrellensis, C. canjerana, V. bicuhyba and S. multijuga grow slowly, resulting in a denser wood with marked growth rings. Probably, this reflects a strategy of slow metabolism and a higher stem resistance to damage or pathogens (Zimmerman et al. 1994).
Our results suggest that these woody species growing in wet climate of the Atlantic forest in southern Brazil, even when there is no water deficit during the less rainy season, can have a marked seasonality in cambium activity and stem growth in the hotter and longer days of summer months. This pattern varies among species and is marginally associated with the tree deciduousness and wood characteristics. In view of the worldwide discussion regarding the future of rainforests, the knowledge of seasonality in tree growth is an important step to dating tropical trees and for providing better comprehension of growth dynamics in tropical ecosystems and its effects on ecosystem processes, such as biomass accumulation, across climate change scenarios.
Author contribution statement
MCMM, PCB and CYS designed the experiment. CYS collected field data. EA, CYS and PCB performed the anatomical analysis. CYS and MCMM performed statistical analysis. All authors together wrote the manuscript and interpreted the data.
References
Alves ES, Angyalossy-Alfonso V (2000) Ecological trends in the wood anatomy of some Brazilian species. I: growth rings and vessels. IAWA J 21:3–30
Baas P, Vetter RE (1989) Growth rings in tropical trees. IAWA Bull 10(2):95–174
Baas P, Wheeler EA (2011) Wood anatomy and climate change. In: Hodkinson TR, Jones MB, Waldren S, Parnell JAN (eds) Climate change, ecology and systematics, Cambridge University Press, New York, pp 141–155
Billings WD (1952) The environment complex in relation to plant growth and distribution. Quart Rev Biol 27:251–265
Borgo M, Tiepolo G, Reginato M, Kuniyoshi YS, Galvão F, Capretz RL, Zwiener VP (2011) Espécies arbóreas de um trecho de Floresta Atlântica do Município de Antonina, Paraná, Brasil. Floresta 41:819–832
Botosso PC, Tomazello-Filho M (2001) Aplicação de faixas dendrométricas na dendrocronologia: avaliação da taxa e do ritmo de crescimento do tronco de árvores tropicais e subtropicais. In: Maia NB, Martos HL, Barella W (eds) Indicadores ambientais: conceitos e aplicações. EDUC, São Paulo, pp 145–17
Botosso PC, Vetter RE (1991) Alguns aspectos sobre a periodicidade e taxa de crescimento em 8 espécies arbóreas tropicais de floresta de Terra Firme (Amazônia). Rev Inst Florest 3:163–180
Botosso PC, Vetter RE, Tomazello-Filho M (2000) Periodicidade e taxa de crescimento de árvores de cedro (Cedrela odorata L., Meliaceae), jacareuba (Calophyllum angulare A.C. Smith, Clusiaceae) e muirapiranga (Eperua bijiga Mart. ex Benth, Leg. Caesalpinioideae) de floresta de terra firme, em Manaus-AM. In: Roig FA (ed) Dendrocronología en América Latina, Mendoza, pp 357–380
Boura A, De Franceschi D (2007) Is porous wood structure exclusive of deciduous trees? C R Palevol 6:385–391
Bruel BO, Marques MCM, Britez RM (2010) Survival and growth of tree species under two direct seedling planting systems. Rest Ecol 18:414–417
Callado CH, Neto SJS, Scarano FR, Costa CG (2001) Periodicity of growth rings in some flood-prone trees of the Atlantic Rain Forest in Rio de Janeiro, Brazil. Trees 15:492–497
Callado CH, Roig FA, Tomazello-Filho M, Barros CF (2013) Cambial growth periodicity studies of South American woody species—a review. IAWA J 34:213–230
Campos JCC (1970) Principais fatores do meio que afetam o crescimento das árvores. Floresta 2:45–52
Cardoso FCG, Marques R, Botosso PC, Marques MCM (2012) Stem growth and phenology of two tropical trees in contrasting soil conditions. Plant Soil 354:269–281
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Ann Rev Ecol Syst 13:229–259
Chitra-Tarak R, Ruiz L, Pulla S, Dattaraja H, Suresh H, Sukumar R (2015) And yet it shrinks: a novel method for correcting bias in forest tree growth estimates caused by water-induced fluctuations. For Ecol Manag 336:129–136
Committee IAWA (1989) IAWA list of microscopic features for hardwood identification. IAWA Bull 10:219–332
Copenheaver CA, Pokorski EA, Currie JE, Abrams MD (2006) Causation of false ring formation in Pinus banksiana: a comparison of age, canopy class, climate and growth rate. For Ecol Manag 236:348–355
Cunningham S, Read J (2003) Comparison of temperate and tropical rainforest tree species: growth responses to temperature. J Biogeogr 30:143–153
David MD, Downes GM (2009) The use of precision dendrometers in research on daily stem size and wood property variation: a review. Dendrochronologia 27:159–172
Dié A, Kitin P, Kouamé FNG, Bulcke JV, Acker JV, Beeckman H (2012) Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann Bot 110:861–873
Dünisch O, Bauch J, Gasparotto L (2002) Formation of increment zones and intra-annual growth dynamics in the xylem of Swietenia macrophylla, Carapa guianensis, and Cedrela odorata (Meliaceae). IAWA J 23:101–119
Escudero A, del Arco JM (1987) Ecological significance of the phenology of leaf abscission. Oikos 49:11–14
Fahn A, Burley J, Longman KA, Mariaux A (1981) Possible contributions of wood anatomy to the determination of the age of tropical trees. In: Bormann FH, Berlyn G (eds) Age and growth rate of tropical trees: new directions for research. Yale University, New Haven, pp 83–100
Ferretti AR, Britez RM (2006) Ecological restoration carbon sequestration and biodiversity conservation: the experience of the Society for Wildlife Research and Environmental Education (SPVS) in the Atlantic Rain Forest of Southern Brazil. J Nat Conserv 14:249–259
Givnish TJ (2002) Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fennica 36:703–743
Gourlay ID (1995) The definition of seasonal growth zones in some african Acacia species: a review. IAWA J 16(4):353–359
Heinrich I, Banks JCG (2005) Dendroclimatological potential of Australian red cedar. Aust J Bot 51:21–32
Jacoby GC (1989) Overview of tree-ring analysis in tropical regions. IAWA Bull 10:99–108
Jurik TW, Briggs GM, Gates DM (1988) Springtime recovery of photosynthetic activity of white pine in Michigan. Can J Bot 66:138–141
Kauano E, Torezan JMD, Cardoso FCG, Marques MCM (2012) Landscape structure in the northern coast of Parana State, a hotspot for the Brazilian Atlantic Forest conservation. Rev Árvore 36:961–970
Keeland B, Sharitz R (1993) Accuracy of tree growth measurements using dendrometer bands. Can J For Res 23:2454–2457
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Academic Press, California
Lamprecht H (1990) Silvicultura nos trópicos; Ecossistemas florestais e respectivas espécies arboreas: possibilidades e métodos de aproveitamento sustentado. Eschborn, Instituto de Silvicultura da Universidade de Gottingen, GTZ, p 130
Larson PR (1995) The vascular cambium. Springer Verlag, Berlin
Liebsch D, Goldenberg R, Marques MCM (2007) Florística e estrutura de comunidades vegetais em uma cronoseqüência de Floresta Atlântica no Estado do Paraná, Brasil. Acta Bot Bras 21:983–992
Liebsch D, Marques MCM, Goldenberg R (2008) How long does the Atlantic Rain Forest take to recover after a disturbance? Changes in species composition and ecological features during secondary succession. Biol Conserv 141:1717–1725
Lisi CS, Tomazello-Filho M, Botosso PC, Roig FA, Maria VRB, Ferreira-Fedele L, Voigt ARA (2008) Tree-ring formation, radial increment periodicity, and phenology of tree species from a seasonal semi-deciduous forest in southeast Brazil. IAWA J 29:189–207
Lopez L, Villalba R, Peña-Claros M (2012) Determining annual periodicity of growth rings in seven tree species of a tropical moist forest in Santa Cruz, Bolivia. For Syst 21:508–514
Lüttge U, Hertel B (2009) Diurnal and annual rhythms in trees. Trees 23:683–700
Marcati CR, Milanez CRD, Machado SR (2008) Seasonal development of secondary xylem and phloem in Schizolobium parahyba (Vell.) Blake (Leguminosae: caesalpinioideae). Trees 22:3–12
Maria VRB (2002) Estudo da periodicidade do crescimento, fenologia e relação com a atividade cambial de espécies arbóreas tropicais de florestas estacionais semideciduais. MSc Dissertation, Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba, University of São Paulo
Mariaux A (1969) La périodicité des cernes dans le bois de limba. Bois et Forêts des Tropiques No 128:39–54
Mariaux A (1970) La périodicité de formation des cernes dans le bois de L’okoume. Bois et Forêts des Tropiques No. 131:37–50
Mariaux A (1977) Marques et rubans dendroètres. Information Technique: 238, CTFT, Morgent-sur-Marne
Mariaux A (1995) Growth periodicity in tropical trees. IAWA J 16:327–328
Marques MCM, Oliveira PEAM (2004) Fenologia de espécies do dossel e do sub-bosque de duas Florestas de Restinga na Ilha do Mel, sul do Brasil. Rev Bras Bot 27:713–723
Marques MCM, Oliveira PEAM (2008) Seasonal rhythms of seed rain and seedling emergence in two tropical rain forests in southern Brazil. Plant Biol 10:596–603
Masiokas M, Villalba R (2004) Climatic significance of intra-annual bands in the wood of Nothofagus pumilio in southern Patagonia. Trees 18:696–704
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons: leaves, stem and wood in relation to taxonomy with notes on economic uses. Clarendon Press, Oxford
Monk CD (1966) An ecological significance of evergreenness. Ecology 47:504–505
Mozeto AA, Fritz P, Moreira MZ, Vetter E, Aravena R, Salati E, Drimmie RJ (1988) Growth rates of natural Amazonian forest trees based on radiocarbon measurements. Radiocarbon 30:1–6
Muller-Landau HC (2004) Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica 36:20–32
O’Brien TP, Feder N, Mccully ME (1965) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
O’Brien JJ, Oberbauer SF, Clark DB, Clark DA (2008) Phenology and stem diameter increment in Costa Rica wet tropical forest. Biotropica 40:151–159
Parolin P, Ferreira LV, Junk WJ (1998) Central Amazonian floodplains: effect of two water types on the wood density of trees. Verh Internat Verein Limnol 26:1106–1112
Poole I, van Bergen PF (2006) Physiognomic and chemical characters in wood as palaeoclimate proxies. Plant Ecol 182:175–195
Priya PB, Bhat KM (1999) Influence of rainfall, irrigation and age on the growth periodicity and wood structure in teak (Tectona grandis). IAWA J 20(2):181–192
Roig FA (2000) Dendrocronología en los bosques del Neotrópico: revisión y prospección futura. In: Roig FA (ed) Dendrocronología en América Latina, Mendoza, pp 307–355
Shimamoto CY, Botosso PC, Marques MCMM (2014) How much carbon is sequestered during the restoration of tropical forests? Estimates from tree species in the Brazilian Atlantic forest. For Ecol Manag 329:1–9
Smith DM (1954) Maximum moisture content method for determining specific gravity of small wood samples. Laboratory report no. 2014. United States Department of Agriculture Forest Service, Forest Products, Madison and Wisconsin
Speer J (2010) Fundamentals of tree-ring research. The University of Arizona Press, Tucson, p 333
Stahle DW (1999) Useful strategies for the development of tropical tree-ring chronologies. IAWA J 20:249–253
Suzuki E (1999) Diversity in specific gravity and water content of wood among Bornean tropical rainforest trees. Ecol Res 14:211–224
Tomazello-Filho M, Lisi CS, Hansen N, Cury G (2004) Anatomical features of increment zones in different tree species in the state of São Paulo, Brazil. Sci Forestalis 66:46–55
van Gelder HA, Poorter L, Sterck FJ (2006) Wood mechanics, allometry, and life-history variation in a tropical rain forest tree community. New Phyt 171:367–378
Vetter RE (2000) Growth periodicity and age of Amazonian tree species. Methods for their determination. In: Roig FA (ed.), Dendrocronología en América Latina, Mendoza, pp 135–155
Vetter RE, Botosso PC (1989) Remarks on age and growth rate determination of Amazonian trees. IAWA Bull 10:133–145
Vogel JC, Fuls A, Visser E (2001) Radiocarbon adjustments to the dendrochronology of a yellowwood tree (Research Letters). S Afr J Sci 97:164–166
Walter H (1983) Vegetation of the earth. Spring-Verlag, Germany
Wheeler E (2011) Insidewood—a web resource for hardwood anatomy. IAWA J 32:199–211
Wheeler E, Baas P (1993) The potentials and limitations of dicotyledonous wood anatomy for climatic reconstructions. Paleobiology 19:487–498
Wimmer R, Strumia G, Holawe F (2000) use false rings in Austrian pine to reconstruct the early growing season precipitation. Can J For Res 30:1691–1697
Worbes M (1989) Growth rings, increment and age of trees in inundation forests, savannas and a mountain forest in the Neotropics. IAWA Bull 10:109–122
Worbes M (1995) How to measure growth dynamics in tropical trees: a review. IAWA J 16:337–351
Worbes M (1999) Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. J Ecol 87:391–403
Worbes M (2002) One hundred years of tree-ring research in the tropics—a brief history and an outlook to future challenges. Dendrochronologia 20:217–231
Worbes M, Staschel R, Roloff A, Junk WJ (2003) Tree ring analysis reveals age structure, dynamics and wood production of a natural forest stand in Cameroon. For Ecol Manag 173:105–123
Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J (2013) Global wood density database 2009. http://hdl.handle.net/10255/dryad.235. Accessed 8 Feb 2013
Zar JH (1999) Biostatistical analysis. Prentice-Hall, New Jersey
Zimmerman JK, Everham EM III, Waide RB, Lodge DJ, Taylor CM, Brokaw NVL (1994) Responses of tree species to hurricane winds in subtropical wet forest in Puerto Rico: implications for tropical tree life histories. J Ecol 82:911–922
Acknowledgments
We thank Juliano Morales de Oliveira, Marcelo Errera, Isabela Galarda Varassin for their suggestions to the first version of the manuscript and to Gabriel A.R. de Paula for support with part of the analysis. We also thank the Brazilian Education Council (Capes/Reuni) for the fellowship to CYS and the Brazilian Agriculture Research Company (Embrapa Floresta) and the NGO Sociedade de Pesquisa em Vida Selvagem e Educação Ambiental (SPVS) for their support and staff assistance during the field work. MCMM received grants from the Brazilian Research Council (CNPq, Grants 304650/2012-9 and 229349-2013-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by G. Piovesan.
Rights and permissions
About this article
Cite this article
Shimamoto, C.Y., Botosso, P.C., Amano, E. et al. Stem growth rhythms in trees of a tropical rainforest in Southern Brazil. Trees 30, 99–111 (2016). https://doi.org/10.1007/s00468-015-1279-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1279-z