Abstract
Key message
Arbuscular mycorrhizal symbiosis can help date palm to cope with the oxidative stress induced by long-term drought due to its capacity to enhance host plant antioxidant defense system.
Abstract
Plant adaptation to water scarcity is mostly the result of existing intrinsic traits. However, mycorrhizal symbiosis is widely believed to provide complementary characteristics that improve host plants protection against the deleterious effect of drought. We previously showed that arbuscular mycorrhizal (AM) date palm seedlings suffered less water stress imposed by short period of water deficiency due to a primary drought avoidance effect by the AM symbiosis related to difference in water and nutrients status between mycorrhizal and non-mycorrhizal plants. The objective of this investigation was to study the impact of long-term drought stress (LTDS, 25 % field capacity) on changes of antioxidant metabolism in non-mycorrhizal and mycorrhizal (colonized with Rhizophagus intraradices or Funneliformis mosseae) date palm seedlings. Obtained results revealed that LTDS induced clear decreases in shoot height (SH), root length (RL) and shoot (SDW) and root (RDW) dry weights. However, AM colonization alleviated the detrimental effect of LTDS on growth performance of date palm seedlings. Moreover, AM colonization mitigated drought-induced oxidative stress by alleviating H2O2 and malondialdehyde (MDA) accumulation and enhancing antioxidant enzymes activities, e.g., catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and guaiacol peroxidase (G-POD). Our results revealed different responses of mycorrhizal seedlings to LTDS depending on AM fungi strains. R. intraradices (Ri) induced the highest SH and RL, the highest SDW and RDW and the highest mycorrhizal dependency. Ri-plants exhibited the lowest MDA and H2O2 contents and the highest CAT, SOD, APX and G-POD activities. Furthermore, Ri-plants showed the lowest oxidative damage and the highest proteins and soluble sugars contents. Thus, AM colonization enhanced date palm strategies involving antioxidant defense system to cope with the LTDS-induced oxidative stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The date palm Phoenix dactylifera L., belonging to the Arecaceae family, represents an important socio-economical and ecological culture for the arid and semi-arid areas of many countries. This tree is considered crucial to the ecosystem as it protects the surrounding vegetation against desert influences and provides adequate microclimate to the under storey crops. Although date palm is thought drought tolerant, water is required to fully realize growth and productivity performance. Indeed, among abiotic stresses, water scarcity is the most severe environmental stress impairing crop development in arid and semi-arid areas where long dry seasons with low water availability adversely affect all plant functions. In response to drought, plants often accumulate reactive oxygen species (ROS) such as superoxide radical (O ·−2 ), hydrogen peroxide (H2O2), hydroxyl radical (OH·) and singlet oxygen (1O2). These harmful species are responsible for many of the degenerative reactions such as lipid peroxidation, protein and nucleic acid oxidization, and metabolic enzyme inactivation (Dhindsa et al. 1981; Smirnoff 1993; Reddy et al. 2004). Plants are endowed with a complex antioxidant system to control the balance between ROS formation and consumption including antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (G-POD), and glutathione reductase (GR) as well as redox metabolites such as ascorbate and glutathione (Polle et al. 1994; Mittler 2002; Roldán et al. 2008). However, under water stress, ROS generation often exceeds the removing capacity of the antioxidant system, leading to oxidative stress and consequently to oxidative damage (Smirnoff 1993; Mittler 2002). It is well established that arbuscular mycorrhizal (AM) fungi can form a symbiotic association with the vast majority of land plants, including those of arid areas. In fact, AM fungi play a critical role in plants’ mineral nutrition and terrestrial ecosystem functioning (Estrada et al. 2013). Once established, AM association benefits host plants not only by improving water uptake and mineral nutrition, but also by increasing plant resistance to drought (Faghire et al. 2010; Estrada et al. 2013; Fouad et al. 2014, Sukesh et al. 2014), soil salinity (Giri et al. 2003) and pathogens (Garmendia et al. 2004). In date palm, AM symbiosis has been recognized to contribute to plantlet establishment and survival as well as improved growth, nutrient and water status under conditions such as drought and poor soil quality (Meddich et al. 2004; Aqqua et al. 2010; Faghire et al. 2010; Baslam et al. 2014). In previous studies mycorrhizal date palm seedlings suffered less water stress imposed by short-term water scarcity. This positive effect was attributed to a primary drought avoidance effect by the mycorrhizal symbiosis related to difference in tissue hydration, cell wall elasticity, leaf water potential and osmotic potential as well as nutrients acquisition, but not related to changes in antioxidant activities between mycorrhizal and non-mycorrhizal plants (Faghire et al. 2010; Baslam et al. 2014). The present investigation assessed the contribution of AM symbiosis to date palm tolerance to long-term drought and the potential induction in mycorrhizal plants of some antioxidant defense involved in oxidative stress alleviation through scavenging ROS accumulation.
Materials and methods
Plant material and treatments
Germinated seeds of date palm (Phoenix dactylifera L.), cv. Khalt, were cultivated in 1 kg pots containing a mixture (v/v) of sand and soil (collected from the palm grove of Marrakesh, Morocco) previously sterilized for 3 h in an oven at 180 °C. Pots were transferred to a greenhouse and placed under a 16 h light regime (600–1400 µmol m−2 s−1), 60–70 % relative humidity, with maximum and minimum temperatures of 32 °C and 24 °C, respectively. Soil characteristics were: pH (H2O) 8.15; 1.25 % organic matter; 4.8 mg kg−1 available phosphorus; 350 mg kg−1 total phosphorus; 141.9 mg kg−1 potassium and 545 µs cm−1 electrical conductivity. After 2 months, the seedlings were divided into three groups of 60 plants each: non-inoculated plants (Nino); plants inoculated with Funneliformis mosseae (Nicolson and Gerdemann) Gerdemann and Trappe (Fm-plant) and plants inoculated with Rhizophagus intraradices (Schenck and Smith) (Ri-plant). The mycorrhizal inocula were provided in the form of spores conserved in sterile soil supplied by the Experimental Station del Zaidin (Granada, Spain), and our own laboratory collection. The AM fungi inocula consisted of rhizospheric soil containing spores and colonized root fragments of Hordeum vulgare L., in amounts of 10 g per pot, which were predetermined to have achieved high levels of root colonization (Faghire et al. 2010). Inoculum from each AM fungus possessed similar infective characteristics (75 % of infected roots and approximately 20 spores g−1 of inoculum). The same amount of autoclaved inoculum was added to Nino-plants. After 3 months, the mycorrhizal and non-mycorrhizal plants were subjected to two water regimes: well water (WW) corresponding to 75 % of field capacity and water stress (WS) corresponding to 25 % of field capacity. The water status of the pots was examined daily and the amount of water lost was replaced (Faghire et al. 2010). Water treatments were applied over 20 weeks.
The experimental design consisted of two water regimes (WW and WS) and three AM fungi treatments (Fm, Ri, and Nino). All treatments were placed randomly in the greenhouse and replicated twenty times.
AM colonization, plant growth and mycorrhizal dependency
At harvest, the fresh roots were washed, cut into 1 cm root pieces, cleared with 10 % (w/v) KOH and then stained with 0.05 % (w/v) trypan blue in lactoglycerol for AM colonization evaluation (Phillips and Hayman 1970). The intensity of root colonization was determined by optical microscopic observations of 20 root fragments. Each root fragment observed was assigned a class note between 0 and 5, corresponding to the estimation of the level of mycorrhizal colonization: Zero was the class note corresponding to 0 % of root colonization, 1 lower than 1 %, 2 between 1 and 10 %, 3 between 10 and 50 %, 4 between 50 and 90 % and 5 more than 90 %. The intensity of root colonization (M %) was calculated using the following formula (Trouvelot et al. 1986):
where N is the total number of fragments observed, and n5, n4, n3, n2 and n1 are the number of fragments rated, respectively, 5, 4, 3, 2 and 1.
Shoot height (SH) and root length (RL) were recorded and then shoot and root materials were dried separately at 80 °C for 48 h to record shoot (SDW) and root (RDW) dry weights. Mycorrhizal dependency was determined as the gain of dry weight (DW) due to mycorrhizal colonization using the following formula (Bagyaraj 1994):
Nutrient content analysis
Dry matter was incinerated at 500 °C for 5 h and the resulting ash digested in 2 M HCl. Phosphorus content was analyzed spectrophotometrically using the molybdate-blue method (Murphy and Riley 1962), K content was estimated using flame spectrophotometer according to Brown and Lilleland (1946) while Ca, Mg, Mn and Cu contents were determined by atomic spectrophotometry (UNICAM 99 AA spectrophotometer).
Antioxidant enzyme assays
Fresh leaves (0.5 g) were frozen in liquid nitrogen and then ground at 4 °C in 5 mL solution containing 0.1 M potassium phosphate buffer (pH 7.0), 0.1 g polyvinylpolypyrrolidone (PVPP), and 0.1 mmol Ethylenediaminetetraacetic acid (EDTA). The homogenate was centrifuged at 18,000×g and 4 °C for 10 min, and the supernatants were kept at −20 °C for subsequent biochemical assays.
Total superoxide dismutase (SOD, EC 1.15.1.1) activity estimation was based on the ability of SOD to inhibit the reduction of nitroblue tetrazolium (NBT) by superoxide radicals generated photochemically (Beyer and Fridovich 1987). One unit of SOD was defined as the amount of enzyme required to halve the reduction rate of NBT at 25 °C.
Catalase (CAT, EC 1.11.1.6) activity was measured by the disappearance of H2O2 in the reaction mixture consisting of 0.1 M potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 20 mM H2O2 (Aebi 1984). The reaction was started by the addition of the enzyme extract and the decrease of H2O2 content was monitored at 240 nm for 3 min.
Ascorbate peroxidase (APX, EC 1.11.1.11) activity was measured in the reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM H2O2, and 0.1 mM ascorbate. Adding the H2O2 started the reaction and the decrease in absorbance at 290 nm was recorded for 1 min to determine the oxidation rate of ascorbate (Amako et al. 1994).
Guaiacol peroxidase (G-POD, EC 1.11.1.7) activity was measured by following the change of absorbance at 470 nm due to guaiacol oxidation. The activity was assayed for 1 min in a reaction solution containing 100 mM potassium phosphate buffer (pH 7.0), 20 mM guaiacol, 10 mM H2O2 and 0.15 ml enzyme extract (Polle et al. 1994). Soluble proteins were measured by the Bradford method (Bradford 1976) using Bovine Serum Albumin as standard.
Hydrogen peroxide (H2O2) content
Hydrogen peroxide content was determined according to Patterson’s method (Patterson et al. 1984) with slight modifications as described by Aroca et al. (2008). Five-hundred milligrams of fresh leaves were homogenized in a cold mortar with 5 mL 5 % TCA containing 0.1 g of activated charcoal and 0.1 g PVPP. The homogenate was centrifuged at 18,000×g for 10 min. The supernatant was filtered through a Millipore filter (0.45 µm). A volume of 1.9 mL of 100 mM potassium phosphate buffer (pH 7) and 1 mL of the colorimetric reagent were added to 0.1 mL of the supernatant. The colorimetric reagent was freshly made by mixing 1:1 (v/v) 0.6 mM potassium titanium oxalate and 0.6 mM 4–2 (2-pyridy-lazo) resorcinol (disodium salt). The samples were incubated at 60 °C for 45 min and the absorbance at 508 nm was recorded. In the blank, leaf extract was replaced by 5 % TCA.
Malondialdehyde (MDA) concentration and oxidative damage to lipid
The malondialdehyde concentration was estimated according to thiobarbituric acid (TBA) reaction described by Dhindsa et al. (1981). Leaf samples (0.5 g) were homogenized in 10 mL of 0.1 % TCA and centrifuged at 18,000×g for 10 min. Two milliliter aliquot of supernatant was mixed with 2 mL of 20 % TCA containing 0.5 % TBA. The mixture was heated at 95 °C for 30 min, cooled and the absorbance of the supernatant was read at 532 nm (A532). The unspecific turbidity was corrected by A600 subtracting from A532. The MDA content was calculated using an extinction coefficient of 155 mM−1 cm−1. Oxidative damage was estimated as the ratio of malondialdehyde to proteins (Dhindsa et al. 1981).
Total soluble sugars and free proline
Total soluble sugars (TSS) and free proline were quantified in 50 mM potassium phosphate buffer (pH = 7.5) extract of fresh leaves (1 g). The extract was filtered through four cheese cloth layers and centrifuged at 38,720×g for 10 min at 4 °C. The supernatant was collected and stored at −20 °C for subsequent analysis. For TSS analysis, 0.1 mL of potassium phosphate buffer (pH = 7.5) extract was mixed with 3 mL freshly prepared anthrone (200 mg anthrone + 100 mL 72 % H2SO4) and placed in a boiling water bath for 10 min according to Irigoyen et al. (1992). After cooling, the absorbance at 620 nm was determined. The calibration curve was made using glucose in the range of 20–400 µg mL−1. Free proline determination was estimated by reacting 5 mL of ninhydrin (3.125 g ninhydrin dissolved in 50 mL of phosphoric acid 6 M and 75 mL of glacial acetic acid) and placed in a boiling water bath for 45 min, then absorbance was read at 515 nm (Paquin and Lechasseur 1979). Free proline concentration was calculated from a calibration curve using proline as standard.
Statistical analysis
Data were analyzed with two-way ANOVA (IBM SPSS 20.0). AM status (A) and Water regime (B) were used as first and second factors, respectively. The significance of differences and interaction between factors were calculated at 5 %. Means comparison was made using least significant difference (LSD) test P < 0.05.
Results
Mycorrhizal colonization, growth parameters and mycorrhizal dependency
The mycorrhizal status of the date palm seedlings was evaluated before and after water treatment application. Microscopic root observations confirmed the absence of mycorrhizal structures in Nino-plants, while Ri-plants and Fm-plants showed typical arbuscular mycorrhizal structures (arbuscules, vesicles and hyphae). The highest root colonization was observed in Ri-plants regardless of water regime (Table 1). Water deficiency significantly reduced the intensity of AM colonization; but the reduction rate was lower in Ri-plants (24 %) compared to Fm-plants (35 %).
Mycorrhizal plants showed higher SH and RL as well as SDW and RDW than Nino-plants both under WW and LTDS (Table 1). Imposed LTDS severely decreased SH, RL, SDW and RDW in Nino-plants; but AM symbiosis significantly improved date palm growth performance. Under a long period of water deficiency, SH, RL, SDW and RDW were, respectively, 2, 2.5, 2.3 and 3.6 times higher in Ri-plants than in Nino-plants (Table 1). Mycorrhizal dependency was highest in Ri-plants under LTDS (Table 1).
Nutrient contents, total soluble sugars and free proline
Leaves’ nutrient contents as well as free proline, proteins and total soluble sugars (TSS) concentrations were very sensitive to LTDS. Water stress induced accumulation of K (Table 2) and proline (Table 3), these increases were more pronounced in mycorrhizal plants than in Nino-plants. On the contrary, a significant decrease of P, Ca, Mg, Mn and Cu contents was observed as a consequence of water stress, but the reduction was more relevant in Nino-plants than in Fm-plants and in Ri-plants. Under LTDS Ri-plants showed the highest K, P, Mg, and Cu contents (Table 2) and the highest proline concentration (Table 3).
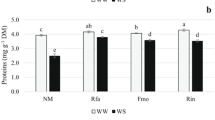
Under WW, soluble proteins and TSS contents were similar in Nino-plants and mycorrhizal plants (Table 3). A significant reduction in proteins and soluble sugars contents both in Nino-plants and AM colonized plants was observed as a consequence of LTDS. This reduction was more prevalent in Nino-plants (37 % for proteins and 44 % for sugars) and lowest in Ri-plants (9.4 % for proteins and 9.6 % for sugars).
Hydrogen peroxide and malondialdehyde contents and oxidative damage
Hydrogen peroxide and MDA levels were steeply increased by LTDS in Nino-plants, but AM colonization alleviated H2O2 and MDA accumulation (Table 4). MDA and H2O2 increases were lower in LTDS Ri-plants (9 and 4.5 %, respectively), than in their relative Nino-plants (52 and 35 %, respectively). The oxidative damage to lipids, estimated as the ratio of MDA to proteins, as a consequence of LTDS was strongly increased in Nino-plants compared to mycorrhizal plants (Table 4). Oxidative damage was two times higher in Nino-plants than in Ri-plants (Table 4).
Antioxidant enzymes (SOD, POD, CAT and APX) activities
Activities of SOD, POD, CAT, and APX significantly increased in mycorrhizal and Nino-plants as a consequence of LTDS (Table 5). This increase was more prominent in mycorrhizal plants especially in water stressed Ri-plant which showed the highest SOD (747.4 U g−1 DM), G-POD (5.87 µmol.g−1 DM min−1), CAT (304.4 nmol g−1 DM min−1) and APX (7.05 µmol g−1 DM min−1) activities (Table 5). Under WW, SOD activity was higher in mycorrhizal than in Nino-plants while the activities of POD, CAT and APX were similar in mycorrhizal and Nino-plant (Table 5).
Discussion
Drought is one of the most adverse factors affecting plants growth and productivity. To adapt and survive periods of drought stress higher plants are endowed with evolved defense mechanisms that include, among other strategies, a battery of enzymatic (e.g., SOD, CAT, APX, G-POD and GR) and non-enzymatic antioxidants, e.g., ascorbate, glutathione (Kar 2011; Sharma et al. 2012). However, long-term drought stress inevitably results in over production of reactive oxygen species (ROS) that can pose a threat to cells by causing oxidization of lipids, DNA, RNA and proteins, leading ultimately to cell death (Smirnoff 1995; Mittler 2002; Cruz de Carvalho 2008; Kar 2011; Sharma et al. 2012).
Arbuscular mycorrhizal (AM) symbiosis is widely believed to provide complementary characteristics that improve host plants’ performance due to the capacity of AM fungi to alleviate the deleterious effects of drought (Wu and Xia 2006; Aqqua et al. 2010; Faghire et al. 2010; Abbaspour et al. 2012; Fouad et al. 2014). The contribution of AM symbiosis to the host plant’s drought tolerance results from a combination of physiological and metabolic effects. This appears to be due to improved water uptake and/or reduced transpiration leading to differences in tissue hydration between mycorrhizal and non-mycorrhizal plants (Ruiz-Lozano 2003; Goicoechea et al. 2004; Faghire et al. 2010; Baslam et al. 2014). However, additional mechanisms have been proposed such as enhanced osmotic adjustment or reduced oxidative damage caused by reactive oxygen species ROS (Ruiz-Lozano 2003; Abbaspour et al. 2012; Fouad et al. 2014).
In a previous study we showed that, under short-term drought, performance of mycorrhizal date palm seedlings was related to their water status (high levels of tissue hydration, water potential, osmotic potential and cell wall elasticity) and nutrients acquisition, but not to changes in their antioxidant metabolism (Baslam et al. 2014). In this study, activity of antioxidant enzymes SOD, CAT, G-POD and APX were higher in mycorrhizal plants than in Nino-plants under LTDS, showing a consistent effect of AM colonization on alleviation of oxidative stress induced by LTDS. Indeed, H2O2 and MDA were less accumulated in water stressed mycorrhizal plants compared to their relative Nino-plants. These results are consistent with previous reports indicating that AM colonization decreased MDA and H2O2 levels and improved membrane integrity in water stressed pistachio plants (Abbaspour et al. 2012). MDA reflects an end product of lipid peroxidation; the low MDA content provides evidence of lower cell membrane damage. Data from this study showed that colonized plants exhibited lower oxidative damage to lipid, expressed as the ratio of MDA to proteins, than Nino-plants.
Avoidance of oxidative stress through preventing ROS accumulation as the most effective approach used by mycorrhizal plants to cope with drought stress has been reported by many authors (Ruiz-Lozano 2003; Reddy et al. 2004; Abbaspour et al. 2012; Fouad et al. 2014). Among the antioxidant enzymes, SOD constitutes the first line of defense against ROS: it converts O2 − into H2O2 which is then eliminated by CAT, APX and G-POD that dismutate H2O2 into water and oxygen (Sharma et al. 2012). The high antioxidant enzyme activities associated with low accumulation of H2O2 and less peroxidation of lipids would explain the performance of mycorrhizal plants, giving proof that AM symbiosis contributed to protect date palm against the oxidative damage induced by long-term drought. This provides evidence that under long periods of drought, AM fungi are essential to help date palm plants to cope with the oxidative stress. However, under short-term drought, performance of mycorrhizal date palm seedlings was found to be related to the high tissue hydration and high nutrient status, but not to changes in antioxidant metabolism (Baslam et al. 2014).
Moreover, P, Mg, Ca and Cu contents were more greatly decreased in Nino-plant than in mycorrhizal plants under LTDS. However, LTDS induced a greater increase of K content in mycorrhizal plants than in Nino-plants. In fact, the most well established benefits of mycorrhizal fungi to the host plant is through the widespread extraradical mycelial network which penetrates more deeply and widely in the soil thereby extending the root surface area and enhancing nutrients acquisition. In addition, mycorrhizal fungi can access forms of nutrients that are unavailable to non-mycorrhizal plants, particularly organic forms of these nutrients, by enhancing decomposition process (Goussous and Mohammad 2009) and increasing root hydraulic conductivity (Graham and Syvertsen 1984). Phosphorus may also be acquired by AM fungal action on increasing dissolution of minerals that contain P (Gholamhoseini et al. 2013). Potassium acquisition may be a consequence of the increased P availability resulting from mycorrhizal activity (Gholamhoseini et al. 2013).
Furthermore, drought stressed mycorrhizal plants showed higher levels of proteins, proline and soluble sugars than their relative Nino-plants. The strong organic osmolytes accumulation in stressed mycorrhizal plants appears to play an important role, jointly with K, in maintaining the cells’ turgor. These osmolytes are also considered as indexes of drought avoidance and antioxidative plant defense responses. Similar results were pointed out by previous reports indicating that AM colonization might alleviate or decrease RNA disassembly by enhancing the non-enzymatic defense system including proline and soluble proteins (Abbaspour et al. 2012).
The high concentrations of TSS found in leaves of inoculated plants may be a consequence of enhanced photosynthetic rates induced by the sink effect of the AM fungal demand for sugars from leaves to roots (Porcel and Ruiz-Lozano 2004). One of the earliest effects of water stress is the decrease of plant photosynthesis that affect the status of root carbohydrates and consequently the rate of AM colonization, since most of the energy used for the development and branching of the hyphae is obtained from photosynthesis (Reddy et al. 2004). In fact, it is widely admitted that drought can reduce AM colonization by inhibiting spores germination and reducing growth and spread of hyphae after colonization has occurred (Abbaspour et al. 2012). However, according to most of the previous reports, there is no threshold value of root colonization for symbiotic efficiency and this depends on the plant and the fungal species involved (Estrada et al. 2013). In this study, AM root colonization of date palm seedlings was significantly reduced by long-term water deficit. These data confirm earlier results in date palm (Meddich et al. 2004; Baslam et al. 2014) and in others species (Goicoechea et al. 2004; Wu and Xia 2006; Lee et al. 2012). Nevertheless, we observed that R. intraradices (Ri) showed a good aptitude to colonize roots of date palm seedlings notably under LTDS conditions, confirming the high level of infectivity of this fungus reported as a better adapted and/or more aggressive colonizer under drought conditions (Roldán et al. 2008). This species (Ri) was also the most efficient in terms of date palm growth performance and protection against the detrimental effects of long period of water deficit. The Ri positive effect is mainly attributed to the improvement of nutrient acquisition and water status under short-term drought (Roldán et al. 2008) as well as to enhanced antioxidant metabolism defense under long-term drought.
Conclusion
The improved growth, the increased soluble sugars, proteins, proline and K contents as well as the enhanced antioxidants enzymes activities and the alleviation of ROS accumulation and oxidative damage in mycorrhizal plants under long-term drought stress is a proof of the capacity of AM symbiosis to increase date palm tolerance to drought stress. R. intraradices was more efficient and better able to support date palm plants to cope with drought than F. mosseae. The contribution of AM fungi to improve date palm tolerance to water stress may be attributed to: (1) enhanced water status and nutrient acquisition under short-term drought (Baslam et al. 2014), and (2) enhanced protection against oxidative stress as evidenced by the increased antioxidant enzyme activities and the alleviation of H2O2 and MDA accumulation in mycorrhizal plants subjected to long-term drought stress.
Author contribution statement
LB and AQ designed the experiments and developed the methodology, LB, MOF and EA collected the data and carried out analysis, LB and AQ prepared the manuscript and CG assisted with data analysis and manuscript preparation.
References
Abbaspour H, Saeidi-Sarb S, Afsharia H, Abdel-Wahhab MA (2012) Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J Plant Physiol 169:704–709
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Aqqua K, Ocampo JA, Garcıa Romera I, Qaddoury A (2010) Effect of saprotrophic fungi on arbuscular mycorrhizal root colonization and seedlings growth in date palm under greenhouse conditions. Acta Hort 882:891–898
Aroca R, Alguacil MD, Vernieri P, Ruiz-Lozano JM (2008) Plant responses to drought stress and exogenous ABA application are modulated differently by mycorrhization in tomato and an ABA deficient mutant (sitiens). Microb Ecol 56:704–719
Bagyaraj DJ (1994) Vesicular arbuscular mycorrhizal: application in agriculture. In: Norris JR, Read DJ, Varma AK (eds) Techniques for the study of mycorrhizal. Academic Press, London, pp 819–833
Baslam M, Qaddoury A, Goicoechea N (2014) Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 28:161–172
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Brown JD, Lilleland O (1946) Uptake determination of potassium and sodium in plant material and soil extracts by Flame photometry. Proc Am Soc 48:341–346
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Estrada B, Aroca R, Barea JM, Ruiz-Lozano JM (2013) Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci 201:42–51
Faghire M, Baslam M, Samri S, Meddich A, Goicoechea N, Qaddoury A (2010) Effect of arbuscular mycorrhizal colonization on nutrient status, water relations and growth of date palm seedlings under water stress. Acta Hortic 882:833–838
Fouad MO, Essahibi A, Benhiba A, Qaddoury A (2014) Effectiveness of arbuscular mycorrhizal fungi in the protection of olive plants against oxidative stress induced by drought. Span J Agric Res 12:763–771
Garmendia I, Goicoechea N, Aguirreolea J (2004) Antioxidant metabolism in asymptomatic leaves of Verticillium-infected pepper associated with an arbuscular mycorrhizal fungus. J Phytopathol 152:593–599
Gholamhoseini M, Ghalavand A, Dolatabadian A, Jamshidi E, Khodaei-Joghan A (2013) Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agr Water Manage 117:106–114
Giri B, Kapoor R, Mukerji KG (2003) Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass and mineral nutrition of Acacia auriculiformis. Biol Fertil Soils 38:170–175
Goicoechea N, Merino S, Sánchez-Díaz M (2004) Contribution of arbuscular mycorrhizal fungi (AMF) to the adaptations exhibited by the deciduous shrub Anthyllis cytisoides under water deficit. Physiol Plant 122:453–464
Goussous SJ, Mohammad MJ (2009) Comparative effect of two arbuscular mycorrhizae and N and P fertilizers on growth and nutrient uptake of onions. Int J Agric Biol 11:463–467
Graham JH, Syvertsen JP (1984) Influence of vesicular-arbuscular mycorrhiza on the hydraulic conductivity of roots of two Citrus root stocks. New Phytol 97:277–284
Irigoyen JJ, Emerich DW, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plantarum 84:67–72
Kar RK (2011) Plant responses to water stress role of reactive oxygen species. Plant Signal Behav 6:1741–1745
Lee BR, Muneer S, Jung WJ, Avice JC, Ourry A, Kim TH (2012) Mycorrhizal colonization alleviates drought-induced oxidative damage and lignification in the leaves of drought-stressed perennial ryegrass (Lolium perenne). Physiol Plant 145:440–449
Meddich A, Oihabi A, Bizid E, Elhadrami I (2004) Role des Champignons mycorhiziens VA dans la tolérance du palmier dattier (Phoenix dactylifera L.) au déficit hydrique. Revue des régions arides 2:640–646
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Paquin R, Lechasseur P (1979) Observations sur une méthode de dosage de la proline libre dans les extraits des plantes. Can J Bot 57:1851–1854
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway Spruce (Picea abies L.). Plant Physiol 106:53–60
Porcel R, Ruiz-Lozano JM (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J Exp Bot 55:1743–1750
Reddy AR, Chaitanya KV, Sumithra JK (2004) Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ Exp Bot 55:33–42
Roldán A, Díaz-Vivancos P, Hernández JA, Carrasco L, Caravaca F (2008) Superoxide dismutase and total peroxidase activities in relation to drought recovery performance of mycorrhizal shrub seedlings grown in an amended semi arid soil. J Plant Physiol 165:715–722
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, antioxidative damage and oxidative defense mechanism in plants under stressful conditions. J Botany. doi:10.1155/2012/217037
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Smirnoff N (1995) Antioxidant systems and plant response to the environment. Smirnoff N (ed) Environment and plant metabolism: Flexibility and acclimation. Bios Scientific Publishers, Oxford, pp 217–243
Sukesh Kavya M, Sharma Samhitha, Chandrashekar KR (2014) Inoculation effect of arbuscular mycorrhizal fungi on the growth of Vatica chinensis L.: a critically endangered species of Western Ghats. Trees 28:381–388
Trouvelot A, Kough J, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un système radiculaire: Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA Press, Paris, pp 217–221
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Acknowledgments
This work has been financially supported by the Moroccan National Center for Scientific and Technical research (CNRST) project RS19/2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The studies presented in this publication were supported financially by the Moroccan National Center for Scientific and Technical Research (Centre National de la Recherche Scientifique et Technique, CNRST) as part of the research project RS19/2011. The terms of the project agreement were reviewed and approved by the University of Marrakech and the CNRST.
Additional information
Communicated by P. Courty.
Rights and permissions
About this article
Cite this article
Benhiba, L., Fouad, M.O., Essahibi, A. et al. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 29, 1725–1733 (2015). https://doi.org/10.1007/s00468-015-1253-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1253-9