Abstract
Background
Levamisole is less expensive and has a better toxicity profile compared to other steroid sparing agents used in nephrotic syndrome. It has a plasma half-life of 2.0 to 5.6 hours, but is conventionally administered on alternate days. We aimed to assess whether daily levamisole is safe and more effective than standard alternate-day therapy in maintaining remission in children with frequently relapsing or steroid-dependent nephrotic syndrome (FR/SDNS).
Methods
An open-label randomized controlled trial was conducted in children with FR/SDNS. Group A received daily while Group B received alternate-day levamisole (2–3 mg/kg/dose) for 12 months. Prednisolone was tapered off by 3 months. Patients were monitored for relapses, further steroid requirement, and adverse effects.
Results
A total of 190 children with FR/SDNS (94 in Group A and 96 in Group B) were analyzed. Sustained remission for 12 months was observed in 36% of Group A and 27% of Group B patients (p = 0.18). Numbers completing 12 months in the study were 67% in Group A and 56% in Group B (p = 0.13). Time to first relapse, persistent FR/SDNS, and withdrawal due to poor compliance were statistically similar in both groups, while relapse rate and cumulative steroid dosage were significantly lower in Group A compared to Group B (p = 0.03 and p = 0.02, respectively). The incidence of adverse effects was comparable in both groups, with reversible leucopenia and hepatic transaminitis being the commonest.
Conclusions
Daily levamisole therapy was not superior to alternate-day therapy in maintaining sustained remission over 12 months. Nevertheless, relapse rate and cumulative steroid dosage were significantly lower without increased adverse effects.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrotic syndrome (NS) is the most common chronic, recurring kidney disease occurring in children. While over 80% patients respond to corticosteroids, more that 50% of these have frequent relapses (FR) or become steroid dependent (SD) and may develop multiple steroid toxicities [1]. Steroid sparing agents (SSA) used effectively and early in FR/SDNS have the potential to prevent excessive steroid exposure [1, 2].
Levamisole (LEV) as a steroid sparing agent has been used for decades in pediatric steroid-sensitive nephrotic syndrome (SSNS). Prior reports show that it is safe and effective [3] and can reduce the relative risk of relapse by 48% [4]. A significant advantage, particularly in developing countries, is its much lower cost in comparison to other SSAs like mycophenolate mofetil (MMF), cyclosporine, tacrolimus, and rituximab. In addition, essential monitoring requirements are simpler, require less infrastructure, and are also less expensive than for most other SSAs. All recent SSNS guidelines include alternate-day LEV as an option for use in FR/SDNS [1, 2, 5].
Conventionally, LEV is administered on alternate days; however, the basis for this dosing is unclear. Pharmacokinetic studies indicate that its plasma half-life is about 2.0 to 5.6 hours [6, 7]. Several observational studies [8,9,10,11,12] and one cross-over study [13] have reported effective use of daily therapy without increase in adverse effects. However, randomized controlled trials (RCT) of daily vs. alternate-day therapy do not exist. The aim of our study therefore was to assess whether daily LEV is safe and more effective than conventional alternate-day therapy, in maintaining remission in FR/SDNS, in an RCT.
Methods
An open-label RCT with 1:1 allocation was conducted in children between 1 to 18 years of age with FR/SDNS. Included patients were required to be steroid sensitive, with normal serum creatinine for age. Patients with secondary NS, steroid-resistant NS, active infections, or other systemic diseases and those receiving SSAs within the last 1 year were excluded.
Standard criteria were used to define NS, steroid sensitivity, relapse, and remission [1, 2]. Frequent relapses were defined as 2 or more relapses in first 6 months after onset, or 3 or more relapses in 1 year (not counting the first episode), and infrequent relapses as < 3 relapses per year [2]. Steroid dependence was defined as 2 or more relapses occurring while on steroid therapy or within 14 days of discontinuation [1]. The study participants were identified and followed up in the out-patient department of the Institute of Child Health, Kolkata, India.
Sample size calculation
A recent RCT dealing with similar subjects of NS treated with alternate-day LEV vs. placebo was used as the reference to assess the sample size [14, 15]. The relapse free rate at the end of 1 year with alternate-day LEV in this RCT was 26%. We hypothesized that a further increase of 20% (to 46%) in the relapse free rate may be possible by our daily dosing intervention. Assuming a superiority margin of 20%, power of the study at 80%, and α of 0.05, we would need a sample size of 174 evaluable subjects, 87 randomized to each group. Assuming an attrition rate of about 10%, we proposed to enroll a minimum of 96 subjects in each arm.
Randomization was by computer-generated sequence and allocation concealment by using serially numbered opaque envelope method. A block randomization strategy was undertaken with a fixed block size of 12. The person allocating the serially numbered envelopes was independent of the physicians responsible for clinical management and follow-up.
The primary outcome measure was the number of participants having sustained remission at 12 months, while secondary outcome measures were (1) time to first relapse, (2) relapse rate per month, (3) cumulative steroid dose required per kg body weight per month, and (4) adverse effects.
Levamisole 2–3 mg/kg/day was started on a daily (Group A) or alternate day (Group B) basis as per randomization, once the child was in NS remission, and continued for 12 months. Home urine monitoring, with recording of urine protein and LEV and prednisolone doses, in “Nephrotic diaries” was maintained on a daily basis by the parents. Prednisolone was weaned off by 3 months. Further relapses were treated with prednisolone 2 mg/kg/day until remission, followed by 1.5 mg/kg/alternate day for 4 weeks, 1.0 mg/kg/alternate day for 2 weeks, 0.5 mg/kg/alternate day for 2 weeks, 0.25 mg/kg/alternate day for 2 weeks. For patients in Group B, LEV was administered on days alternating with prednisolone while they were on steroids.
Monthly review of clinical status, complete blood count, and liver function tests were performed for 12 months. During the COVID-19 lockdown period, monitoring was performed by phone and video calls, in association with local laboratories and pediatricians, and data crosschecked from diaries at the next physical visit. Any adverse effects were recorded, and participants were requested to report/attend in case of any rash, infectious symptoms, joint pain, NS relapse, or any other unexpected event.
Early withdrawal from study was planned if there was persistent FR/SDNS despite LEV therapy or significant adverse effects, including severe infection requiring admission, leucopenia/ neutropenia, or hepatic transaminitis persisting for ≥ 2 weeks. Leucopenia was defined as total leukocyte count < 4000/ml, neutropenia as absolute neutrophil count < 1500/ml, and hepatic transaminitis as alanine transferase (ALT) level more than 3 times normal laboratory range. Levamisole intervention was considered to have failed in patients who required early withdrawal due to the above reasons. On the other hand, LEV intervention was considered successful if there were no or infrequent relapses, and these patients were continued in the study for 12 months.
Statistical analysis
Categorical data were summarized using frequencies and percentages while continuous data were summarized using median and interquartile ranges (IQR), since the majority of the data had non-normal distribution pattern. Chi-squared test was used to compare proportions, while Mann–Whitney U test was used for intergroup comparison of non-parametric variables. Log rank test was used for comparing the time to event occurrence between the groups, and the Kaplan–Meier plot was used to plot the trends in the time to event analysis. Statistical significance was considered at p < 0.05. IBM SPSS version 17 software was used for statistical analysis.
Ethics
Informed consent/assent was obtained from all guardians/participants and the Declaration of Helsinki was adhered to. Approval was obtained from the institutional review board of the Institute of Child Health, Kolkata, and the study was registered in the Clinical Trials Registry of India (CTRI/2015/10/006257).
Results
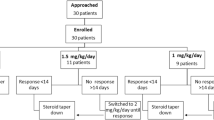
Between May 2017 to Feb 2020 and Jan 2021 to April 2022, a total of 216 FR/SDNS patients were approached for trial entry (recruitment stopped between March 2020 and December 2020 due to COVID-19 pandemic issues). Of these, 196 consented to participate and started on LEV after randomization (98 in each group).
Four patients (2 in Group A and 2 in Group B) were subsequently excluded for follow-up of less than 2 months post randomization. Two further patients from Group A were excluded as they were changed to alternate-day therapy within the first 2 months by their local doctors, and steroids were not tapered. Thus, a total of 190 patients who had therapy as per study protocol were analyzed for outcome measures up to study completion or study withdrawal (Fig. 1).
The median age at NS onset was 2.67 (IQR 2 to 4) years, median age at study entry was 4.54 (3.2 to 6.1) years, and 62% of the subjects were male. In Group A, 2 patients had previously received MMF, and one had received cyclophosphamide, while in Group B, 2 patients previously received cyclophosphamide, all more than 1 year prior to study entry. For all other patients, LEV was the first SSA prescribed. Baseline characteristics at study entry were similar in both groups (Table 1).
Levamisole was given orally at median dose of 2.52 (2.49 to 2.82) mg/kg daily in Group A and 2.68 (2.41 to 3.01) mg/kg every alternate day in Group B.
Comparison of efficacy
The total number of patients completing 12 months on LEV, with no or infrequent relapses, was 117 (62%). This included 63 (67%) of Group A and 54 (56%) of Group B (p = 0.13) (Fig. 1). Of these patients, at the end of 1 year of study participation, 60 (32%) children were in sustained remission, 34 (36%) of Group A and 26 (27%) of Group B (p = 0.18) (Supplementary Table 1).
Eighteen (19%) patients in Group A and 27 (28%) patients in Group B were withdrawn from the study due to persistent FR/SDNS (p = 0.19). In addition, 8 (8%) patients in Group A and 10 (10%) in group B were withdrawn due to poor compliance/follow-up (p = 0.65) (Fig. 1). The median time to first relapse computed using Kaplan–Meier and log rank test was 8 (95% CI, 4.29 to 11.72) months in Group A and 5 (95% CI, 3.11 to 6.9) months in Group B (p = 0.162) (Fig. 2).
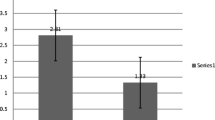
The NS relapse rate was 0.08 (0 to 0.17) per month in Group A vs. 0.08 (0 to 0.32) per month in Group B (p = 0.03). The cumulative steroid dosage was 6.7 (3.03 to 12.86) mg/kg/month in Group A vs. 9.3 (3.96 to 19.87) mg/kg/month in Group B (p = 0.02). Both the latter were significantly lower in Group A compared to Group B (Figs. 3 and 4 and Supplementary Table 1).
Exploration of efficacy in sub-groups
Assessment of factors that may predict LEV response revealed that children with sustained remission on LEV were older, with lower number of relapses and less steroid requirement prior to study inclusion. Overall, 50/130 (38%) children with FRNS vs. 10/60 (17%) children with SDNS at baseline were in sustained remission at 12 months (p = 0.003) (Supplementary Table 2). In patients with prior FRNS, 26/67 (39%) in Group A vs. 24/63 (38%) in Group B had sustained remission (p = 0.94), while in patients with prior SDNS, 8/27 (30%) in Group A vs. 2/33 (6%) in Group B had sustained remission (p = 0.015) (Supplementary Table 3).
Comparison of safety
The majority of patients tolerated LEV without any adverse effects. Four patients (3 in Group A and 1 in Group B) had neutropenia, which reversed within 2 weeks of stopping LEV in 2 and within 4 weeks in 2 patients. Levamisole was restarted in the first 2 patients where the neutropenia was mild, associated with upper respiratory tract infection symptoms, and there was no recurrence of neutropenia subsequently.
Gradual reduction of hemoglobin from 13.2 g/dl to 9.6 g/dl occurred over 4 months in one girl on daily LEV, RBC indices and morphology were normal, and investigations did not reveal any specific cause; stoppage of LEV resulted in normalization of hemoglobin level.
Hepatic transaminitis was seen in 2 patients in Group A and 3 in Group B. A maximum ALT level of 620 IU/L within 1 month of study entry occurred in one patient in Group B; searches for other etiological factors were negative. In all patients, the ALT level reverted to normal within 4 weeks of stopping LEV.
One child in Group A had Varicella infection; LEV was stopped for 3 weeks and then restarted, and she completed 1 year of study participation with no further relapses. One child in Group A developed a rash with fever and a clinical diagnosis of Stevens-Johnson syndrome was made; he recovered with supportive treatment and was withdrawn from the study. Another patient in Group B developed a rash, tested positive for ANA, and was withdrawn from study. Both the latter 2 patients were negative for ANCA tests (Supplementary Table 4).
Discussion
Levamisole is an accepted therapy in FR/SDNS, for its steroid sparing effects. Based on prior evidence, guidelines suggest its alternate-day dosing, despite a relatively short plasma elimination half-life [1, 2, 5]. Our RCT compared LEV administered on a daily basis to a conventional alternate-day regimen, in a real-world situation. The results show that the number of NS patients who had sustained remission in the 12 months of study participation was statistically similar in both groups. Time to first relapse and incidence of adverse effects were also similar; however, the relapse rate as well as the cumulative steroid dosage was significantly reduced in patients who received daily LEV.
Levamisole, a synthetic imidazole derivative, has been used as an anthelminthic, reportedly since 1969. While its definitive mechanism of action in SSNS is unknown, it has been shown to have immunomodulatory effects by altering Th1 and Th2 immune responses. Effects on B cell activity and direct effects on the podocytes have also been demonstrated [16, 17].
The first RCT showing benefit of LEV in SDNS was published by the British Association for Pediatric Nephrology in 1991 [18]. The Cochrane meta-analysis, updated in 2020, analyzed a total of 8 RCTS and concluded that LEV “compared with steroids or placebo may reduce the number of children with relapse during treatment (RR 0.52, 95% CI 0.33 to 0.82) (low certainty evidence)” [4]. The RCT published by Gruppen et al. in 2018 was a well powered study with minimal bias, comparing alternate-day LEV therapy to placebo. This demonstrated that time to relapse was significantly increased in the LEV group (hazard ratio 0.22 [95% confidence interval 0.11–0.43]), while 6% of placebo patients vs. 26% LEV patients had sustained remission at 12 months [14, 15]. The latter results are similar to our control patient group who received alternate-day therapy and had sustained remission in 27%.
The reason for conventional intermittent therapy of LEV in NS (given twice weekly in some early publications and subsequently every alternate day) is unclear. Pharmacokinetic studies in adults (healthy or suffering from cancer or malaria) indicate that peak plasma concentrations are reached within 2 hours of receiving a single oral dose of 150 mg or 2.5 mg/kg, and the elimination half-life of LEV ranges from 4 to 5.6 hours. In contrast, a recent study in a pediatric NS population indicated that median peak plasma concentration of LEV occurred at 1.65 (IQR 1.32–2.0) hours while median plasma elimination half-life was 2.6 (IQR 2.06–3.65) hours [6]. Although it is acknowledged that biological half-life may be longer than these reported plasma elimination half-lives, probably due to effect of LEV metabolites, this is yet untested. Thus, it was logical to assess clinically whether daily LEV therapy may yield additional benefits in FR/SDNS.
Several observational studies in FR/SDNS have reported that daily LEV therapy is safe [8, 10], with some [9, 12] suggesting that daily therapy may reduce relapse rate and steroid dosage in patients where alternate-day therapy has not been effective. Further, a cross-over study in patients who were relapsing on alternate-day LEV reported reduction in relapses with daily LEV plus alternate-day steroids [13]. One small RCT found daily LEV to be non-inferior to MMF in reducing relapses [19]. However, a head-to-head RCT comparing daily with alternate-day LEV in FR/SDNS has not been conducted before. Our RCT failed to show superiority of daily LEV in maintaining sustained remission in FR/SDNS patients. Although daily dosing may improve compliance, alternate-day dosing reduces cost by half which is a definite advantage. Therefore, we would suggest that patients continue to be treated initially with alternate-day dosage as per current guidelines. However, since relapse rate and cumulative steroid doses were reduced on daily LEV therapy in our study, this schedule may be considered in patients who relapse on alternate-day therapy, prior to switching to other SSAs. This could be a valuable option in developing countries as other SSAs are associated with significantly increased cost factors.
Our exploration into effect in subgroups showed that, overall, LEV was more effective in maintaining sustained remission in FRNS compared to SDNS. This effect has also been noted in previous studies [8, 15]. In addition, our results suggest that daily LEV may be more effective than alternate-day LEV in SDNS; trials with larger numbers of SDNS patients are required to confirm this effect.
In agreement with previous studies, the commonest adverse effects in our patients were neutropenia and hepatic transaminitis [1,2,3]. White blood count and liver function tests returned to normal ranges within a few weeks of cessation of LEV. One patient developed rash with ANA positivity, and another was admitted with probable Stevens-Johnson syndrome. ANCA tests in both these participants were negative. Although no other patient in the study had clinical features suggestive of ANCA-related vasculitis, in cognizance with recent guidelines [1, 2], 20 of the last patients to complete the trial had ANCA tests at 12 months. Of these, pANCA was positive in 1 patient on daily and 1 patient on alternate-day LEV and in both became negative on re-testing after 3 months. These patients are included in a separate report on ANCA results in patients on long-term LEV [20]. A couple of recent articles have reported a rise in creatinine on LEV [21, 22], but with cystatin C levels remaining normal [22]. Our protocol did not include monitoring for kidney function other than to confirm normal baseline levels for age at study entry, or if clinically indicated thereafter, and no clinical issues with significant rise in serum creatinine occurred.
Limitations
This is a single-center study with an open-label design; therefore, bias cannot be completely eliminated; however, blinding was not possible for us due to logistic reasons. In addition, the limitations encountered in the COVID pandemic caused a break in recruitment for 10 months and hampered physical follow-up and monitoring, maximally during the lockdown period of March to May 2020. During those months, monitoring shifted to online phone calls and videos; tests were done locally and sent to us by WhatsApp and email and medications adjusted if needed in coordination with local pediatricians; patients were asked to attend physically only if in dire need. However, at the earliest opportunity, patient diaries for ascertaining relapses/remission and drug doses were physically reviewed and data confirmed. Our sample size calculation allowed for an attrition rate of 10%, with a final 87 patients in each arm, and we were able to achieve numbers close to this projection (86 in each arm after attritions), despite the COVID pandemic (Fig. 1).
Conclusions
Our RCT results indicate that in FR/SDNS patients treated with LEV, 36% of patients on daily therapy vs. 27% of patients on alternate-day therapy had sustained remission at 12 months (the primary outcome measure); however, this difference was not statistically significant. In contrast, secondary outcome measures such as monthly relapse rate and cumulative steroid dosage were significantly reduced in the daily LEV group, without increase in adverse effects. Thus, daily LEV therapy may be considered in patients who have persistent FR/SDNS despite treatment with alternate-day LEV, prior to changing to other SSAs. Further evidence is required to assess if daily LEV may be more effective than alternate-day therapy in SDNS patients specifically.
Data availability
All original data is available for review with the Research Advisory Committee of Institute of Child Health, Kolkata, India, email: ichrac23@gmail.com.
References
Sinha A, Bagga A, Banerjee S et al (2021) Steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatr 58:461–481. https://doi.org/10.1007/s13312-021-2217-3
Trautmann A, Boyer O, Hodson E et al (2023) IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 38:877–919. https://doi.org/10.1007/s00467-022-05739-3
Bhatt GC, Patel B, Das RR, Malik S, Bitzan M, Mishra NR (2023) Efficacy and safety of levamisole in childhood nephrotic syndrome: a meta-analysis. Indian J Pharmacol 55:43–52. https://doi.org/10.4103/ijp.ijp_673_21
Larkins NG, Liu ID, Willis NS, Craig JC, Hodson EM (2020) Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 4(4):CD002290. https://doi.org/10.1002/14651858.CD002290.pub5
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO (2021) (2021) clinical practice guideline for the management of glomerular diseases. Kidney Int 100:S1–S276. https://doi.org/10.1016/j.kint.2021.05.021
Kreeftmeijer-Vegter AR, Dorlo TP, Gruppen MP, de Boer A, de Vries PJ (2015) Population pharmacokinetics of levamisole in children with steroid-sensitive nephrotic syndrome. Br J Clin Pharmacol 80:242–252. https://doi.org/10.1111/bcp.12607
Kouassi E, Caillé G, Léry L, Larivière L, Vézina M (1986) Novel assay and pharmacokinetics of levamisole and p-hydroxylevamisole in human plasma and urine. Biopharm Drug Dispos 7:71–89. https://doi.org/10.1002/bdd.2510070110
Ekambaram S, Mahalingam V, Nageswaran P, Udani A, Geminiganesan S, Priyadarshini S (2014) Efficacy of levamisole in children with frequently relapsing and steroid-dependent nephrotic syndrome. Indian Pediatr 51:371–373. https://doi.org/10.1007/s13312-014-0419-7
Fu LS, Shien CY, Chi CS (2004) Levamisole in steroid-sensitive nephrotic syndrome children with frequent relapses and/or steroid dependency: comparison of daily and every-other-day usage. Nephron Clin Pract 97:c137–c141. https://doi.org/10.1159/000079172
Sümegi V, Haszon I, Iványi B, Bereczki C, Papp F, Túri S (2004) Long-term effects of levamisole treatment in childhood nephrotic syndrome. Pediatr Nephrol 19:1354–1360. https://doi.org/10.1007/s00467-004-1608-8
Moorani KN, Zubair AM, Veerwani NR, Hotchandani HJ (2020) Efficacy of levamisole in children with frequent relapsing and steroid dependent nephrotic syndrome at tertiary care center-Karachi. Pak J Med Sci 36:1193–1198. https://doi.org/10.12669/pjms.36.6.2337
Kiruba Samuel EM, Krishnamurthy S, Bhanudeep S, Muske S (2017) Levamisole in frequently-relapsing and steroid-dependent nephrotic syndrome. Indian Pediatr 54:831–834. https://doi.org/10.1007/s13312-017-1144-9
Abeyagunawardena AS, Karunadasa U, Jayaweera H, Thalgahagoda S, Tennakoon S, Abeyagunawardena S (2017) Efficacy of higher-dose levamisole in maintaining remission in steroid-dependant nephrotic syndrome. Pediatr Nephrol 32:1363–1367. https://doi.org/10.1007/s00467-017-3616-5
Gruppen M, Davin JC, Bouts A (2016) Levamisole increases time to relapse in children with steroid sensitive idiopathic nephrotic syndrome: results of a multicenter double blind placebo controlled randomized control trial [Abstract]. Pediatr Nephrol 31:1753
Gruppen MP, Bouts AH, Jansen-van der Weide MC et al (2018) A randomized clinical trial indicates that levamisole increases the time to relapse in children with steroid-sensitive idiopathic nephrotic syndrome. Kidney Int 93:510–518. https://doi.org/10.1016/j.kint.2017.08.011
Mühlig AK, Lee JY, Kemper MJ et al (2019) Levamisole in children with idiopathic nephrotic syndrome: clinical efficacy and pathophysiological aspects. J Clin Med 8:860. https://doi.org/10.3390/jcm8060860
Khan GH, Veltkamp F, Scheper M, LEARNS Consortium et al (2023) Levamisole suppresses activation and proliferation of human T cells by the induction of a p53-dependent DNA damage response. Eur J Immunol 53:e2350562. https://doi.org/10.1002/eji.202350562
British Association for Paediatric Nephrology (1991) Levamisole for corticosteroid-dependent nephrotic syndrome in childhood. Lancet 337:1555–1557
Singh J, Afzal K, Abqari S (2020) Daily levamisole versus mycophenolate mofetil in patients with frequently relapsing or steroid-dependent nephrotic syndrome: an open-label non-inferiority randomized controlled trial. Asian J Pediatr Nephrol 3:43–48. https://doi.org/10.4103/2589-9309.305896
Sinha R, Sarkar S, Banerjee S et al (2024) Antineutrophil cytoplasmic antibody in children with nephrotic syndrome treated with levamisole: a cross-sectional cohort study. Pediatr Nephrol. https://doi.org/10.1007/s00467-024-06362-0
Hoogenboom LA, Webb H, Tullus K, Waters A (2021) The effect of levamisole on kidney function in children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 36:3799–3802. https://doi.org/10.1007/s00467-021-05231-4
Veltkamp F, Bökenkamp A, Slaats J, Hamer H, Bouts AHM, LEARNS Consortium (2022) Levamisole causes a transient increase in plasma creatinine levels but does not affect kidney function based on cystatin C. Pediatr Nephrol 37:2515–2519. https://doi.org/10.1007/s00467-022-05547-9
Acknowledgements
We sincerely thank Ms. Adrita Saha for administrative coordination and Dr Sanjukta Poddar for assistance with data collection. We are very grateful to Dr Elisabeth M Hodson (Children’s Hospital at Westmead, Sydney, Australia) and Dr Susan Samuels (University of Calgary, Alberta, Canada), for reviewing the manuscript and for their valuable suggestions.
Funding
This was an academic, investigator-initiated trial supported by internal research funds of the Institute of Child Health, Kolkata, India.
Author information
Authors and Affiliations
Contributions
SB, JS, RSi, and AP conceptualized the study. SC contributed to study design, protocol drafting, randomization process, and statistical analysis. All other authors were involved in protocol writing, clinical follow-up, and data collection. The first draft of the manuscript was written by SB and subsequently reviewed and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banerjee, S., Sengupta, J., Sinha, R. et al. Daily compared with alternate-day levamisole in pediatric nephrotic syndrome: an open-label randomized controlled study. Pediatr Nephrol 39, 2969–2977 (2024). https://doi.org/10.1007/s00467-024-06402-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-024-06402-9