Abstract
Hypertension in children with neuroblastoma is uncommon and can be difficult to control due to the potential for multiple underlying causes. We present the case of a pediatric patient with high-grade neuroblastoma which was complicated by hypertensive emergency. The patient had imaging suggestive of renal artery compression, as well as significantly elevated normetaphrine levels. Multiple anti-hypertensive agents, including an angiotensin converting enzyme inhibitor, α- and β-adrenergic receptor blockers, and a tyrosine hydroxylase inhibitor, were initiated prior to tumor excision. While her blood pressure improved during the post-operative period, she continued to require multiple antihypertensive medications due to residual tumor burden. In this report, we highlight the importance of careful, multidisciplinary management to avoid peri-operative complications in patients with catecholamine-producing tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case
Initial presentation
An 8-year-old female who recently emigrated to a new country to seek treatment for an intra-abdominal high-risk neuroblastoma presented to the emergency department with elevated blood pressure (BP), headache, and confusion. Her parents report that she had been diagnosed with hypertension and started on captopril 2 months prior at the time of her oncologic diagnosis in their home country. While arranging subspecialty appointments in this new country, her parents had been monitoring BP at home and stated that her systolic pressures typically ranged from 140–160 mmHg but have been higher in the past 24 h. She was not seen by a Pediatric Nephrologist before this presentation and had no prior workup for secondary causes of her hypertension.
In the emergency department, her initial BP was 185/130 mmHg when using an auscultatory device and appropriately sized cuff on the right arm. She complained of a headache and was slow to respond to questions, but a review of the systems was otherwise negative for vision changes, sweating, palpitations, abdominal pain, nausea, vomiting, diarrhea, and hematuria.
Hospital course
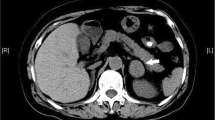
Due to concern for hypertensive emergency, she was transferred to the intensive care unit. Initial workup for etiology of hypertension revealed serum potassium was 4.1 mmol/L, bicarbonate level was 26 mmol/L, and serum creatinine level was 0.38 mg/dL. Urinalysis had no protein or blood. Plasma renin activity was 0.88 ng/mL/hr (normal 0.25–5.82) and plasma aldosterone concentration was undetectable (normal ≤ 9 ng/dL). Spectral Doppler ultrasound of the left kidney was abnormal with elevated peak systolic velocity of 822 cm/s (normal 100–180) in the left renal artery and an elevated renal to aortic ratio of 7.1 (normal < 3.5). Spectral Doppler of the right kidney was normal with peak systolic velocity of 157 cm/s. The resistive indices of right and left renal arteries were normal at 0.62 and 0.55 (normal 0.5–0.7), respectively. This suggested left renal artery compression by the tumor as a potential cause of hypertension.
To safely lower blood pressure in the setting of hypertensive emergency, the patient was first treated with a nicardipine drip. Once BP had stabilized, enteral medications were titrated to sustain normotension. In addition, she was continued on captopril due to beneficial effects in the setting of suspected unilateral renal artery compression. At this time, she was receiving amlodipine 0.5 mg/kg every 12 h, labetalol 15 mg/kg/day, captopril 0.75 mg/kg every 8 h, and a 0.2 mg/day clonidine patch.
Computed tomography scan imaging of the tumor showed a large, multi-lobulated, retroperitoneal mass that was encasing major vasculature including the aorta and bilateral renal arteries. The patient was started on induction chemotherapy (including cyclophosphamide, topotecan, cisplatin, etoposide, vincristine, doxorubicin, and dexrazoxane). Upon completion of induction, it was determined that the tumor could not be completely resected due to its relationship with major blood vessels. She was scheduled for surgical debulking of the mass with a plan for eventual stem cell collection and tandem autologous stem cell transplant.
Despite improvement in overall blood pressure control during her hospitalization, the patient continued to have intermittent episodes of extreme hypertension lasting between 5 min and several hours. These episodes were accompanied by severe headaches, altered mental status, and facial flushing. Based on these symptoms, the decision was made to evaluate for excess catecholamine production. Laboratory testing of fractionated plasma metanephrine levels revealed normal free metanephrine level 0.39 nmol/L (normal < 0.5 nmol/L) and elevated free normetanephrine levels 24 nmol/L (normal < 0.9 nmol/L). The episodes of symptomatic hypertension responded well to treatment with intravenous phentolamine (0.02 mg/kg).
To prepare this patient with multifactorial hypertension for surgical tumor debulking, multi-disciplinary discussions took place between Anesthesia, Critical care, Nephrology, Endocrinology, General surgery, and Pharmacy. The surgical team anticipated that complete resection would be impossible due to the tumor’s relationship to major abdominal vasculature and a left nephrectomy would be required. Resecting a large portion of the tumor would hopefully decrease, although not completely eliminate, the exogenous catecholamine production. The nephrology team recommended a radionucleotide study with Tc-99 m MAG3 to assess the contribution of the patient’s left kidney to overall renal function. It showed differential renal function of 89% on the right and 11% on the left with a calculated glomerular filtration rate of 60.2 ml/min/1.73 m2. This suggested that the patient could likely tolerate a left nephrectomy without a large decrease in kidney function. Discussion with the anesthesia and endocrinology teams emphasized the importance of minimizing the physiologic impact of catecholamine release by maximizing pharmacologic α-adrenergic and β-adrenergic blockade because manipulation of the tumor during surgical resection can cause additional catecholamine release and dangerous hypertension. With this in mind, the critical care team altered the patient’s existing antihypertensive medication regimen using guidance from an institutional algorithm for peri-operative management of catecholamine-secreting tumors (Fig. 1). This included starting doxazosin (a selective α1 blocker) and titrating her other anti-hypertensives to target BP between 25 and 50th percentile, minimize tachycardia, and allow for normalization of intravascular volume. Despite maximally tolerated doses of these medications, the patient continued to have hypertension > 95th percentile, so she was initiated on metyrosine for catecholamine depletion 12 days prior to surgery. Metyrosine was up-titrated in accordance with institutional protocol until the patient had an acute dystonic reaction with neck stiffening. At this point, metyrosine was held for 24 h and then restarted at the previously tolerated dose without further up-titration. Her final doses of anti-hypertensive medications prior to surgery were as follows: metyrosine 155 mg every 6 h, labetalol 15 mg/kg/day, doxazosin 1 mg every 8 h, captopril 0.75 mg/kg every 8 h, and 0.1 mg/day clonidine patch. She was no longer receiving amlodipine. Peri-operative, her blood pressure ranged from 94 to 112/50 to 73 mmHg.
The patient received all of her enteral antihypertensive medications on the morning of the procedure. She underwent partial tumor resection (greater than 50% tumor burden was removed) and left nephrectomy without intra-operative hemodynamic instability. Post-operatively, she remained persistently hypertensive and BP was controlled with esmolol and nicardipine drips until she could tolerate oral medications. After recovery from surgery, her antihypertensive regimen included amlodipine 0.5 mg/kg/day, labetalol 15 mg/kg/day, doxazosin 2 mg twice a day, and a 0.1 mg/day clonidine patch. Her blood pressures 2 weeks after surgery ranged from 92 to 124/49 to 85 mmHg without episodes concerning for catecholamine surge.
Discussion
We describe the case of a child with neuroblastoma complicated by hypertension due to catecholamine release and left renal artery compression. Throughout her hospital course, she received multiple classes of medications aimed at controlling her multifactorial hypertension, including an angiotensin converting enzyme (ACE) inhibitor, α- and β-adrenergic receptor blockers, a calcium channel blocker, an α2-agonist, and a tyrosine hydroxylase inhibitor.
The initial medication started in this patient by previous providers was captopril, an ACE inhibitor. Common first-line medications for primary hypertension include ACE inhibitors, angiotensin receptor blockers, or calcium channel blockers [1]. However, medication choice may differ if secondary hypertension due to a specific cause is suspected. As in this patient with imaging findings suggestive of unilateral renal artery compression, renin–angiotensin–aldosterone–system blockade may be considered for first-line therapy [2].
Despite initial improvement in overall blood pressure control, this patient continued to have frequent short bouts of severe and symptomatic hypertension during the time that she remained inpatient for chemotherapy. The classic triad of episodic headache, sweating, and tachycardia with paroxysmal hypertension should prompt evaluation for a catecholamine-secreting tumor [3]. Although catecholamine excess is more commonly associated with pheochromocytomas or paragangliomas, neuroblastomas have also been associated with hypertension due to elevated circulating catecholamines [4, 5]. In addition, catecholamine release peaks more frequently during chemotherapy due to tumor tissue remodeling [3].
Hospitals commonly utilize an algorithm or standardized protocol for peri-operative blood pressure management in patients with catecholamine-secreting tumors (Fig. 1). However, this patient had hypertension due to multiple etiologies and she was already receiving an extensive regimen of antihypertensive medications when excess catecholamine production was diagnosed. This made it less straightforward to follow the algorithm and necessitated careful interdisciplinary discussions. Multi-disciplinary care is essential for the management of patients with severe hypertension due to neuroblastoma to minimize the increased risk of peri-operative morbidity and mortality.
Summary
What is new?
-
Rarely, neuroblastomas can be catecholamine-secreting tumors. Prior to tumor removal, patients require optimal α-adrenergic blockade and catecholamine depletion. Careful, multidisciplinary peri-operative management is essential in avoiding complications.
References
Fox C (2021) Pediatric hypertension. Prim Care 48:367–378. https://doi.org/10.1016/j.pop.2021.04.001
Mannemuddhu SS, Ojeda JC, Yadav A (2020) Renovascular hypertension. Prim Care 47:631–644. https://doi.org/10.1016/j.pop.2020.08.009
Kwok SY, Cheng FW, Lo AF, Leung WK, Yam MC, Li CK (2014) Variants of cardiomyopathy and hypertension in neuroblastoma. J Pediatr Hematol Oncol 36:e158–e161. https://doi.org/10.1097/MPH.0b013e318290c628
Liu J, Zurakowski D, Weldon C, Umaretiya P, Holzman R, Lin YC (2023) Perioperative hypertension and anesthetic management in patients undergoing resection of neuroblastoma. Paediatr Anaesth 33:577–582. https://doi.org/10.1111/pan.14673
Garcia-Carbonero R, Matute Teresa F, Mercader-Cidoncha E, Mitjavila-Casanovas M, Robledo M, Tena I, Alvarez-Escola C, Arístegui M, Bella-Cueto MR, Ferrer-Albiach C, Hanzu FA (2021) Multidisciplinary practice guidelines for the diagnosis, genetic counseling and treatment of pheochromocytomas and paragangliomas. Clin Transl Oncol 23:1995–2019. https://doi.org/10.1007/s12094-021-02622-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elliott, E., Rogal, S., Song, B. et al. Blasting off: persistent hypertension in a child with neuroblastoma. Pediatr Nephrol 39, 1085–1088 (2024). https://doi.org/10.1007/s00467-023-06188-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06188-2