Abstract
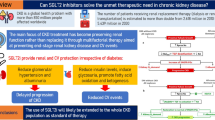
Chronic kidney disease (CKD) is a global public healthcare concern in the pediatric population, where glomerulopathies represent the second most common cause. Although classification and diagnosis of glomerulopathies still rely mostly on histopathological patterns, patient stratification should complement information supplied by kidney biopsy with clinical data and etiological criteria. Genetic determinants of glomerular injury are particularly relevant in children, with important implications for prognosis and treatment. Targeted therapies addressing the primary cause of the disease are available for a limited number of glomerular diseases. Consequently, in the majority of cases, the treatment of glomerulopathies is actually the treatment of CKD. The efficacy of the currently available strategies is limited, but new prospects evolve. Although the exact mechanisms of action are still under investigation, accumulating data in adults demonstrate the efficacy of sodium-glucose transporter 2 inhibitors (SGLT2i) in slowing the progression of CKD due to diabetic and non-diabetic kidney disease. SGLT2i has proved effective on other comorbidities, such as obesity, glycemic control, and cardiovascular risk that frequently accompany CKD. The use of SGLT2i is not yet approved in children. However, no pathophysiological clues theoretically exclude their application. The hallmark of pediatric CKD is the inevitable imbalance between the metabolic needs of a growing child and the functional capacity of a failing kidney to handle those needs. In this view, developing better strategies to address any modifiable progressor in kidney disease is mandatory, especially considering the long lifespan typical of the pediatric population. By improving the hemodynamic adaptation of the kidney and providing additional beneficial effects on the overall complications of CKD, SGLT2i is a candidate as a potentially innovative drug for the treatment of CKD and glomerular diseases in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a global public healthcare concern [1, 2]. As the burden of disease is constantly increasing across all age groups, shortcomings in health care systems become evident, calling for changes in kidney care delivery, management, and prevention. Although representing a small proportion of the total CKD population, children warrant special attention. Slowing the progression of kidney disease would have substantial benefits in delaying the need for kidney replacement therapy [3, 4] and the associated morbidity and mortality. The efficacy of the currently available strategies is limited, but new prospects evolve. Accumulating data in adults demonstrate the efficacy of sodium-glucose transporter 2 inhibitors (SGLT2i), also known as glifozins, in slowing the progression of CKD due to diabetic and non-diabetic kidney disease. Here, we review the available evidence about the efficacy of this class of drugs in adults and discuss their mechanisms of action as well as the potential implications for the use of SGLT2i in the pediatric population, with a particular focus on patients affected by glomerulopathies.
Glomerulopathies in children
Glomerulopathies represent the second most common cause of CKD in the pediatric population, following congenital anomalies of the kidney and urinary tract (CAKUT) [1, 2, 5, 6]. Other causes include ciliopathies, atypical hemolytic-uremic syndrome, and nephrolithiasis/nephrocalcinosis that collectively contribute to less than 10% of cases [1]. The hallmark of glomerulopathies is urinary abnormalities, which can develop profound clinical manifestations, e.g., overt nephrotic syndrome. However, since proteinuria, hematuria, and mild to moderate CKD are frequently asymptomatic, the epidemiological burden of glomerulopathies, and their role as causes of CKD is probably underestimated.
Pathomechanisms and disease progression
Current classifications of glomerulopathies relate mostly to a small number of different histopathological patterns, but attempts are being made to define categories that better link pathogenesis, treatment options, and prognosis. For example, glomerulopathies include (1) podocytopathies, encompassing a broad spectrum of etiologies, including genetic abnormalities, circulating/permeability factors and immunologic dysfunction, infectious agents, and toxic drugs [7], with different prevalence across infancy and childhood; (2) Immune-mediated primary glomerular diseases (e.g., IgA nephropathy, membranous nephropathy, C3 glomerulopathy), all showing immune deposits on kidney biopsy; (3) systemic diseases with secondary glomerular involvement (e.g., lupus nephritis, vasculitis, metabolic storage diseases, diabetic nephropathy). Diagnosis and patient stratification should complement information supplied by kidney biopsy with clinical data and etiological criteria [7]. Of note, genetic determinants of glomerular injury are particularly relevant in children, comprising abnormalities that are responsible for kidney damage per se (e.g., rare variants with large effect-size on podocyte, collagen, and other phenocopy genes) [8,9,10] and common and polymorphic variants conferring susceptibility to glomerular diseases and CKD (e.g., risk alleles in APOL1, polymorphisms in UMOD, or in complement genes) [11]. In addition, increasing evidence suggests that multiple mechanisms with different effect-size can determine or amplify glomerular injury from other causes, prompting multifactoriality even in children [12]. Prematurity and low birth weight can be associated with low nephron endowment even in the absence of clinically detectable CAKUT. Low nephron mass leads to podocyte damage, proteinuria, and secondary focal segmental glomerulosclerosis (FSGS) [13]. A subclinical low number of nephrons lacks the capacity to sufficiently compensate upon damage-related nephron loss [14,15,16]. In addition, the overall survival of children treated in pediatric Intensive Care Units for complex kidney diseases, as well as in oncohematology settings, has markedly improved over time [2, 17]. ICU care involves exposure to nephrotoxic medications, episodes of acute kidney injury (AKI), invasive surgery, and procedures that significantly contribute to early-determined kidney damage by causing nephron loss. Furthermore, obesity represents a rising plague in the pediatric population. Besides predisposing to diabetes and hypertension, obesity is a recognized determinant of glomerular hyperfiltration, increased podocyte shear stress, and accelerated CKD progression [18]. Irrespective of the pathophysiology of glomerular injury, all of these conditions can cause accumulating damage, which implies a reduction of viable kidney cells and functional nephrons, ultimately leading to an imbalance between the metabolic needs of the body and the functional capacity of a failing kidney. It is of note that in growing children, metabolic needs substantially increase with time, while at the same time in progressive CKD the kidney’s capacity to handle metabolic load declines. This imbalance rapidly exploits all adaptive mechanisms of the remaining nephrons to maximize filtration and reabsorption. As a result, the hemodynamic and metabolic load per nephron increases as the child grows and as more nephrons get lost. Therefore, single nephron overload is a shared pathomechanism and potential target for therapeutic intervention in all forms of CKD irrespective of its underlying causes such as podocytopathies, immune-mediated diseases, or systemic disorders [19], not only in adults but in particular in growing children.
Current treatment options
The ideal goal of treatment is complete remission of proteinuria and excretory kidney function especially when kidney disease develops secondary to a systemic disorder [20, 21]. Complete remission of the disease is paramount in minimizing injury-related nephron loss so that the additional workload for the remaining nephrons remains within their capacity for adaptation, which could ensure favorable long-term kidney and global outcomes. Treatment must target the cause of glomerular injury whenever possible. This fits quite well with immune-mediated glomerulopathies (either primary or secondary) that can be effectively addressed with immunosuppressive therapies (i.e., steroids and second-line immunosuppressants, plasma exchange, eculizumab) that should anyway be tailored to each individual patient in order to obtain clinically relevant results while limiting toxicity. Enzyme replacement therapy in Fabry disease and coenzyme Q10 supplementation in FSGS due to coenzyme Q10-related gene mutations are illustrative of other disease-targeted treatments [7]. In addition, in some cases, the primary cause cannot be addressed (e.g., genetic podocytopathies, Alport syndrome) or is putative or unknown (e.g., non-genetic FSGS), hampering the possibility to target it and dramatically limiting treatment efficacy. For all these reasons, whenever interventions aimed at targeting the specific cause of glomerulopathies are limited or inefficient, focusing on slowing the progression toward dialysis or transplantation represents the primary goal of treatment.
So far, renin–angiotensin–aldosterone system inhibitors (RAASi) have represented the only available strategy to reduce cardiovascular (CV) morbidity and mortality, control hypertension, and slow disease progression in different types of glomerulopathies, including genetic podocytopathies, as well as in other forms of CKD [19]. However, a subset of patients with glomerular diseases progress despite optimal RAASi therapy, suggesting that current treatment options are largely insufficient in halting CKD and ensuring a favorable long-term prognosis [2]. Additional strategies to address this shortcoming are based on intensive blood pressure control and amelioration of metabolic balance [19], but the results are mostly unsatisfying. While treatment with vitamin D receptor activators [22], selective endothelin receptor antagonists [23, 24], finerenone [25], and bardoxolone [26] are under investigation, SGLT2i have been proposed recently as an additional treatment for controlling CKD progression in diabetic and non-diabetic glomerular diseases in the adult population [12, 27]. Although the exact mechanisms of action are still under investigation, the promising therapeutic profile renders SGLT2i as intriguing new therapeutic options for glomerular diseases handling and CKD management, with potential applications also in children.
Efficacy of SGLT2i in the adult population
A number of large randomized controlled trials demonstrated the efficacy of SGLT2i on CV and kidney outcomes in patients with type 2 diabetes mellitus (T2DM) at an unprecedented rate [28, 29] (Table 1). In particular, the CREDENCE trial demonstrated that patients with T2DM, CKD, and albuminuria receiving canagliflozin in addition to RAASi had a lower risk of the composite outcome of kidney replacement therapy (KRT), CKD progression, or death from kidney or CV causes in comparison to controls [29]. Interestingly, upon an immediate decline of estimated glomerular filtration rate (eGFR), the slope of decline in eGFR was lower compared to controls, suggesting a nephroprotective effect of SGLT2i, which should translate into a longer kidney life-span. Of note, the initial drop in eGFR was associated with a reduction in albuminuria. A post hoc analysis showed that early reduction in albuminuria is independently associated with favorable long-term kidney and CV outcomes [30]. Recent data provided evidence that the nephroprotective effect of dapaglifozin was remarkable across all levels of eGFR and proteinuria [31, 32].
The beneficial effects of SGLT2i observed in the high-risk population for CKD progression and kidney failure included in the CREDENCE trial (T2DM with CKD) raised the question whether SGLT2i efficacy would translate also to CKD patients without diabetes. Indeed, this SGLT2 transporter could be involved in the progression of CKD from other causes [12]. Recently, the potential role of SGLT2i in CKD was investigated in a randomized, double-blind, placebo-controlled, multicenter clinical trial, the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD). This trial represents an innovative point of view on the pathophysiology, use, efficacy, and safety of this class of drugs in patients with CKD and nephropathies different from the diabetic one [33]. The trial aimed at evaluating the effects of dapagliflozin on kidney and CV events in patients affected by different nephropathies, with eGFR between 25 to 75 mL per minute per 1.73 m2 of the body surface area and proteinuria measured as an albumin-to-creatinine ratio of 200–5000 mg/g at enrollment [33, 34]. Underlining the importance of therapy with RAAS-blocking drugs, ACE inhibitors (ACE-I) and angiotensin receptor blockers (ARB) were used in all patients enrolled unless contraindicated, suggesting that the hemodynamic and metabolic effects exerted by SGLT2i may have a greater weight on the progression of CKD. The DAPA-CKD trial proved the efficacy of SGLT2i in reducing kidney disease progression, CV events, and deaths in patients with CKD, irrespective of the presence of T2DM and the degree of CKD when added to RAASi. Of note, the trial included patients with different glomerulopathies, such as IgA nephropathy (IgAN), FSGS, membranous nephropathy (MN), and minimal-changes (MC) [33]. Interestingly, 270 patients with IgAN (biopsy proven in 94% of cases) as a main cause of CKD were enrolled, representing the third-largest group of participants [35, 36]. A pre-specified analysis of this group showed a reduction in the risk of progression of CKD (eGFR decline, occurrence of KRT) in patients treated with SGLT2i, with an effect size of 57% on the composite kidney endpoint and a good safety profile [36]. Patients who had previously undergone immunosuppressive therapy were excluded, eliminating the confounding effect of any previous treatment different from ACE-I or ARB and emphasizing the combined effect of these classes of drugs with SGLT2i. Of note, several trials (STOP-IgAN trial and TESTING) have questioned the effectiveness of immunosuppressive therapy in IgAN, especially when balancing the risk of side effects [37, 38]. In this view, the results provided by the DAPA-CKD trial suggest a profound revision of the therapeutic approach to IgAN.
Although striking, the results of the DAPA-CKD trial in the group of patients with IgAN do present some limitations. Firstly, kidney biopsy was performed a long time before enrollment in the study in many patients; therefore, other factors contributing to CKD progression cannot be ruled out, limiting the generalizability of the results. As an example, almost one-third of patients with IgAN also had T2DM that could have acted as an accelerator of disease progression. Secondly, the adverse events in the IgAN population enrolled were very low (3 participants died—2 from CV death and 1 was hospitalized for heart failure) and the slope of eGFR reduction in the placebo group was significantly higher than that reported in other clinical studies on IgAN (STOP-IgAN). This makes it difficult to assess the real effects of the drug in this specific subgroup, which could therefore have been overestimated [36].
The DAPA-CKD trial also included 115 patients with FSGS, 43 with MN and 11 with MC [35]. Although no specific sub-analysis for each of these categories of patients is available, the cumulative effect of SGLT2i was comparable to that in IgAN patients. Previous study reported a reduced expression of SGLT2 either in humans or in rodents with FSGS not associated with diabetes, hypothesizing that SGLT2i may not affect renal hemodynamic function or proteinuria in these patients [39]. Of note, it was an explorative study with ten patients and 8 weeks of observation. In fact, the same group then published the DIAMOND trial, the first randomized double-blind clinical study of SGLT2i on 53 patients with non-diabetic CKD (including patients with IgAN, FSGS, hypertensive nephropathy, and other nephropathies), who were on stable RAASi blockers at baseline. The authors found that 6 weeks of treatment with dapagliflozin did not affect proteinuria but induced an acute and reversible decline in measured GFR and a reduction in body weight, paving the way for exploring the results in subsequent clinical trials, including the DAPA-CKD trial [40]. Although additional studies are needed to verify these observations, understanding the role and the response of SGLT2i in patients with FSGS and other non-diabetic nephropathies is crucial to frame their therapeutic space in slowing CKD progression, especially when immunosuppressive therapy fails to obtain remission.
Finally, patients with advanced CKD (i.e., stage IV CKD) are very vulnerable to CV events and mortality, including those due to progression of kidney damage. In previous trials, such as CREDENCE, only patients with CKD up to stage III with diabetes were enrolled [29]. The DAPA-CKD enrolled patients with CKD stage IV (14% of patients included in the study), showing favorable efficacy and safety of SGLT2i also in this population. These results are consistent with those provided by studies evaluating the effect of glifozins in patients with heart failure. In particular, the EMPEROR study showed a significant improvement in kidney endpoints and CV mortality in the treatment arm in comparison to placebo in patients with hypertension, left ventricular hypertrophy, and preserved ejection fraction [41]. Of interest, the latter are typical features of patients with CKD.
The DAPA-CKD trial has recognized SGLT2i as a promising class of drugs, not only in helping to slow CKD progression, but also acting on other comorbidities (obesity, glycemic control, CV risk) that frequently accompany CKD. Of note, adult patients with normal kidney function and normoalbuminuria, type 1 diabetes mellitus (T1DM), autosomal dominant polycystic kidney disease, lupus nephritis, and vasculitis, who could theoretically benefit from the same therapeutic effects of glifozins, were not included in the DAPA-CKD study. Therefore, the role of these drugs in slowing the progression of kidney damage is debated and still under investigation [42].
Mechanisms of action of SGLT2i and their role in CKD progression
In the last decade, basic research studies provided evidence of the possible mechanisms of action of SGLT2i in reducing proteinuria and limiting CKD progression. More recently, their use in large clinical trials confirmed their effectiveness in treating diabetes, heart, and kidney diseases [28]. While the diuretic effect can explain the benefits observed in patients with heart failure and CV disease, the mechanisms in support of the results in patients with isolated kidney disorders may involve other biological effects.
Most studies of this class of drugs have been conducted in experimental and human models of diabetes mellitus [43]. By selectively blocking the SGLT2 protein, glifozins prevent sodium-coupled glucose reabsorption in the proximal tubule, leading to glycosuria, lowering blood glucose, and facilitating weight loss [19]. Diabetic rodents showed an increased expression of SGLT2 in the proximal tubule [27]. Conversely, results on protein expression in humans have been conflicting [39, 44]. However, the clinical evidence of effectiveness also in non-diabetic patients affected by CKD made clear that the nephroprotective effects of SGLT2i are probably independent of the glucose-lowering effect [27]. Indeed, many other possible mechanisms of action have been proposed and the list is yet to be completed (Fig. 1) [45]. Since the mechanisms of action of SGLT2i can be modulated during kidney growth, their role can putatively change over time from childhood to adulthood.
The tubulo-glomerular feedback mechanism
The most commonly accepted and important protective mechanism of SGLT2i is the modulation of tubulo-glomerular feedback. Tubulo-glomerular feedback is a pivotal mechanism of homeostasis since it allows GFR to adapt to modifications in blood pressure and volume. There is a paucity of data about the modifications of this mechanism during kidney development and growth in humans. However, studies performed in ovines suggested that the tubulo-glomerular feedback is active during fetal life and that a reduction in sensitivity allows GFR to increase after birth [46, 47]. Emerging evidence suggests the involvement of tubulo-glomerular feedback in the progression of CKD. Nephron loss of any cause leads to a functional overload in the remaining nephrons, causing intraglomerular hypertension, glomerular hypertrophy, and further glomerular damage [48]. By blocking glucose reabsorption, SGLT2i induces a coupled reduction in sodium reuptake in the proximal tubule. The increased concentration of sodium in the tubular lumen is sensed by the macula densa, which in turn activates negative feedback (tubulo-glomerular feedback) that reduces intraglomerular pressure, hyperfiltration, and proteinuria [49]. The main mediator of tubulo-glomerular feedback is thought to be adenosine, a potent vasoconstrictor of the glomerular afferent artery [50, 51]. The rationale for using SGLT2i also in non-diabetic subjects comes from the observation that hyperfiltration is common also in different conditions, such as obesity and hypertension without overt concomitant proteinuria or CKD [52, 53]. In these patients, studies on lithium handling by kidney tubules showed an increase in reabsorption in the proximal tubule, which represents an indirect sign of hyperfiltration. Moreover, these patients show an increased risk of developing microalbuminuria, further suggesting glomerular overload [52]. In obese patients, reducing proximal tubule sodium reabsorption by acetazolamide has provided evidence that modulation of tubulo-glomerular feedback is possible, supporting the rationale of SGLT2i implementation in the treatment of glomerular diseases other than diabetic nephropathy [54]. Furthermore, there is experimental evidence that chronic use of SGLT2i reduces the activity of the Na ++ /H + exchanger 3 in the proximal tubule, potentially leading to improvement in controlling acid–base balance and protecting from CKD progression [55]. The net result of all glomerular hemodynamic changes induced by SGLT2i is responsible for the clinical manifestation of an eGFR drop of approximately 4–6 mL/min/1.73 m2 recorded in clinical trials in diabetic and non-diabetic subjects at the beginning of the treatment [45, 49]. Of note, this eGFR reduction is transitory, suggesting that it is secondary to a hemodynamic effect and not evidence of kidney injury [56]. Furthermore, the drop is reversible and the decreased rate of annual eGFR reduction observed during follow-up may actually be the expression of kidney function preservation [56].
Proximal tubule workload reduction
In the last decade, there has been a shift of attention to the proximal tubule as the primary sensor and effector in the progression of CKD as well as AKI. An interesting hypothesis of the protective mechanism of SGLT2i is related to metabolic and workload changes in the proximal tubule. The reduction of sodium and glucose reabsorption mediated by SGLT2i leads to a decrease in proximal tubule energy demand, reducing cellular workload and limiting the risk of developing hypoxia [57]. Indeed, the lower incidence of AKI observed in large clinical trials in treated arms and the reduction in the release of urinary markers of proximal tubular damage during SGLT2i therapy support this hypothesis [57, 58]. In addition, the reduced uptake of metabolites in proximal tubules leads to an increase in the metabolic activity of the thick ascending limb of the loop of Henle and of oxygen requirements in the outer medulla, resulting in the activation of the hypoxia-inducible-factor 2 (HIF-2) pathway. This brings about a reduction of anemia and a rise in erythropoietin production and hematocrit in patients on SGLT2i [59]. Since metabolic stress is directly involved in hypoxia signaling and inflammation, this mechanism of nephroprotection probably applies to all forms of CKD.
The “fuel” theory
In addition to the aforementioned metabolic changes, the “fuel hypothesis” has been proposed as an additional theory to explain the mechanism of action of SGLT2i in the kidney. Briefly, by reducing glycemia, glycosuria would cause an increase in glucagon and a reduction in insulin secretion, that in turn result in an increase in lipid oxidation and production of ketone bodies, in particular β-OH-butyrate. The shift in kidney metabolism from fat and glucose oxidation toward energy-efficient ketogenesis is thought to improve cellular efficiency and function [56]. This mechanism is present also in non-diabetics, albeit attenuated by higher insulin levels [45]. Furthermore, it has been proposed that ketogenesis actually represents a marker of transcriptional changes of protective compensatory mechanisms for the kidney via the sirtuin 1-AMPK-HIF-2a axis [45, 60]. According to this, the urinary level of β-OH-butyrate correlates to the increase in erythropoietin [57]. Moreover, ketones such as β-OH-butyrate also suppress kidney inflammation, e.g., through attenuating the activation of the NLRP3 inflammasome and IL-1beta production in monocytes [61]. Finally, ketones indicate improved mitochondrial functioning possibly linked to the metabolic adjustment induced by the SGLT2i [57]. These mechanisms need to be more extensively studied in humans in order to characterize all the complex pathways involved [45, 60].
Improvement of blood pressure control
A large meta-analysis in patients with T2DM showed that SGLT2i induces a mean reduction in systolic and diastolic blood pressure of approximately 2.5 and 1.5 mmHg, respectively [62]. There is also evidence that nocturnal dipping is re-established in some patients [62]. These effects seem independent of the concomitant use of other antihypertensive medications and the starting blood pressure levels [62]. Blood pressure reduction by SGLT2i is likely a multifactorial mechanism. Recently, the DAPASALT trial showed that in a strictly selected cohort of diabetic patients without CKD, dapaglifozin does not increase net urinary sodium loss, probably by activating distal tubular reabsorption, thus questioning the causal relationship between natriuresis and blood pressure control elicited by these drugs [63]. The effects of SGLT2i on the RAAS system are still unclear and need to be further evaluated in relation to pre-existing therapy with RAASi. In fact, conflicting results emerged from the assessment of systemic and intrarenal RAAS activation in diabetic patients, impairing the extension of the observations to other patient populations [64]. Beside this, therefore, weight loss, improved glycemic control, the resulting reduction in arterial inflammation and stiffness, and improved endothelial regulation have been proposed as additional factors participating in blood pressure control beside osmotic diuresis [65, 66].
Additional actions and potential effects in CKD
The clinical use of SGLT2i provided evidence of additional effects that could widen their therapeutic fan, even besides the role in slowing kidney disease and CKD progression. A common class effect of SGLT2i reported in clinical trials is the reduction in blood uric acid levels. This is thought to be linked to the reduced reabsorption of urate by GLUT-9 [67]. Moreover, it has been observed that SGLT2i can have an additive effect on other drugs such as verinarurad, a novel blocker of urate reuptake [68]. A post hoc analysis of the CREDENCE trial recently highlighted that canagliflozin reduced the risk of hyperkalemia in the enrolled population [69]. This effect could be at least in part due to the kaliuretic effect [69]. Although these data need to be confirmed with additional studies, this further effect could prove useful in the management of CKD, potentially helping the maximization of RAASi and aldosterone antagonists that are generally suspended in advanced stages of CKD. As further support, other reports previously ruled out the increased risk of hyperkalemia with SGLT2i [69].
Potential applications and drawbacks of SGLT2i in the treatment of pediatric glomerulopathies and CKD
In the adult population, the use of SGLT2i has retraced the path of RAASi, having initially been used in patients with diabetes mellitus and CKD and subsequently employed in other categories of patients. Indeed, it seems reasonable to hypothesize a similar fate also in children.
The assessment of the risk-to-benefit ratio of new drugs is crucial and requires caution and patience before the use can be extended to the pediatric population. Due to the lack of data in children, we can only try to project evidence about safety and efficacy from the adult population to envisage potential fields of application of SGLT2i therapy in pediatric glomerulopathies and CKD. SGLT2i generally showed a favorable safety profile in clinical trials in adults. Nevertheless, as they are intended as a long-term treatment, even small side effects can become relevant. The most frequently reported side effects were an overall increased risk of urogenital infections [70], polyuria and pollakiuria affecting the quality of life, hypovolemia and hypotension when used in addition to diuretics or blood pressure medications, euglycemic diabetic ketoacidosis, AKI, and hypovolemia [70]. Among the associated side effects, some deserve a specific consideration in the pediatric population. Urogenital infections are common and can be more severe in children. In addition, they might become even more frequent and harmful in patients on steroids or other immunosuppressants as a treatment for glomerular diseases, even if data in adults does not suggest this as a relevant risk since patients on immunosuppressive therapy were mostly excluded from the clinical trials. Euglycemic diabetic ketoacidosis, precipitated by severe acute illness, dehydration, extensive exercise, and surgery [70], suggests the need for preventing even subclinical hypovolemia in children, bearing in mind that ketoacidosis is a serious concern in T1DM more than T2DM. As an additional unfavorable aspect concerning their employment, SGLT2i showed only a moderate antidiabetic potential.
As therapeutic tools of nephrologists, SGLT2i were welcomed to prevent the progression of kidney disease in adult patients with diabetes mellitus. Therefore, it is reasonable to consider the use of gliflozins also in the pediatric population. Luckily, diabetic kidney disease in children is rare (Fig. 2). Nevertheless, children with diabetes may represent the first group to consider for this group of drugs, basically for two reasons. Firstly, kidney disease is not already established at the time of diagnosis, neither from diabetes itself nor from other comorbidities, making limitations to their prescription extremely few. Secondly, the advantages of preserving kidney function are crucial in the expected long duration of the disease. In addition, pediatric diabetes is on the rise, with type T2DM increasing faster than T1DM, probably related to the epidemic increase in obesity and overweight among children [71]. Kidney involvement in youth onset-T2DM occurs early in the disease course and the progression is similar to that seen in the T2DM adult population [72]. Unfortunately, oral antidiabetic monotherapy in children with T2DM does not seem to be as effective in glucose control as in adults [73], pushing us to explore association therapy to prevent complications. From this perspective, SGLT2i may play a crucial role. In adults with T1DM and obesity, glifozins seem to play a role as an adjunctive treatment option when insulin alone does not provide adequate glycaemic control despite optimal therapy [74]. These observations have potentially important implications for the pediatric population, where T1DM is the most prevalent form of diabetes. However, the risk-to-benefit profile in T1DM is controversial even in adults [75] and more studies are needed before the use of glifozins in this subgroup could be hypothesized to be transferred to children.
Primary kidney disease distribution at the start of kidney replacement therapy in adults and children in Europe. Primary kidney disease distribution at the start of kidney replacement therapy in Europe in the adult population (left panel). Data from ERA-EDTA Registry, Annual Report 2019. Primary kidney disease distribution at the start of kidney replacement therapy in Europe in the pediatric population (right panel). Data from ESPN/ERA-EDTA Registry, Annual Report 2018. Green to yellow to red scale identifies the likelihood of implementation of SGLT2i in each group of primary kidney disease. ADPD, autosomal dominant polycystic kidney disease; CAKUT, congenital anomalies of the kidney and urinary tract; DM, diabetes mellitus; GN, glomerulonephritis; HUS, hemolytic uremic syndrome; SRNS, steroid-resistant nephrotic syndrome; uCKD, chronic kidney disease of unknown origin
As previously happened with RAASi, after their successful use in diabetic nephropathy SGLT2i have been tested in proteinuric glomerulopathies of different etiology, demonstrating that they reduce the progression of kidney disease, in particular in adult patients with IgAN [12]. Indeed, patients with IgAN and other proteinuric nephropathies represented a significant part of the population enrolled in the DAPA-CKD trial (695 out of 4304 patients, 16%), which proved the efficacy of SGLT2i in reducing the risk of CKD progression and CV morbidity, independently of the concomitant diagnosis of diabetes mellitus. This opens an interesting window on the potential application of this class of drugs in the pediatric population that could be even wider than in adults (Fig. 2). Indeed, in children, teenagers, and young adults, primary glomerular diseases account for a higher percentage of CKD and progression to KRT than in the adult population [1] (Fig. 2). As previously mentioned, the genetic make-up of children with glomerulopathies can be complex, with low effect-size genetic polymorphisms influencing nephron endowment, metabolic balance, and other aspects, probably contributing to determining the overall predisposition to CKD progression. As a consequence, therapy innovation in this field is of great interest in the pediatric population. Moreover, due to the burden of side effects, immunosuppressive therapy has progressively been reconsidered and limited to a selection of patients and clinical situations in many glomerulopathies, prompting the optimization of antiproteinuric treatments together with risk factor reduction [20]. The burden of side effects related to immunosuppressive treatments in children reasonably worries pediatric nephrologists. Luckily, previous experience of antiproteinuric therapy in primary glomerular diseases in children borrowed from adults as in IgAN is very encouraging [76].
Most importantly, given the physiological aspects of SGLT2i shown in the previous sections, these drugs may potentially be applied in all proteinuric diseases in children, whether the cause of glomerular injury leading to CKD is primary or secondary (Fig. 2). Unfortunately, no etiologic therapy is available for the majority of cases of CKD in children (e.g., CAKUT, genetic podocytopathies) and supportive therapy together with appropriate planning of KRT are the only current options to contain morbidity and mortality. Nevertheless, these diseases are characterized by the presence of proteinuria of various degrees, ranging from subnephrotic to massive proteinuria, due to the disease itself and other factors specific to the patient (body mass index, blood pressure control, and diet compliance). Proteinuria may be considered as a sign of intraglomerular failure and per se leads to a progression of kidney disease. Thus, RAASi represent the only available therapy to slow the pace of CKD progression in children at this time. SGLT2i may follow this path and reach this target in all patients with indications of RAASi, finding a key role in addressing the leading causes of CKD in children (Fig. 2). Moreover, many patients are treated with RAASi as long as possible during the decline of kidney function, accepting the implementation of diuretics wasting potassium as well as potassium binders to safely continue these therapies in order to keep proteinuria under control [20]. In this view, SGLT2i could represent adjuvant treatment to help control the metabolic complications of CKD progression.
Conclusions
Developing better strategies to address CKD progression in children is an important unmet medical need. Considering the long lifespan typical of the pediatric population, it is fundamental to address any modifiable progressor in kidney disease to preserve every milliliter of eGFR. Briefly, to summarize the observations about the potential fields of application of SGLT2i in the treatment of pediatric glomerulopathies and CKD, we could hypothesize an outline of priority groups to extend the use of this class of drugs:
-
1.
Children with glomerular causes of CKD: e.g., IgAN, FSGS, genetic podocytopathies, especially those progressing rapidly in terms of proteinuria and eGFR slope, and not responding well to target therapy or RAASi.
-
2.
Children with CKD at high CV risk: e.g., obese patients with T2DM, metabolic syndrome, and poorly controlled hypertension.
-
3.
Children with CAKUT and secondary causes of glomerular proteinuria: e.g., severely hypodysplastic CAKUT with proteinuria, discrepancy between metabolic needs, and filtering nephrons (preterm and low birth weight, low nephron endowment, obesity without T2DM, etc.).
On the other hand, caution should prevail in children with low risk of progressing CKD and on target with RAASi monotherapy, children under steroids and other immunosuppressants and with a specific red flag for severe urinary tract infection susceptibility, and children with T1DM. Finally, for tubulointerstitial and cystic forms of CKD, and secondary immune-mediated diseases data are still lacking.
In conclusion, SGLT2i recently emerged as promising therapeutic tools. Although their use in the pediatric population has not yet started, there are no pathophysiological clues to theoretically exclude their application in children. Indeed, by improving the hemodynamic adaptation elicited by RAASi, providing additional beneficial effects on overall metabolic homeostasis, blood pressure, weight control, and limiting complications of CKD, SGLT2i potentially could be applied to a wide range of contexts in the treatment of CKD and glomerular diseases in children.
References
Becherucci F, Roperto RM, Materassi M, Romagnani P (2016) Chronic kidney disease in children. Clin Kidney J 9:583–591. https://doi.org/10.1093/ckj/sfw047
Romagnani P, Remuzzi G, Glassock R, Levin A et al (2017) Chronic kidney disease. Nat Rev Dis Primers 3:17088. https://doi.org/10.1038/nrdp.2017.88
Kula AJ, Somers MJG, American Society of Pediatric Nephrology (2021) Children with CKD are not little adults with CKD: pediatric considerations for the Advancing American Kidney Health Initiative. Clin J Am Soc Nephrol 16:470–472. https://doi.org/10.2215/CJN.11540720
Ingelfinger JR (2018) A disturbing legacy of childhood kidney disease. N Engl J Med 378:470–471. https://doi.org/10.1056/NEJMe1716499
Rheault MN, Wenderfer SE (2018) Evolving epidemiology of pediatric glomerular disease. Clin J Am Soc Nephrol 13:977–978. https://doi.org/10.2215/CJN.06220518
O’Shaughnessy MM, Hogan SL, Poulton CJ, Falk RJ et al (2017) Temporal and demographic trends in glomerular disease epidemiology in the Southeastern United States, 1986–2015. Clin J Am Soc Nephrol 12:614–623. https://doi.org/10.2215/CJN.10871016
Kopp JB, Anders HJ, Susztak K, Podestà MA et al (2020) Podocytopathies Nat Rev Dis Primers 6:68. https://doi.org/10.1038/s41572-020-0196-7
Landini S, Mazzinghi B, Becherucci F, Allinovi M et al (2020) Reverse phenotyping after whole-exome sequencing in steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 15:89–100. https://doi.org/10.2215/CJN.06060519
Warejko JK, Tan W, Daga A, Schapiro D et al (2018) Whole exome sequencing of patients with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 13:53–62. https://doi.org/10.2215/CJN.04120417
Becherucci F, Landini S, Cirillo L, Mazzinghi B, Romagnani P (2020) Look alike, sound alike: phenocopies in steroid-resistant nephrotic syndrome. Int J Environ Res Public Health 17:8363. https://doi.org/10.3390/ijerph17228363
Wuttke M, Schaefer F, Wong CS, Köttgen A (2015) Genome-wide association studies in nephrology: using known associations for data checks. Am J Kidney Dis 65:217–222. https://doi.org/10.1053/j.ajkd.2014.09.019
Anders HJ, Peired AJ, Romagnani P (2020) SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, ‘diabetic nephropathy’, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfaa329
Luyckx VA, Perico N, Somaschini M, Manfellotto D et al (2017) A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390:424–428. https://doi.org/10.1016/S0140-6736(17)30576-7
Khalsa DD, Beydoun HA, Carmody JB (2016) Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr Nephrol 31:1509–1516. https://doi.org/10.1007/s00467-016-3384-7
Low Birth Weight and Nephron Number Working Group (2017) The impact of kidney development on the life course: a consensus document for action. Nephron 136:3–49. https://doi.org/10.1159/000457967
Ruggajo P, Svarstad E, Leh S, Marti HP, Reisæther AV, Vikse BE (2016) Low birth weight and risk of progression to end stage renal disease in IgA nephropathy–a retrospective registry-based cohort study. PLoS One 11:e0153819. https://doi.org/10.1371/journal.pone.0153819
Nada A, Jetton JG (2021) Pediatric onco-nephrology: time to spread the word-part II: long-term kidney outcomes in survivors of childhood malignancy and malignancy after kidney transplant. Pediatr Nephrol. https://doi.org/10.1007/s00467-021-05172-y
Panwar B, Hanks LJ, Tanner RM, Muntner P et al (2015) Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int 87:1216–1222. https://doi.org/10.1038/ki.2014.384
Perico N, Ruggenenti P, Remuzzi G (2017) ACE and SGLT2 inhibitors: the future for non-diabetic and diabetic proteinuric renal disease. Curr Opin Pharmacol 33:34–40. https://doi.org/10.1016/j.coph.2017.03.006
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group (2021) KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 100:S1–S276
Trautmann A, Vivarelli M, Samuel S, Gipson D et al (2020) IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 35:1529–1561. https://doi.org/10.1007/s00467-020-04519-1
de Zeeuw D, Agarwal R, Amdahl M, Audhya P et al (2010) Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376:1543–1551. https://doi.org/10.1016/S0140-6736(10)61032-X
Mann JF, Green D, Jamerson K, Ruilope LM et al (2010) Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21:527–535. https://doi.org/10.1681/ASN.2009060593
Wenzel RR, Littke T, Kuranoff S, Jürgens C et al (2009) Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20:655–664. https://doi.org/10.1681/ASN.2008050482
Bakris GL, Agarwal R, Anker SD, Pitt B et al (2020) Effect of Finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383:2219–2229. https://doi.org/10.1056/NEJMoa2025845
Chin MP, Bakris GL, Block GA, Chertow GM et al (2018) Bardoxolone methyl improves kidney function in patients with chronic kidney disease stage 4 and type 2 diabetes: post-hoc analyses from bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes study. Am J Nephrol 47:40–47. https://doi.org/10.1159/000486398
Panchapakesan U, Pollock C (2021) Organ protection beyond glycaemic control with SGLT2 inhibitors. Nat Rev Nephrol 17:223–224. https://doi.org/10.1038/s41581-020-00373-4
Zinman B, Wanner C, Lachin JM, Fitchett D et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128. https://doi.org/10.1056/NEJMoa1504720
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu P-L, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H,Zinman B, Meininger G, Brenner BM, Mahaffey KW (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380:2295–2306. https://doi.org/10.1056/NEJMoa1811744
Oshima M, Neuen BL, Li J, Perkovic V, Charytan DM et al (2020) Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE Trial. J Am Soc Nephrol 31:2925–2936. https://doi.org/10.1681/ASN.2020050723
Heerspink HJL, Jongs N, Chertow GM, Langkilde AM et al (2021) Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 9:743–754. https://doi.org/10.1016/S2213-8587(21)00242-4
Jongs N, Greene T, Chertow GM, McMurray JJV et al (2021) Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 9:755–766. https://doi.org/10.1016/S2213-8587(21)00243-6
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM et al (2020) Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383:1436–1446. https://doi.org/10.1056/NEJMoa2024816
Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R et al (2020) Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35:274–282. https://doi.org/10.1093/ndt/gfz290
Wheeler DC, Stefansson BV, Batiushin M, Bilchenko O et al (2020) The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 35:1700–1711. https://doi.org/10.1093/ndt/gfaa234
Wheeler DC, Toto RD, Stefánsson BV, Jongs N et al (2021) A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int 100:215–224. https://doi.org/10.1016/j.kint.2021.03.033
Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JFE, Hilgers R-D, Floege J (2015) Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373:2225–2236. https://doi.org/10.1056/NEJMoa1415463
Lv J, Zhang H, Wong MG, Jardine MJ et al (2017) Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 318:432–442. https://doi.org/10.1001/jama.2017.9362
Rajasekeran H, Reich HN, Hladunewich MA, Cattran D et al (2018) Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Renal Physiol 314:F412–F422. https://doi.org/10.1152/ajprenal.00445.2017
Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D et al (2020) Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 8:582–593. https://doi.org/10.1016/S2213-8587(20)30162-5
Anker SD, Butler J, Filippatos G, Ferreira JP et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461. https://doi.org/10.1056/NEJMoa2107038
Herrington WG, Preiss D, Haynes R, von Eynatten M et al (2018) The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 11:749–761. https://doi.org/10.1093/ckj/sfy090
Miyata N, Zhang SL, Chan JSD (2021) The rationale and evidence for SGLT2 inhibitors as a treatment for nondiabetic glomerular disease. Glomerular Dis 1:21–33. https://doi.org/10.1159/000513659
Srinivasan Sridhar V, Ambinathan JPN, Kretzler M, Pyle LL, Bjornstad P et al (2019) NephRenal SGLT mRNA expression in human health and disease: a study in two cohorts. Am J Physiol Renal Physiol 317:F1224–F1230. https://doi.org/10.1152/ajprenal.00370.2019
Sen T, Heerspink HJL (2021) A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab 33:732–739. https://doi.org/10.1016/j.cmet.2021.02.016
Brown RD, Turner AJ, Carlström M, Persson AE, Gibson KJ (2011) Tubuloglomerular feedback response in the prenatal and postnatal ovine kidney. Am J Physiol Renal Physiol 300:F1368–F1374. https://doi.org/10.1152/ajprenal.00019.2011
Deng A, Hammes JS, Thomson SC (2002) Hemodynamics of early tubuloglomerular feedback resetting during reduced proximal reabsorption. Kidney Int 62:2136–2143. https://doi.org/10.1046/j.1523-1755.2002.00682.x
Brenner BM, Mackenzie HS (1997) Nephron mass as a risk factor for progression of renal disease. Kidney Int Suppl 63:S124–S127
Cherney DZ, Perkins BA, Soleymanlou N, Maione M et al (2014) Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129:587–597. https://doi.org/10.1161/CIRCULATIONAHA.113.005081
Kidokoro K, Cherney DZI, Bozovic A, Nagasu H et al (2019) Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 140:303–315. https://doi.org/10.1161/CIRCULATIONAHA.118.037418
Rajasekeran H, Lytvyn Y, Bozovic A, Lovshin JA et al (2017) Urinary adenosine excretion in type 1 diabetes. Am J Physiol Renal Physiol 313:F184–F191. https://doi.org/10.1152/ajprenal.00043.2017
Nosadini R, Semplicini A, Fioretto P, Lusiani L et al (1991) Sodium-lithium countertransport and cardiorenal abnormalities in essential hypertension. Hypertension 18:191–198. https://doi.org/10.1161/01.hyp.18.2.191
Chagnac A, Herman M, Zingerman B, Erman A et al (2008) Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant 23:3946–3952. https://doi.org/10.1093/ndt/gfn379
Zingerman B, Herman-Edelstein M, Erman A, Bar Sheshet Itach S, Ori Y, Rozen-Zvi B, Gafter U, Chagnac A (2015) Effect of acetazolamide on obesity-induced glomerular hyperfiltration: a randomized controlled trial. PLoS One 10:e0137163. https://doi.org/10.1371/journal.pone.0137163
Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M et al (2020) A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol 319:F712–F728. https://doi.org/10.1152/ajprenal.00264.2020
Wanner C, Heerspink HJL, Zinman B, Inzucchi SE et al (2018) Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol 29:2755–2769. https://doi.org/10.1681/ASN.2018010103
Mulder S, Heerspink HJL, Darshi M, Kim JJ et al (2019) Effects of dapagliflozin on urinary metabolites in people with type 2 diabetes. Diabetes Obes Metab 21:2422–2428. https://doi.org/10.1111/dom.13823
Neuen BL, Young T, Heerspink HJL, Neal B et al (2019) SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 7:845–854. https://doi.org/10.1016/S2213-8587(19)30256-6
Oshima M, Neuen BL, Jardine MJ, Bakris G et al (2020) Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol 8:903–914. https://doi.org/10.1016/S2213-8587(20)30300-4
Qiu H, Novikov A, Vallon V (2017) Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 33(5). https://doi.org/10.1002/dmrr.2886
Youm YH, Nguyen KY, Grant RW, Goldberg EL et al (2019) The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21:263–269. https://doi.org/10.1038/nm.3804
Mazidi M, Rezaie P, Gao HK, Kengne AP (2017) Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc 6:e004007. https://doi.org/10.1161/JAHA.116.004007
Scholtes RA, Muskiet MHA, van Baar MJB, Hesp AC et al (2021) Natriuretic effect of two weeks of dapagliflozin treatment in patients with type 2 diabetes and preserved kidney function during standardized sodium intake: results of the DAPASALT Trial. Diabetes Care 44:440–447. https://doi.org/10.2337/dc20-2604
Schork A, Saynisch J, Vosseler A, Jaghutriz BA et al (2019) Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol 18:46. https://doi.org/10.1186/s12933-019-0852-y
Striepe K, Jumar A, Ott C, Karg MV et al (2017) Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation 136:1167–1169. https://doi.org/10.1161/CIRCULATIONAHA.117.029529
Herat LY, Magno AL, Rudnicka C, Hricova J et al (2020) GLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci 5:169–179. https://doi.org/10.1016/j.jacbts.2019.11.007
Novikov A, Fu Y, Huang W, Freeman B et al (2019) SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol 316:F173–F185. https://doi.org/10.1152/ajprenal.00462.2018
Stack AG, Han D, Goldwater R, Johansson S et al (2021) Dapagliflozin added to verinurad plus febuxostat further reduces serum uric acid in hyperuricemia: the QUARTZ study. J Clin Endocrinol Metab 106:e2347–e2356. https://doi.org/10.1210/clinem/dgaa748
Neuen BL, Oshima M, Perkovic V, Agarwal R et al (2021) Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J 42:4891–4901. https://doi.org/10.1093/eurheartj/ehab497
Toyama T, Neuen BL, Jun M, Ohkuma T et al (2019) Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab 21:1237–1250. https://doi.org/10.1111/dom.13648
Centers for Disease Control and Prevention (2021) https://www.cdc.gov/. Accessed 30 Sept 2021
Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P (2021) Long-term complications in youth-onset type 2 diabetes. N Engl J Med 385:416–426. https://doi.org/10.1056/NEJMoa2100165
Zeitler P, Hirst K, Pyle L, Linder B et al (2012) A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 366:2247–2256. https://doi.org/10.1056/NEJMoa1109333
Paik J, Blair HA (2019) Dapagliflozin: a review in type 1 diabetes. Drugs 79:1877–1884. https://doi.org/10.1007/s40265-019-01213-x
Taylor SI, Blau JE, Rother KI, Beitelshees AL (2019) SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol 7:949–958. https://doi.org/10.1016/S2213-8587(19)30154-8
Coppo R, Peruzzi L, Amore A, Piccoli A et al (2007) IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol 18:1880–1888. https://doi.org/10.1681/ASN.2006040347
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cirillo, L., Ravaglia, F., Errichiello, C. et al. Expectations in children with glomerular diseases from SGLT2 inhibitors. Pediatr Nephrol 37, 2997–3008 (2022). https://doi.org/10.1007/s00467-022-05504-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05504-6