Abstract
Background
Glucocorticoid discontinuation, a challenge in systemic lupus erythematosus (SLE), might be achievable with the advent of new therapeutic options.
Methods
This single-center study included 31 children with newly diagnosed pediatric SLE between 2002 and 2021, after the exclusion of patients who were followed for less than 1 year after treatment initiation and those lost to follow-up. Patient characteristics, clinical course including flares, treatment, glucocorticoid discontinuation, and outcomes were retrospectively analyzed.
Results
Glucocorticoids could be discontinued in 19 (61%) patients during a median observation period of 105.5 (range, 17–221) months. Of these, 5 (26%), 12 (63%), and 18 (95%) patients could discontinue glucocorticoids in 3, 5, and 10 years from treatment initiation, respectively. Additionally, 18 of the 19 patients did not experience flares after glucocorticoid discontinuation during a median duration of 37.2 (7.2–106.8) months. Three of the nineteen patients achieved drug-free remission. At last follow-up, all patients achieved low disease activity with or without glucocorticoids and 19, 8, and 1 patient were receiving mycophenolate mofetil (MMF), MMF plus tacrolimus, and MMF plus ciclosporin A, respectively. Flares were observed in 15 patients during the observation period. MMF as initial immunosuppressant (P = 0.01) and shorter interval between therapy initiation and achieving maintenance prednisolone dose of 0.1–0.15 mg/kg/day (P = 0.001) were associated with significantly reduced flare risk. Femoral head necrosis was observed in two patients.
Conclusion
Despite the small sample size, these results support glucocorticoid discontinuation as a therapeutic target in pediatric SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE), a complex autoimmune disease with multi-organ involvement, is characterized by a chronic relapsing course [1]. Pediatric-onset SLE (p-SLE) is a rare presentation with an incidence of 0.3–2.2 per 100,000 children-years [1,2,3]. Outcomes of p-SLE, particularly in patients with multi-organ and kidney involvement, remain unsatisfactory, although the morbidity and mortality rates have significantly improved with the development of aggressive new regimens in recent decades [4,5,6].
Glucocorticoids remain an essential therapeutic option for remission induction and maintenance therapy in patients with SLE; however, there is no consensus on optimal glucocorticoid dosage for maintenance therapy [6, 7], which should employ the lowest dosage necessary to control disease activity. The ultimate goal is to discontinue glucocorticoids as recommended in the treat-to-target (T2T) strategy [8, 9]. In recent years, the emergence of new therapeutic options, including immunosuppressants and biologics, has made glucocorticoid discontinuation a realistic goal [10, 11]. However, few studies have focused on glucocorticoid discontinuation in p-SLE [6]. In addition, flare rate and outcomes after glucocorticoid discontinuation have not been extensively clarified in patients with SLE [6, 7]. Therefore, some physicians are more likely to hesitate to discontinue glucocorticoids or may continue administering low-dose glucocorticoids even to patients in remission [9]. Due to the severe adverse effects of glucocorticoids, such as growth impairment, femoral head necrosis, and glaucoma, simultaneous achievement of glucocorticoid minimization and flare prevention can improve long-term outcomes and quality of life in patients with SLE [7, 12]. Although, glucocorticoid discontinuation remains a challenge especially in patients with p-SLE, understanding the factors associated with patient outcome in glucocorticoid discontinuation in p-SLE is important.
We therefore conducted a retrospective, single-center study to elucidate patient outcomes following glucocorticoid tapering and discontinuation in patients with p-SLE.
Methods
Participants and data collection
This was a retrospective study including the analysis of patient characteristics, clinical course including flares, treatment approaches, and outcomes of patients with newly diagnosed p-SLE between May 1, 2002, and May 31, 2021, at the National Center for Child Health and Development. All patients included in the study were diagnosed with SLE before the age of 20 years. Patients who were followed for less than 1 year from treatment initiation and those who were lost to follow-up were excluded from the study.
Definitions
The diagnosis of SLE was based on the American College of Rheumatology criteria [13]. The diagnosis of lupus nephritis was based on kidney biopsy and categorized as class I, II, III, IV, or V according to the International Society of Nephrology/Renal Pathology Society classification system [14]. Disease activity was evaluated based on the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [15] and classified as mild, moderate, or severe. Specifically, mild disease activity was defined as the presence of minor clinical symptoms and/or minor abnormalities in laboratory markers. Moderate disease activity was defined as the presence of organ involvement including lupus nephritis (class I, II, or V). Severe disease activity was defined as the presence of severe organ involvement including lupus nephritis (class III or IV), neurological disease, and/or pulmonary hemorrhage. Drug-free remission was defined as the absence of clinical activity based on a SLEDAI score 0 with no treatment with glucocorticoids or immunosuppressants. Low disease activity was defined as the presence of a SLEDAI score of ≤ 4 with good tolerance to treatment with ≤ 7.5 mg prednisolone and immunosuppressants [16]. Flares were defined as clinical, renal, or serological exacerbation requiring additional treatment [16]. Serological flare in asymptomatic cases was defined as persistent and severe worsening of laboratory markers, including complement (C3, C4, and CH50), anti-double-stranded DNA antibody level, and erythrocyte sedimentation ratio. Body mass index (BMI) was calculated using the following formula: BMI = weight/height2 (kg/m2).
Treatment strategy
Induction therapy
Oral prednisolone (initial dose of 1 mg/kg/day, followed by tapering every month until maintenance dosage is reached) with or without oral immunosuppressants was used for remission induction therapy in patients with mild p-SLE. Patients with moderate p-SLE were treated with several courses of intravenous methylprednisolone pulse therapy (MPT; 30 mg/kg/day for 3 days; maximum 1 g/day), followed by oral immunosuppressants and oral prednisolone, whereas those with severe p-SLE were treated with several courses of intravenous MPT with or without intravenous cyclophosphamide therapy (IVCY; maximum six doses at 500 mg/m2, administered at 1-month intervals), followed by oral immunosuppressants and oral prednisolone. The starting dose of oral prednisolone after MPT was 1 mg/kg/day (maximum, 30 mg/day) for 1 month, which was slowly tapered every month until a maintenance prednisolone dosage of 0.1–0.15 mg/kg/day was achieved in about 6 months in patients without a flare. In most patients, oral administration of initial immunosuppressants was started at the time of induction therapy and was added as maintenance therapy in advance. Mycophenolate mofetil (MMF; 500–1000 mg/m2/day; maximum, 2000 mg/day), mizoribine (10 mg/kg/day; maximum, 300 mg/day), azathioprine (AZA; 1 mg/kg/day; maximum 100 mg/day), ciclosporin A (CsA; initial trough level, 100–120 μg/L), or tacrolimus (trough level, 5 ng/mL) was chosen by the attending physician. A similar regimen was previously reported by Kizawa et al. [17]. Subsequently, all patients were switched from immunosuppressants to MMF or MMF in combination with a calcineurin inhibitor. Following its approval in 2015 in Japan, hydroxychloroquine sulfate (HCQ) has been used from the initial treatment of SLE at onset. Therefore, most patients in the present study had not previously received HCQ. Rituximab was not administered in this cohort because it has not been approved for the treatment of SLE in Japan.
Maintenance therapy
Maintenance therapy comprised low doses of oral prednisolone and oral immunosuppressants, including MMF, AZA, mizoribine, CsA, and tacrolimus. However, all patients who were initially treated with AZA and mizoribine were switched to MMF (800–1200 mg/m2/day) even in the absence of flares after its approval for SLE treatment in 2016 in Japan. In patients who could be tapered to a maintenance prednisolone dose of 0.1–0.15 mg/kg/day, further tapering with a slow dose reduction in 1-mg decrements over several months was followed until discontinuation was achieved. If possible, immunosuppressants were carefully tapered and discontinued after the discontinuation of prednisolone.
Flare management
The clinical management of flares was individualized. Briefly, additional MPT or increased prednisolone dosage was administered during flares, followed by switching the immunosuppressive regimen to MMF, adding tacrolimus to the current immunosuppressive regimen, or adding HCQ. The prednisolone dosage was tapered to the maintenance level after achieving remission. The patients with serological flares in asymptomatic cases were treated in advance, and treatment was revised to switch to or add immunosuppressants.
Statistical analysis
Values were expressed as medians (minimum–maximum). Fisher’s exact test was used to compare categorical variables, and the Mann–Whitney U test was used to compare continuous variables. Proportions of patients experiencing a flare and those who discontinued glucocorticoids were calculated with the Kaplan–Meier method using the time from treatment initiation. A two-sided P value of < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using the JMP Pro 14 software package for Windows (SAS Institute Japan, Tokyo, Japan).
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Health, Labor, and Welfare, Japan, and was approved by the institutional ethics committee of the National Center for Child Health and Development (approval no. 1868). Informed consent for participating in the study was not required in accordance with the abovementioned guidelines.
Results
Patient characteristics and initial treatment
During the study period, 31 of 37 children who were diagnosed with SLE in our institution fulfilled the inclusion criteria, after the exclusion of 2 patients who were followed for less than 1 year and 4 patients who were lost to follow-up (Fig. 1). Four and two of the six patients who were excluded had moderate and severe SLE, respectively.
Table 1 summarizes the clinical characteristics of the study cohort of 31 patients, including sex, age at onset, lupus nephritis class, laboratory and urinary markers and SLEDAI score at diagnosis, and follow-up duration after treatment initiation. Briefly, 19 (61%) patients had severe p-SLE; 12 (39%) and 7 (23%) patients had active class III and IV lupus nephritis, respectively. Although kidney biopsy was performed for all patients, including those without abnormal urinary findings, one patient did not undergo kidney biopsy because the parents did not provide consent. He had normal urinary findings, and his SLEDAI score was 11 at onset. As remission induction therapy, 30 (97%) patients received several courses of MPT whereas 15 (48%) patients with severe p-SLE received IVCY. A median of 6 (range, 4–6) IVCY treatments was administered. All patients were treated with oral prednisolone. Thirty (97%) patients started oral immunosuppressant therapy together with remission induction therapy, which comprised MMF, AZA, mizoribine, and CsA in 18, 6, 5, and 1 patient, respectively. The median initial dose of MMF was 676 mg/m2 (range, 478–981 mg/m2). The median maximum dose of MMF during the clinical course was 1195 mg/m2 (range, 561–1546 mg/m2). The mycophenolic acid level was not measured. Clinical course, data, and outcomes at last follow-up were shown in Table 2.
Low disease activity and drug-free remission
All 31 patients achieved low disease activity at last follow-up. Among 25 patients who were observed for more than 5 years, 22 patients (88%) maintained low disease activity for five consecutive years. Additionally, 3 (10%) patients who achieved drug-free remission for 3.7, 5.3, and 6.4 years did not experience a flare for 5.8, 6.4, and 8.9 years, respectively, after the discontinuation of glucocorticoids and immunosuppressants. Immunosuppressants used at last follow-up were MMF, MMF in combination with tacrolimus, and MMF in combination with CsA in 19, 8, and 1 patient, respectively.
Discontinuation of prednisolone
The Kaplan–Meier curve for time to discontinuation of prednisolone from initiation of treatment is shown in Fig. 2A. During the study period, 19 (61%) patients could discontinue prednisolone by the last follow-up evaluation. The rates of prednisolone discontinuation were 26% (5/19), 63% (12/19), and 95% (18/19) in 3, 5, and 10 years after treatment initiation, respectively.
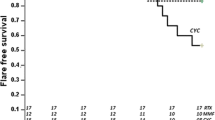
Flares
The rates of flare-free survival are shown in Fig. 2B. Briefly, 15 (48%) patients experienced 34 flares during the entire observation period, including 7 (47%) and 11 (73%) patients who experienced flares within 1 and 2 years following treatment initiation, respectively. Additionally, 8 patients experienced flares after achieving low disease activity. Only 1 of the 19 patients who discontinued prednisolone experienced flare whereas the remaining 18 patients did not experience flares during the median observation period of 37.2 (7.2–106.8) months after the discontinuation of prednisolone. Among these 18 patients, none of the three patients who achieved drug-free remission experienced flares after the discontinuation of glucocorticoids and immunosuppressants. Of the 34 flares, 10 were serological. The median SLEDAI score of the 10 serological flares was 4.5 (range, 2–5).
Patient outcomes
All patients in the study cohort were alive with normal kidney function at last follow-up. The median observation period from treatment initiation was 105.5 (range, 17–221) months. Follow-up kidney biopsy was conducted in six patients. Lupus nephritis improved in all patients on follow-up kidney biopsy (class IV to III, class IV to II, class V to II, and class III to II in 2, 2, 1, and 1 patient, respectively). The median height was + 0.01 (range, − 2.9 to 3.1) standard deviation (SD) before treatment and − 0.01 (range, − 2.6 to 3.1) SD at last follow-up. The median BMI was 18.1 (range, 13.0–28.2) kg/m2 before treatment and 20.3 (range, 15.4–28.9) kg/m2 at last follow-up.
Glucocorticoid-related severe adverse effects
The adverse effects of glucocorticoid treatment are shown in Table 3. A total of ten glucocorticoid-related severe adverse effects were observed in nine patients during the entire observation period. These events were hypertension, glaucoma, osteoporosis, and femoral head necrosis in 1, 5, 2, and 2 cases, respectively. Growth failure, defined as lower than − 2 SD change in height, was observed in two patients. The two patients with femoral head necrosis required surgery, but the prognosis was not poor and one of the two patients could discontinue prednisolone at last follow-up. The median cumulative doses of oral prednisolone and intravenous MPT over the study period were 9.5 (range, 4.6–44.2) g and 6.0 (range, 0.0–24.0) g, respectively.
Comparison between the patients with and without flares
The comparison between the patients with and without flares is shown in Table 4. MMF as initial immunosuppressant (P = 0.01) and shorter interval between therapy initiation and achieving maintenance dose (P = 0.001) significantly reduced flare risk. A younger age (P = 0.06) and AZA as initial immunosuppressant (P = 0.01) were also risk factors for flare.
Comparison between the patients with and without prednisolone discontinuation
The comparison between the patients with and without prednisolone discontinuation is shown in Table 5. There were no significant differences between the patients with and without prednisolone discontinuation. Young age tended to be a risk factor for prednisolone discontinuation (P = 0.08).
Discussion
In the present retrospective observational study, we evaluated the clinical course, including remissions and flares, prednisolone discontinuation, and outcomes in patients with p-SLE. We found that all 31 patients included in the study were able to maintain low disease activity with or without prednisolone. We also found that 3 (10%) patients achieved drug-free remission and that 19 (61%) patients successfully discontinued prednisolone during the median observation period of 105.5 months. Moreover, 18 (95%) patients did not experience any flares after the discontinuation of prednisolone. Due to the increased availability of therapeutic options, glucocorticoid discontinuation might be feasible even in patients with severe p-SLE.
In adult-onset SLE, the rate of prolonged drug-free remission, defined as the absence of flares for more than five consecutive years, is only 2–7% [18, 19] whereas the rate of prolonged remission with prednisolone and/or immunosuppressants is 4.5–30.3% [18, 19]. In the present study, 22 (88%) of the 25 patients who were followed for more than 5 years maintained low disease activity for five consecutive years, which was better than that reported in previous studies. Early and aggressive induction therapy in patients with severe p-SLE was reported to be associated with better outcomes and fewer flares [20]. In the present study, the remission induction therapy comprised MPT and oral immunosuppressants with or without IVCY in moderate and severe cases. Furthermore, in most patients receiving IVCY, oral immunosuppressant administration as remission maintenance therapy was initiated simultaneously with IVCY. This intensified induction therapy approach might result in favorable outcomes, consistent with the findings of a previous report [17].
Moroni et al. reported a case series of patients with adult-onset SLE who completely stopped glucocorticoid and immunosuppressive therapy [10]. The authors suggested that discontinuation of all treatments should be attempted only in certain patients, i.e., those who received maintenance therapy for at least 5 years and achieved complete renal remission for at least 3 years. In the present study, three patients who were able to achieve drug-free remission did not experience subsequent flares during the observational periods of 5.8, 6.4, and 8.9 years after therapy discontinuation. The duration of maintenance therapy and the length of discontinuation of all medications in these three patients were consistent with the recommendations by Moroni et al. Additionally, Basu et al. recently reported the benefits of rituximab for glucocorticoid discontinuation by comparing rituximab to IVCY and MMF used in remission induction therapy in a retrospective cohort study [11] and found that the glucocorticoid-free ratio at 36 months from onset was 82% in patients treated with rituximab. Although rituximab was not used in the present study, 19 (61%) of the 31 patients could discontinue prednisolone during the median observation period of 105.5 months and 18 of the 19 patients did not experience any flares after prednisolone discontinuation. From the perspective of preventing severe flares and organ damage, discontinuing all treatment remains challenging; however, the results of the study by Basu et al. as well as the present study suggest that treatment discontinuation may be achievable in patients with excellent disease control.
Although there were no significant differences between the patients with and without prednisolone discontinuation, MMF as initial immunosuppressant (P = 0.01) and shorter interval between therapy initiation and achieving maintenance dose (P = 0.001) significantly reduced flare risk. On the other hand, AZA as initial immunosuppressant was a risk factor for flare (P = 0.01). These results are consistent with a meta-analysis demonstrating the superiority of MMF to AZA as remission maintenance therapy for lupus nephritis [21]. At last follow-up, immunosuppressants as maintenance therapy in the present study were MMF alone or MMF in combination with a calcineurin inhibitor. Tacrolimus in combination with MMF is more effective than IVCY for remission induction and is also effective as maintenance therapy in adult patients with SLE [22]. In addition, MMF has fewer side effects than other immunosuppressants [23]. Therefore, MMF, alone or in combination with a calcineurin inhibitor, should be considered an excellent option for tapering glucocorticoid use in pediatric patients with refractory SLE.
In children, growth impairment is a critical adverse effect of glucocorticoids [7, 12]. Furthermore, obesity and cushingoid appearance are often related to poor compliance, especially in adolescent patients, and may lead to increased rate of flares [24]. In the present study, the median final height and BMI at last follow-up were satisfactory despite the long-term glucocorticoid exposure. Although femoral head necrosis developed in two patients who required surgery, other critical complications including cardiovascular issues were not observed in the present study.
Based on the findings of the present study, the points for the treatment strategy for p-SLE in our institution are summarized as follows. Intense remission induction therapy that included MPT and a prompt initiation of an immunosuppressant might allow for a lower starting dose of glucocorticoids, reduction of total IVCY dosage, and faster tapering and eventual discontinuation of glucocorticoids. In cases with a strong suspicion for a serological flare and showing evident disease activity, early intervention, including increasing glucocorticoid dosage and changing or adding other immunosuppressants, should be considered. These strategies might aid in minimizing increases in glucocorticoid administration, in reducing total glucocorticoid dosage, and in decreasing organ damage. It should be noted that this strategy resembles the 2019 European League Against Rheumatism recommendations [16].
There are several limitations in the present study. First, this was a single-center, retrospective study which enrolled a small number of patients, all of whom were Japanese. There might be racial differences in response to treatment for SLE [25]. Second, the impact of new medications, such as rituximab and HCQ, was not evaluated. The advent of these new therapeutic options may contribute to further improvements. Third, the selection of immunosuppressants administered at the onset of disease was not based on a uniform approach due to historical backgrounds, such as the delay of approval for immunosuppressant use in Japan.
In conclusion, approximately 60% of the patients with p-SLE in the present study were able to discontinue glucocorticoids due to advances in treatment, demonstrating the possibility of discontinuing glucocorticoid use in some patients with p-SLE.
Data availability
Not applicable.
Code availability
Not applicable.
References
Levy DM, Kamphuis S (2012) Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am 59:345–364. https://doi.org/10.1016/j.pcl.2012.03.007
Mackie FE, Kainer G, Adib N, Boros C, Elliott EJ, Fahy R, Munro J, Murray K, Rosenberg A, Wainstein B, Ziegler JB, Singh-Grewal D (2015) The national incidence and clinical picture of SLE in children in Australia - a report from the Australian Paediatric Surveillance Unit. Lupus 24:66–73. https://doi.org/10.1177/0961203314552118
Hiraki LT, Feldman CH, Liu J, Alarcón GS, Fischer MA, Winkelmayer WC, Costenbader KH (2012) Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum 64:2669–2676. https://doi.org/10.1002/art.34472
Hersh AO, von Scheven E, Yazdany J, Panopalis P, Trupin L, Julian L, Katz P, Criswell LA, Yelin E (2009) Arthritis Rheum 61:13–20. https://doi.org/10.1002/art.24091
Urowitz MB, Gladman DD, Tom BD, Ibañez D, Farewell VT (2008) Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 35:2152–2158. https://doi.org/10.3899/jrheum.080214
Felten R, Scher F, Sibilia J, Chasset F, Arnaud L (2019) Advances in the treatment of systemic lupus erythematosus: from back to the future, to the future and beyond. Joint Bone Spine 86:429–436. https://doi.org/10.1016/j.jbspin.2018.09.004
Moroni G, Gatto M, Raffiotta F, Binda V, Frangou E, Lightstone L, Boumpas DT (2018) Can we withdraw immunosuppressants in patients with lupus nephritis in remission? An expert debate. Autoimmun Rev 17:11–18. https://doi.org/10.1016/j.autrev.2017.11.003
van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrøm K, Aringer M, Bootsma H, Boumpas D, Bruce IN, Cervera R, Clarke A, Costedoat-Chalumeau N, Czirják L, Derksen R, Dörner T, Gordon C, Graninger W, Houssiau F, Inanc M, Jacobsen S, Jayne D, Jedryka-Goral A, Levitsky A, Levy R, Mariette X, Morand E, Navarra S, Neumann I, Rahman A, Rovensky J, Smolen J, Vasconcelos C, Voskuyl A, Voss A, Zakharova H, Zoma A, Schneider M (2014) Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 73:958–967. https://doi.org/10.1136/annrheumdis-2013-205139
Walsh M, Jayne D, Moist L, Tonelli M, Pannu N, Manns B (2010) Practice pattern variation in oral glucocorticoid therapy after the induction of response in proliferative lupus nephritis. Lupus 19:628–633. https://doi.org/10.1177/0961203309356292
Moroni G, Gallelli B, Quaglini S, Banfi G, Rivolta E, Messa P, Ponticelli C (2006) Withdrawal of therapy in patients with proliferative lupus nephritis: long-term follow-up. Nephrol Dial Transplant 21:1541–1548. https://doi.org/10.1093/ndt/gfk073
Basu B, Roy B, Babu BG (2017) Efficacy and safety of rituximab in comparison with common induction therapies in pediatric active lupus nephritis. Pediatr Nephrol 32:1013–1021. https://doi.org/10.1007/s00467-017-3583-x
Rygg M, Pistorio A, Ravelli A, Maghnie M, Di Iorgi N, Bader-Meunier B, Da Silva C, Roldan-Molina R, Barash J, Dracou C, Laloum SG, Jarosova K, Deslandre CJ, Koné-Paut I, Garofalo F, Press J, Sengler C, Tauber T, Martini A, Ruperto N; Paediatric Rheumatology International Trials Organisation (PRINTO) (2012) A longitudinal PRINTO study on growth and puberty in juvenile systemic lupus erythematosus. Ann Rheum Dis 71:511-517https://doi.org/10.1136/annrheumdis-2011-200106
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725. https://doi.org/10.1002/art.1780400928
Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M; International Society of Nephrology Working Group on the Classification of Lupus Nephritis; Renal Pathology Society Working Group on the Classification of Lupus Nephritis (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65:521-530. https://doi.org/10.1111/j.1523-1755.2004.00443.x
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35:630–640. https://doi.org/10.1002/art.1780350606
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, Houssiau F, Jayne D, Kouloumas M, Kuhn A, Larsen JL, Lerstrøm K, Moroni G, Mosca M, Schneider M, Smolen JS, Svenungsson E, Tesar V, Tincani A, Troldborg A, van Vollenhoven R, Wenzel J, Bertsias G, Boumpas DT (2019) 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 78:736–745. https://doi.org/10.1136/annrheumdis-2019-215089
Kizawa T, Nozawa T, Kikuchi M, Nagahama K, Okudela K, Miyamae T, Imagawa T, Nakamura T, Mori M, Yokota S, Tsutsumi H (2015) Mycophenolate mofetil as maintenance therapy for childhood-onset systemic lupus erythematosus patients with severe lupus nephritis. Mod Rheumatol 25:210–214. https://doi.org/10.3109/14397595.2014.950810
Steiman AJ, Urowitz MB, Ibañez D, Papneja A, Gladman DD (2014) Prolonged clinical remission in patients with systemic lupus erythematosus. J Rheumatol 41:1808–1816. https://doi.org/10.3899/jrheum.131137
Zen M, Iaccarino L, Gatto M, Bettio S, Nalotto L, Ghirardello A, Punzi L, Doria A (2015) Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis 74:2117–2122. https://doi.org/10.1136/annrheumdis-2015-207347
Otten MH, Cransberg K, van Rossum MA, Groothoff JW, Kist-van Holthe JE, Ten Cate R, Van Suijlekom-Smit LW (2010) Disease activity patterns in juvenile systemic lupus erythematosus and its relation to early aggressive treatment. Lupus 19:1550–1556. https://doi.org/10.1177/0961203310374485
Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, Eitner F, Appel GB, Contreras G, Lisk L, Solomons N; ALMS Group (2011) Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 365:1886-1895. https://doi.org/10.1056/NEJMoa1014460
Li X, Ren H, Zhang Q, Zhang W, Wu X, Xu Y, Shen P, Chen N (2012) Mycophenolate mofetil or tacrolimus compared with intravenous cyclophosphamide in the induction treatment for active lupus nephritis. Nephrol Dial Transplant 27:1467–1472. https://doi.org/10.1093/ndt/gfr484
Jiang YP, Zhao XX, Chen RR, Xu ZH, Wen CP, Yu J (2020) Comparative efficacy and safety of mycophenolate mofetil and cyclophosphamide in the induction treatment of lupus nephritis: a systematic review and meta-analysis. Medicine 99:e22328. https://doi.org/10.1097/MD.0000000000022328
Bengtsson C, Bengtsson A, Costenbader Kh, Jönsen A, Rantapää-Dahlqvist S, Sturfelt G, Nived O (2011) Systemic lupus erythematosus and cardiac risk factors: medical record documentation and patient adherence. Lupus 20:1057–1062. https://doi.org/10.1177/0961203311403639
Yen EY, Shaheen M, Woo JMP, Mercer N, Li N, McCurdy DK, Karlamangla A, Singh RR (2017) 46-year trends in systemic lupus erythematosus mortality in the United States, 1968 to 2013: a nationwide population-based study. Ann Intern Med 167:777–785. https://doi.org/10.7326/M17-0102
Acknowledgements
The authors would like to thank Drs. Kentaro Ogata and Kentaro Matsuoka for their academic contribution and pathological diagnosis. The authors would also like to thank Mr. James R. Valera for his assistance with editing of the manuscript.
Author information
Authors and Affiliations
Contributions
KN prepared the first draft of the manuscript and performed data collection and analysis. MOg, TK, SIs, MOk, SY, TN, and MS edited and reviewed the manuscript. SIt supervised and revised the manuscript. KI and KK revised and oversaw the work. All authors contributed to the study conception and design and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study (No. 1868) was approved by the Ethics Committee of the National Center for Child Health and Development.
Consent to participant
Informed consent for participating in this study was not required in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Health, Labor, and Welfare.
Consent for publication
Consent for publication was waived in accordance with the guidelines.
Conflict of interest
Koichi Kamei has received research funding from the Terumo Foundation for Life Sciences and Arts, Public Foundation of Vaccination Research Center, and Taiju Life Social Welfare Foundation and donations from Ono Pharmaceutical Co., Ltd. Kenji Ishikura has received lecture fees from Asahi Kasei Pharma, Chugai Pharmaceutical Co., Ltd, Zenyaku Kogyo Co., Ltd, and Novartis Pharma K.K. and grants from Asahi Kasei Pharma, Chugai Pharmaceutical Co., Ltd., Novartis Pharma, and Zenyaku Kogyo Co., Ltd. Shuichi Ito has received honoraria and research funding from Asahi Kasei Pharma, Chugai Pharmaceutical Co., Ltd, Zenyaku Kogyo Co., Ltd, and Astellas PHARMA Co., Ltd. Other authors have no potential conflicts of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishi, K., Ogura, M., Ishiwa, S. et al. Glucocorticoid discontinuation in pediatric-onset systemic lupus erythematosus: a single-center experience. Pediatr Nephrol 37, 2131–2139 (2022). https://doi.org/10.1007/s00467-021-05350-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05350-y