Abstract
Background
Acid-base balance is maintained by kidney excretion of titratable acids and bicarbonate reabsorption. Metabolic alkalosis is uncommon in dialysis-treated patients. The aim of this retrospective study was to assess the rate of metabolic alkalosis in pediatric patients treated with peritoneal dialysis.
Methods
Medical records of children treated with peritoneal dialysis in Shaare Zedek Medical Center from January 2000 to June 2021 were reviewed and compared with young adults currently treated with peritoneal dialysis. Demographic, clinical, and peritoneal dialysis characteristics were extracted from the medical records.
Results
Thirty chronic peritoneal dialysis patients were included in our study, seven under 2 years, 13 between 2 and 18 years, and 10 adults. 90.3% of the measurements in infants showed metabolic alkalosis compared to 32.3% in the 2–18-year group and none in the adult group. Higher size-adjusted daily exchange volume, lack of urine output, and high lactate-containing dialysate were associated with metabolic alkalosis. Alkalosis was not explained by vomiting, diuretic therapy, or carbonate-containing medications. High transport membrane, low dietary protein, and malnutrition, all previously reported explanations for metabolic alkalosis, were not found in our study.
Conclusions
Metabolic alkalosis is common in infants treated with peritoneal dialysis as opposed to older children and adults. High lactate-containing dialysate is a possible explanation. Higher size-adjusted daily dialysate exchange volume, which may reflect higher bicarbonate absorption, is another independent predictor of alkalosis. Acid-base status should be closely followed in infants, and using a dialysis solution with lower bicarbonate or lactate level should be considered.
Graphical abstract
A higher resolution version of the graphical abstract is available as Supplementary Information

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid-base balance is maintained by the kidney, via excretion of ammonium and titratable acids (mainly phosphoric acids) and bicarbonate reabsorption. At the initial stages of chronic kidney disease (CKD), single nephron ammonium excretion rate increases, preserving acid excretion and maintaining homeostasis. When glomerular filtration rate (GFR) decreases below 40 to 50 mL/min/1.73 m2 (CKD stage 3), ammonium and titratable acid excretion begins to fall and metabolic acidosis may be noted. The prevalence of serum bicarbonate concentration < 22 mEq/L is < 5% in CKD stage 1–2 and increases linearly to approximately 25% in nondialysis-dependent CKD stage 5 [1]. After the initiation of dialysis therapy, metabolic acidosis usually improves secondary to additional base load delivered in the dialysate. Metabolic acidosis can persist in dialysis patients, typically those with high protein intake which causes greater nitrogen appearance rate and hydrogen ion production or due to dialysis inadequacy [2].
Metabolic alkalosis, which is uncommon in dialysis-treated patients, can be associated with vomiting and diuretic therapy.
The aim of this retrospective study was to assess the rate of metabolic alkalosis in pediatric patients treated with peritoneal dialysis (PD) for kidney failure and to evaluate possible predictors.
Methods
Medical records of all children treated with PD in the pediatric dialysis unit, Shaare Zedek Medical Center, from January 2000 to June 2021 were reviewed. For comparison purposes, medical records of a selected sample of young adult patients (younger than 50 years) currently treated with PD in the adult dialysis unit were also reviewed. Patients were divided into 3 groups: infants 0–2 years, children and adolescents 2–18 years, and young adults over 18 years. Patients treated with PD for less than 3 months or those with persistent vomiting were excluded.
The study was approved by the IRB at the Shaare Zedek Medical Center (approval number: 0272-19-SZMC). The IRB waived the informed consent for retrospective data. Currently, treated patients, or their guardians, provided written informed consent.
PD routine follow-up visits were done at least once monthly and included clinical information, current medications, weight and height, PD prescription, and blood gas and electrolyte measurements. The analysis included the first quarterly record for each patient, starting from the second quarter (3–6 months) of dialysis treatment. Metabolic alkalosis was defined as bicarbonate level > 24 mmol/L under the age of 2 years, and > 26 mmol/L over the age of 2 years [3]. We chose to focus on bicarbonate level rather than pH level because infants tend to cry during blood drawing resulting in hyperventilation, hypocapnia, and respiratory alkalosis.
Peritoneal equilibration test (PET), normalized protein catabolic rate (nPCR) and weekly Kt/V were assessed using PatientOnLine©, PD management software, Fresenius after at least 3 months of treatment, and repeated according to clinical needs. For analysis purposes, we used nPCR and PET results that were measured at the nearest time point to the blood gas measurement.
Nutritional status was defined separately for each age group. In infants 0–2 years, we used the WHO classification of height to weight percentiles: malnutrition (less than 2%), healthy (2–98%), and overweight (> 98%). In children and adolescents between 2 and 18 years, we utilized the CDC classification of BMI percentiles: malnutrition (< 5%), healthy (5–85%), overweight and obese (> 85%). In adults over 18 years, we used the standard NIH classification of BMI: malnutrition (up to 18.5), healthy (18.5 to 25), and overweight (> 25).
To assess the influence of age on the risk of metabolic alkalosis, we analyzed all quarterly measurements according to the patient’s actual age at the time the measurement was done. This resulted in some patients having measurements in more than one age group.
Urine output was defined as a binary variable, with urine output of ≥ 0.5 mL/kg/h considered positive.
Dialysate volume was analyzed as milliliters per square meter per day. Target dwell volume according to National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines for peritoneal dialysis adequacy is 600–800 mL/m2 body surface area (BSA) for infants younger than 2 years and 1000–1200 mL/m2 BSA for patients over 2 years old. We therefore adjusted the dialysate volume in the infant group by a factor of 1.57 (adult target divided by infant target) for use in comparative analysis.
Statistical analysis
Continuous variables were described as mean and standard deviation (SD) or median and interquartile range (IQR). Categorical data were presented as percentiles. Differences between groups were analyzed using ANOVA, Mann-Whitney, or Kruskal-Wallis nonparametric test with Bonferroni correction. Univariate association was assessed using chi-square or Fisher’s exact tests. Multivariate analysis was performed using logistic regression models. All statistical analyses were performed using SPSS© software version 25 (IBM).
Results
Thirty chronic peritoneal dialysis patients were included in our study. Seven (23%) under the age of 2 years, 13 (43%) between 2 and 18 years, and ten (33%) adults. Demographic characteristics are presented in Table 1, concise dialysis characteristics are presented in Table 2. Comprehensive PD characteristics are available in Supplemental Table 1.
Quarterly measurements of blood gases and electrolytes are presented in Table 3.
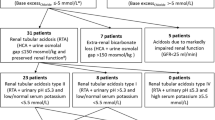
The prevalence of alkalosis in various age groups is presented in Fig. 1. Most of the measurements (90.3%) taken from patients under 2 years of age showed metabolic alkalosis compared to 32.3% in the 2–18-year group and none in the adult group (p < 0.001 for differences between all groups). Of note, the median age of patients with alkalosis in the 2–18-year group was significantly lower than in patients without alkalosis (5.5 years (range 2.1–14.5), compared to 12.1 years (2.1–17.9), respectively).
Of the 30 patients in our study, five were treated with sodium bicarbonate, all of whom were in the 2–18 group. None of them had persistent metabolic alkalosis. Calcium carbonate or sevelamer carbonate was given to three patients in the 0–2 group, ten patients in the 2–18 group, and four patients in the adult group. No association was found between calcium carbonate or sevelamer carbonate treatment and persistent metabolic alkalosis (p = 0.45). In a per-sample analysis, patients receiving carbonate-containing medications were less likely to have metabolic alkalosis (23 of 49 samples (47%) vs. 47/58 (81%), p < 0.001). No patient received diuretic therapy.
Of the 17 patients treated with a dialysate solution containing lactate concentration of 40 mEq/L (Dianeal PD1, Baxter©), 10 (59%) had metabolic alkalosis as opposed to none of the patients treated with a dialysate solution containing lactate concentration of 35 mEq/L (CAPD4, Fresenius©) (p <0.001). Metabolic alkalosis was also highly associated with dialysate lactate concentration in a per-sample analysis (Table 6).
nPCR data was available for 16 out of 30 patients, presented in Table 4. We found a significant difference in nPCR values between the three groups (p < 0.001). Post hoc analysis with Bonferroni correction also revealed a significant difference between all groups (p < 0.01). Surprisingly, alkalosis was associated with low nPCR levels (Table 6).
Nutritional status, presented in Table 5, was studied quarterly starting in the second quarter (3–6 months) of dialysis treatment. As each measurement was assigned to the appropriate age group, measurements from the same child can appear, over time, in two age groups. A significant difference in nutritional status between the three age groups was found (p = 0.002). Post hoc analysis revealed a significant difference between the 0–2 group and both the 2–18 group (p = 0.03) and the > 18 group (p = 0.001), with a higher percentage of underweight among younger infants. Malnutrition was not associated with metabolic alkalosis (Table 6).
No significant difference was found in weekly Kt/V, membrane transport type, or urine output between the three age groups (Table 2). A significant difference in daily dialysate volume per body weight across the age groups was noted (p < 0.001), as well as a significant difference in volume per BSA after adjustment of values in the infant group, as described in the “Methods” section (p = 0.002). Statistical significance of differences between specific age groups is presented in Table 2. Lack of urine output and daily dialysate volume (mL/m2/day) were significantly associated with alkalosis (Table 6). Of note, BSA-corrected daily dialysate volume was higher in anuric patients (mean 9070.1 vs. 8042.2, p = 0.03).
In a multivariate model adjusted for sex and ethnicity, significantly independent predictors for alkalosis were dialysate lactate concentration, lack of urine output, and daily dialysate volume per body surface area, as presented in Table 7. Since age was highly correlated with daily dialysate volume (r = − 0.76, p < 0.001), the model was not adjusted for age to avoid colinearity.
Discussion
PD involves the transport of solutes and water across the peritoneal membrane between the blood in the peritoneal capillaries and the dialysis solution in the peritoneal cavity. Uremic solutes and potassium diffuse from the peritoneal capillary blood into the dialysis solution, whereas glucose, lactate, or bicarbonate and calcium diffuse in the opposite direction to maintain homeostasis. In fact, PD was shown to be quite efficient in controlling metabolic acidosis [4]. In a small study comparing different dialysis modalities (continuous ambulatory peritoneal dialysis (CAPD), automated peritoneal dialysis (APD), and hemodialysis (HD)) in pediatric patients, metabolic acidosis was better controlled in PD patients compared to HD patients. Although serum bicarbonate levels were higher in PD patients in comparison to HD patients (24 ± 2 for CAPD, 22 ± 2 for APD, and 21 ± 2 mEq/L for HD (p value < 0.05)), metabolic alkalosis was not noted [4].
In the current study, we found a significantly higher rate of metabolic alkalosis among PD-treated infants compared to children, adolescents, and adults.
Persistent chronic metabolic alkalosis (serum bicarbonate > 26 mEq/L) was associated with increased risk of heart failure events and mortality in a large adult CKD cohort [5]. Severe acute metabolic alkalosis was also associated with substantial mortality [6]. It is accepted to view severe alkalosis with concern, and to consider correction when the arterial blood pH exceeds 7.55 [7].
No specific symptoms were attributed to metabolic alkalosis in our cohort, but the data may be incomplete due to the retrospective design of this study.
NKF KDOQI guidelines recommend lower target dwell volumes for infants under 2 years of age. This is in accordance with a study by Warady et al. that found higher mass transfer area coefficients (MTAC) in infants, reflecting a higher solute transport capacity in younger children [8]. The difference in MTAC may be explained by maturational changes in the peritoneal membrane. We therefore adjusted the dialysate volume in infants to allow comparison with the older patients. Higher daily exchange volume enables higher solute elimination and lactate or bicarbonate absorption. In our study, adjusted daily dialysate volume per body surface area was negatively correlated with age, with the youngest patients having the highest size-adjusted volume. This reflects a higher number of short dwells in infants as can be seen in Supplemental Table 1. Frequent dialysate exchanges enable maintaining maximal concentration gradient during a large part of dialysis treatment, which can result in higher bicarbonate absorption. Similarly, chloride concentration was lowest in the young age group, possibly reflecting a higher elimination rate.
Lack of urine output was also a significant predictor of metabolic alkalosis in our cohort. Mean dialysate exchange volume was higher in anuric patients, but lack of urine output remained a significant independent predictor in the multivariate model.
PD fluid contains electrolytes, an osmotic agent such as dextrose and lactate or bicarbonate as a buffer. A higher base concentration in the dialysate solution can cause metabolic alkalosis, as reported in adult CAPD patients whose dialysate solution was changed from high calcium-low lactate concentration (35 mmol/L) to low calcium-high lactate (40 mmol/L) concentration [9]. High lactate concentration in the dialysate was also associated with metabolic alkalosis in our study.
Alkalosis in our cohort was not explained by increased hydrogen loss via vomiting or diuretic therapy. Patients treated with carbonate-containing medications such as sodium bicarbonate, calcium carbonate, or sevelamer carbonate were significantly less likely to be found with metabolic alkalosis; hence, carbonate-containing medications were not the explanation for metabolic alkalosis. None of our patients were treated with parenteral nutrition, which is another potential source of base intake.
A number of additional potential mechanisms which may be responsible for metabolic alkalosis in the context of PD are not supported by our findings.
High dietary protein increases net acid generation, resulting in metabolic acidosis [2]. PD patients require high protein intake due to protein losses through the peritoneal membrane. According to NKF KDOQI guidelines, dietary protein intake should be 100% of the dietary reference intake (DRI) for ideal body weight plus an allowance for dialytic protein and amino acid losses [10]. Normalized protein catabolic rate (nPCR) is a formula commonly used to assess dietary nutritional adequacy in dialysis patients, and reported in grams of urea nitrogen per kilogram body weight per day. It is an indirect measurement of protein intake. Most adult guidelines specify maintaining nPCR values above 1.2 g/kg/day in dialysis [11] with values less than 0.8 g/kg/day being equated with protein malnutrition. In our study, nPCR values were significantly higher in the youngest age group, which had the highest rate of metabolic alkalosis. This suggests that nutritional inadequacy or protein malnutrition were not the cause of metabolic alkalosis in this group. However, nPCR is a measure derived from adult data and has been shown to be an inaccurate measure of nutritional status in young hemodialysis patients [12]. Additional study is recommended to clarify this association. In a study by Kung [13] that followed 43 PD patients for 1 year, metabolic alkalosis was found in severely malnourished patients but not in well-nourished patients or patients with mild-to-moderate malnutrition. This was explained by low protein diet associated with lower acid production. However, malnutrition was not associated with metabolic alkalosis in our study.
PET is a semiquantitative assessment of peritoneal transport function in patients on PD. Solute transport rates are evaluated by the equilibration between the peritoneal capillary blood and dialysate. Kang et al. reported that lactate and dialytic base gain, resulting in increased pH and bicarbonate levels, were significantly higher in CAPD patients with high transporter membrane type compared to patients with low transporter membrane type [14]. We did not find significant difference in membrane transport type between the age groups; likewise, membrane type was not associated with metabolic alkalosis. However, PET was performed infrequently in our study group and membrane type may have changed over time.
The limitations of this study include a retrospective design and a relatively small number of participants.
In conclusion, we report that metabolic alkalosis is quite prevalent among infants treated with PD. Possible predictors include dialysate with high lactate concentration, lack of urine output, and high BSA-adjusted daily dialysate volume, which may reflect higher bicarbonate absorption. Acid-base status should be closely followed in this age group and using a dialysis solution with lower bicarbonate or lactate level should be considered. A larger prospective study that systematically explores factors that may lead to metabolic alkalosis among infants on PD is warranted.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- CKD:
-
Chronic kidney disease
- GFR:
-
Glomerular filtration rate
- PD:
-
Peritoneal dialysis
- IRB:
-
Institutional Review Board
- PET:
-
Peritoneal equilibration test
- nPCR:
-
Normalized protein catabolic rate
- WHO:
-
World Health Organization
- CDC:
-
Centers for Disease Control and Prevention
- BMI:
-
Body mass index
- NIH:
-
National Institute of Health
- NKF KDOQI:
-
National Kidney Foundation Kidney Disease Outcomes Quality Initiative
- BSA:
-
Body surface area
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- CAPD:
-
Continuous ambulatory peritoneal dialysis
- APD:
-
Automated peritoneal dialysis
- HD:
-
Hemodialysis
- DRI:
-
Dietary reference intake
References
Kovesdy CP (2012) Metabolic acidosis and kidney disease: does bicarbonate therapy slow the progression of CKD? Nephrol Dial Transplant 27:3056–3062
Uribarri J, Levin NW, Delmez J, Depner TA, Ornt D, Owen W, Yan G (1999) Association of acidosis and nutritional parameters in hemodialysis patients. Am J Kidney Dis 34:493–499
Tschudy MM, Arcara KM (2012) The Harriet Lane handbook: a manual for pediatric house officers, 19th edn. Elsevier, Philadelphia, p 641
Kaiser BA, Polinsky MS, Stover J, Morgenstern BZ, Baluarte HJ (1994) Growth of children following the initiation of dialysis: a comparison of three dialysis modalities. Pediatr Nephrol 8:733–738
Dobre M, Yang W, Pan Q, Appel L, Bellovich K, Chen J, Feldman H, Fischer MJ, Ham LL, Hostetter T, Jaar BG, Kallem RR, Rosas SE, Scialla JJ, Wolf M, Rahman M, CRIC Study Investigators (2015) Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): a report from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Heart Assoc 4:e001599
Anderson LE, Henrich WL (1987) Alkalemia-associated morbidity and mortality in medical and surgical patients. South Med J 80:729–733
Galla JH (2000) Metabolic alkalosis. J Am Soc Nephrol 11:369–375
Warady BA, Alexander SR, Hossli S, Vonesh E, Geary D, Watkins S, Salusky IB, Kohaut EC (1996) Peritoneal membrane transport function in children receiving long-term dialysis. J Am Soc Nephrol 7:2385–2391
Tattersall JE, Dick C, Doyle S, Greenwood RN, Farrington K (1995) Alkalosis and hypomagnesaemia: unwanted effects of a low-calcium CAPD solution. Nephrol Dial Transplant 10:258–262
KDOQI Working Group (2009) KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis 53:S11–S104
KDOQI Working Group (2000) KDOQI Clinical practice guideline for nutrition in CKD. Am J Kidney Dis 3576(3 supp1):S1-S107
Juarez-Congelosi M, Orellana P, Goldstein SL (2007) Normalized protein catabolic rate versus serum albumin as a nutrition status marker in pediatric patients receiving hemodialysis. J Ren Nutr 17:269–274
Kung SC, Morse SA, Bloom E, Raja RM (2001) Acid-base balance and nutrition in peritoneal dialysis. Adv Perit Dial 17:235–237
Kang DH, Yoon KI, Lee HY, Han DS (1998) Impact of peritoneal membrane transport characteristics on acid-base status in CAPD patients. Perit Dial Int 18:294–302
Author information
Authors and Affiliations
Contributions
STB and EBS contributed to the study conception and design. STB, AB, and EBS performed data collection and analysis. The first draft of the manuscript was written by STB, and AB, RBC, CR, JWG, SC, LS, YF, and EBS commented on previous versions of the manuscript. All authors read and approved the final manuscript. As the corresponding author, Shimrit Tzvi-Behr confirms that the manuscript has been read and approved for submission by all the named authors.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board (IRB) at the Shaare Zedek Medical Center (Approval number: 0272-19-SZMC). We declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tzvi-Behr, S., Bnaya, A., Becker-Cohen, R. et al. Metabolic alkalosis in infants treated with peritoneal dialysis. Pediatr Nephrol 37, 1889–1895 (2022). https://doi.org/10.1007/s00467-021-05344-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05344-w