Abstract
Background
Corticosteroid minimisation immunosuppressive protocols (CMP) for children are an approach to safely reduce unwanted medication side effects associated with long-term exposure following kidney transplantation. Here, we provide data regarding the incidence of acute rejection and growth over an extended follow-up in children receiving the CMP used in our centre.
Methods
We retrospectively analysed all children treated with a CMP who received a kidney transplant and had follow-up care in our centre between 2009 and 2019. Data were compared to 5 control groups from recent studies.
Results
Ninety-nine kidney allograft recipients were included in the study (mean follow-up 4.4 years). There was no difference in the cumulative frequency of acute rejection in CMP-treated graft recipients compared to controls. Graft function at latest follow-up was significantly lower in graft recipients experiencing acute rejection compared to those without acute rejection (53.7 mL/min/1.73 m2 vs. 66.8 mL/min/1.73 m2, p = 0.021). Children experiencing >1 acute rejection episode had a greatly elevated risk of graft failure (p = 0.0009, OR 68.25). At latest follow-up, 64/90 (71.1%) graft recipients had a normal height, and younger graft recipients demonstrated greater catch up growth than older children. CMP-treated graft recipients showed a reduced rate of height deficit (28.9% vs. 55.1%, p = 0.0025), less obesity (12.2% vs. 23.9%, p = 0.031), and reduced rates of hypertension (35.4% vs. 68.2%, p< 0.0001).
Conclusions
Children treated with a CMP show greater height attainment, lower frequency of obesity, and reduced rates of hypertension, without an increased risk of acute rejection.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approaches to improving the long-term outcome of paediatric kidney allograft recipients have included attempts to ameliorate the deleterious effects of specific immunosuppressive medication. Corticosteroids have played a principal role in reducing rates of early rejection following transplantation but are associated with problematic side effects including reduced linear growth velocity, hypertension, osteoporosis, obesity, and diabetes mellitus [1].
Several groups have developed corticosteroid avoidance/early withdrawal strategies: a meta-analysis involving 5 randomised controlled trials showed this strategy could be achieved without an increase in acute rejection [2,3,4,5,6,7]. The Trial to Assess the Impact of Early Steroid Withdrawal on Growth in Paediatric Renal Transplantation (TWIST) demonstrated that an immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil (MMF), 2 doses of daclizumab, and corticosteroids until day 4 could lead to improved growth without an increased rate of acute rejection or graft failure at 2 years post-transplant [3, 8]. Another study demonstrated that corticosteroid withdrawal within the first 6 months post-transplant was associated with the eventual attainment of normal adult height in 94% of kidney allograft recipients transplanted before the onset of puberty [9].
Short stature and obesity continue to be a common problem among paediatric kidney transplant recipients. European data involving over 3000 paediatric kidney transplant recipients demonstrated that only 55.1% of children have a height within the normal range [10], and UK data reported that 52.8% of paediatric transplant recipients are overweight or obese 4 years post-transplant [11].
Limited data are available on the frequency of acute rejection and growth outcomes associated with the TWIST protocol, as the initial investigation provided complete 2-year follow-up data on only 53 graft recipients [3]. The aim of the current study was to report the rate of acute rejection and growth outcomes with extended follow-up among paediatric kidney transplant recipients receiving the TWIST immunosuppressive regimen.
Methods
Study design
This is a retrospective cohort analysis of children who underwent a kidney transplant and received follow-up care at the Royal Manchester Children’s Hospital (RMCH) (UK). Case notes were reviewed from June 2009 to January 2019 and graft recipients who met the following requirements were included in the study: (i) Aged < 19 years at time of transplantation; (ii) a minimum of 12 months follow-up data; and (iii) treated with TWIST immunosuppressive regimen. This cohort is described as the ‘study cohort’ in the manuscript. For graft recipients who received more than one transplant, the first transplant was evaluated.
Clinical and serological data from time of transplant to latest follow-up were evaluated from case notes. Estimated glomerular filtration rate (eGFR) of patients younger than 18 years was calculated using the revised Schwartz formula [12]. eGFR of patients older than 18 years was calculated using the modification of diet in renal disease (MDRD) study equation [13]. Graft failure was defined as the requirement to return to dialysis after the first week post-transplantation. Kidney biopsies were performed if a graft recipient recorded a > 15% rise in plasma creatinine once infection, obstruction, and drug toxicity had been excluded. Biopsy-proven acute rejection (BPAR) was diagnosed by a pathologist according to the respective most recent Banff classification [14]. Corticosteroid-resistant BPAR was defined as the failure of serum creatinine to revert to within 20% of baseline at least 3 days after completion of 3–5 days of intravenously administered methylprednisolone (15 mg/kg, maximum 750 mg) [15]. The study was reviewed by the Clinical Trial Management Department (Manchester University NHS Foundation Trust) who waived the requirement for ethical approvement for this study involving the collection of anonymised data. They concluded that informed consent was not required from study participants. The study was performed in accordance with Manchester University NHS Foundation Trust guidelines. No patient identifiable data were recorded.
Immunosuppressive regimen
Children were treated with the TWIST protocol. This involves induction therapy with the interleukin 2 receptor monoclonal antibody antagonist, basiliximab, on day 0 and day 4 post-transplantation, coupled with a rapidly weaning course of prednisolone which is discontinued on day 5. Long-term immunosuppression is provided by tacrolimus and MMF [8]. Target plasma trough levels of tacrolimus were 8–12 μg/L for weeks 1–3 post-transplant and 5–8 μg/L later than 3 weeks post-transplant. MMF target dosing was 600 mg/m2 twice daily (bd) during the first 2 weeks post-transplant and 300 mg/m2 bd after this period.
Analysis of height, weight, BMI, and hypertension
Standard deviation scores (SDS) for height, weight, and BMI were calculated for study participants using the following formula: SDS = (value of individual − value in standard population)/standard deviation (SD) standard population. Changes in SDS score at 1 year, 2 year, and latest follow-up were calculated using the following formula: SDS at study time point (i.e. 1 year, 2 year, latest follow-up) − SDS at the time of transplant (‘baseline’). SDS values were calculated from British 1990 reference values [16, 17]. Height was measured in the children while standing against the vertical plane of a stadiometer with feet flat on the floor without shoes and socks.
Height deficit was defined as height SDS < −1.88 SDS, and further subdivided into moderate (−1.88 > SDS > −3) or severe (< −3 SDS) [10]. The International Obesity Taskforce (IOTF) definition was used to define overweight and obesity. Obesity was defined as BMI (body mass index) SDS score ≥ 2.25 in girls and ≥ 2.37 in boys. Overweight is defined as BMI SDS score ≥ 1.19 in girls and ≥ 1.30 in boys. Underweight is defined as BMI score < −2.16 for boys and < − 2.00 for girls [11, 18].
Hypertension was defined as the need for treatment with antihypertensive medicines [8].
Control groups
Rates of BPAR were compared to data from an American multicentre trial comparing a corticosteroid-based to a corticosteroid-free immunosuppressive regimen with a 3-year follow-up period (‘control group 1’) [5]. The study cohort results were compared to the corticosteroid-based group (n = 70).
Changes in height in the study cohort were compared to data from the European Society for Paediatric Nephrology/European Renal Association and European Dialysis and Transplant Association (ESPN/ERA-EDTA) registry analysis of 3492 paediatric transplant recipients transplanted between 1990 and 2012 (‘control group 2’) [10].
‘Control group 3’ consisted of data from children in the corticosteroid continuation arm of the TWIST study (n = 59 at 1-year follow-up; n = 53 at 2-year follow-up) [3].
Weight/BMI data from the study cohort were compared to an analysis of 159 paediatric transplant recipients from 12 centres in the UK (‘control group 4’) [11].
Use of antihypertensive medication was compared to a study from the Cooperative European Paediatric Renal Transplant Initiative Registry (CERTAIN) involving 336 paediatric transplant recipients with 3 years follow-up (‘control group 5’) [19].
Statistical analysis
Univariate analysis of risk factors was performed using Fisher’s exact test (two-tailed) or chi-squared test for categorical variables, and the nonparametric Mann–Whitney U test for quantitative variables. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated using the Baptista-Pike method [20].
Results
Study population
Ninety-nine graft recipients were eligible for study inclusion with a mean follow-up of 4.4 years (Table 1). The mean age of study participants at time of transplant was 10.8 years. Seven out of ninety-nine (7.1%) graft recipients experienced graft failure, at a mean time of 44.0 months post-transplant (range 18–70 months). There were 2 deaths in the cohort. One graft recipient with methylmalonic acidaemia died 14 months post-transplant from acute pancreatitis, encephalopathy, and pneumonia. The second graft recipient with mosaic variegated aneuploidy and renal dysplasia died 49 months post-transplant from acute lymphoblastic leukaemia.
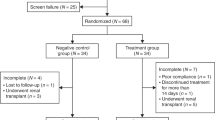
At latest follow-up, 53/99 (53.5%) of graft recipients were receiving tacrolimus and MMF; 12/99 (12.1%) of graft recipients were treated with tacrolimus, MMF, and prednisolone. In graft recipients no longer receiving TWIST at latest follow-up, the most common reason necessitating a medication change was MMF intolerance (Fig. 1). At latest follow-up, 42/99 (42.4%) of graft recipients were receiving corticosteroids as part of their immunosuppressive regimen (Table 1).
Control groups
The mean number of HLA matches between allograft donors and recipients was higher in the study cohort compared to control group 1 for both living donors (4.0 vs. 1.9, p < 0.0001) and deceased donors (4.0 vs. 0.7, p < 0.0001) (Online Resource 1). A lower proportion of study subjects in control groups 1, 2, and 3 received their graft from a living donor compared to the study cohort (Online Resources 1, 2, and 3). There was no difference between the age at transplant and gender distribution in control groups 1, 2, and 3 compared to the study cohort. There was no difference in baseline height between the study cohort and control groups 2 and 3 (Online Resources 2 and 3).
Acute rejection
There were 33 episodes of BPAR in 27/99 graft recipients (27.3%), occurring between 7 days and 5.6 years post-transplant. Fifteen out of thirty-three (45.4%) total BPAR episodes occurred within 6 months of transplant and 20/33 (60.6%) total BPAR episodes occurred within the first 12 months. The annual incidence of BPAR is shown in Fig. 2. Twenty-five out of thirty-three (77.8%) episodes of BPAR occurred in graft recipients receiving tacrolimus and MMF; 2/33 (6.1%) episodes occurred in graft recipients receiving tacrolimus, MMF, and prednisolone.
Incidence of BPAR and graft failure over time. a Percentage of total cohort experiencing BPAR over specified time periods. If a graft recipient had > 1 BPAR episode in a time period, the graft recipient is only recorded once for that period. b Risk of BPAR over time for whole cohort. c Kaplan-Meier estimate of risk of graft failure for graft recipients experiencing no BPAR, single BPAR, and ≥ 2 BPAR episodes (curves compared using log-rank/Mantel-Cox test, p < 0.0001)
The frequency of BPAR in the first year was 18/99 (18.2%). In the sub-cohort of graft recipients who completed at least 36 months of follow-up, 15/68 (22.1%) graft recipients experienced at least 1 BPAR episode within the first 3 years. There was no difference in cumulative BPAR frequency between the study cohort and control group 1 at 1 year (frequency in control group 1 = 18.6%, p = 1.0), or at 3 years post-transplant (frequency in control group 1 = 28.6%, p = 0.44). Of the graft recipients who completed ≥ 60 months follow-up in the study cohort, the BPAR incidence between 25 and 60 months was 2/35 (5.7%).
Donor-specific antibodies (DSAs) were checked in all graft recipients during the episode of BPAR and 5/27 (18.5%) graft recipients recorded positive results. Apart from 1 child who recorded a positive DSA result in the fourth month following transplant, all other positive DSA results occurred later than 1 year post-transplant.
Seven out of thirty-three (21.2%) episodes of BPAR occurred > 2 years post-transplant, involving 5 graft recipients. Two out of five (40.0%) of these graft recipients recorded positive DSA results. Two out of seven graft recipients who developed BPAR later than 2 years post-transplant (aged 15 years and 21 years) had multiple undetectable blood tacrolimus levels and high tacrolimus intra-patient variability (mean absolute deviation 55.8% and 74.2%, based on 3 consecutive readings around time of BPAR). This is indicative of treatment non-adherence. One of these graft recipients subsequently lost their graft due to chronic antibody-mediated rejection (ABMR).
Twenty-five out of thirty-three (75.8%) BPAR episodes were diagnosed as acute T-cell-mediated rejection (TCMR) (Banff Category 4), 4/33 (12.1%) episodes were borderline acute TCMR (Banff Category 3), 2/33 (6.1%) acute (active) ABMR (Banff Category 2), and 2/33 (6.1%) acute vascular rejection. Of the 25 graft recipients with TCMR, Category 3 type is as follows: 12/25 Banff Ia, 10/25 Banff 1b, 1/25 Banff IIa, 1/25 Banff IIb, 1/25 Banff type not specified in report. Five out of twenty-seven (18.5%) graft recipients experienced an episode of corticosteroid-resistant BPAR. Four out of five (80.0%) episodes of corticosteroid-resistant BPAR occurred within the first 6 months post-transplant, with the remaining episode occurring 29 months post-transplant. Two out of five (40.0%) graft recipients with corticosteroid-resistant BPAR recorded a positive DSA result.
No difference in the following characteristics was identified between those experiencing BPAR and those without BPAR: age at transplant, sex, ethnicity, allograft donor (living/deceased), cytomegalovirus serostatus of donor and recipient, cold ischemia time, trough plasma tacrolimus level, frequency of corticosteroids at latest follow-up, rate of graft failure, length of follow-up, and mismatches for human leukocyte antigen (HLA) A, B, and DR (Table 1 and Online Resource 4).
Graft outcome
eGFR at latest follow-up was significantly lower in graft recipients experiencing BPAR compared to those without an episode of BPAR (53.7 mL/min/1.73 m2 vs. 66.8 mL/min/1.73 m2, p = 0.021) (Table 1). Although the eGFR at latest follow-up was lower in the five graft recipients with corticosteroid-resistant BPAR, this was not statistically different to those with corticosteroid-responsive BPAR (39.4 vs. 57.0 mL/min/1.73 m2, p = 0.17).
The proportion of graft recipients who experienced graft failure among those who experienced BPAR (4/27, 14.8%) was numerically larger, but not statistically significantly different, compared to those without BPAR (3/72, 4.2%) (p = 0.086). Three graft recipients with BPAR experienced graft failure due to DSA+ chronic ABMR (all 3 graft recipients experiencing ≥ 2 BPAR episodes). Three graft recipients without BPAR suffered graft failure due to the following reasons: vascular disease/chronic allograft nephropathy (CAN); primary disease recurrence (IgA nephropathy); and DSA+ chronic ABMR following a period of reduced immunosuppression due to Epstein-Barr virus infection. The remaining graft failure was due to CAN (in a graft recipient experiencing a single BPAR episode).
In the 27 graft recipients with at least one episode of BPAR, the rate of graft failure among participants with an episode of corticosteroid-resistant BPAR was 2/5 (40.0%) compared to 2/22 (9.1%) in those with corticosteroid-responsive BPAR (p = 0.14, OR 6.67, 95% CI 0.74–50.35). In the whole cohort of 99 graft recipients, 5/94 (5.3%) graft recipients without an episode of corticosteroid-resistant BPAR experienced graft loss compared to 2/5 (40.0%) in graft recipients with an episode of corticosteroid-resistant BPAR (p = 0.039, OR 11.89, 95% CI 1.70–65.84).
As a previous study suggested multiple BPAR episodes, and first BPAR episode > 6 months post-transplant is associated with an increased risk of graft failure, we investigated whether this was also true in our cohort [21].
In the 27 graft recipients with at least one episode of BPAR, the rate of graft failure among participants with two or more BPAR episodes was 3/4 (75.0%), compared to 1/23 (4.3%) in graft recipients with only a single BPAR episode (p = 0.0053, OR 66.0, 95% CI 4.1–861.7) (Online Resource 5). In the whole cohort of 99 graft recipients, 3/4 graft recipients (75.0%) with two or more BPAR episodes suffered graft failure compared to 4/95 (4.2%) of graft recipients with a single or no BPAR episodes (p = 0.00090, OR 68.25, 95% CI 7.50–882.70) (Fig. 2).
In the 27 graft recipients with at least one episode of BPAR at some point during follow-up, the rate of graft failure among graft recipients experiencing BPAR within the first 6 months post-transplant was 1/15 (6.7%), compared to 3/12 (25.0%) graft recipients whose first BPAR episode occurred after 6 months post-transplant (p = 0.29, OR 0.21, 95% CI 0.02 –1.7).
Linear growth
We compared growth outcomes in graft recipients treated with the corticosteroid-minimisation TWIST regimen to control groups receiving immunosuppressive regimens involving continuous corticosteroid exposure. Six out of ninety-nine graft recipients in the study cohort with the following conditions were excluded from these analyses due to severe short stature associated with the following conditions: Jeune syndrome, Sensenbrenner syndrome, renal dysplasia associated with mosaic variegated aneuploidy.
At latest follow-up, a larger proportion of children in the study cohort recorded a height within the normal range (64/90, 71.1%) compared to control group 2 (1924/3492, 55.1%) (p = 0.0025) (Table 2). The proportion of graft recipients with a severe height deficit in control group 2 was 590/3492 (16.9%) compared to 9/90 (10.0%) in the study cohort at latest follow-up.
There was significantly improved growth in the study cohort subjects at 2 years and latest follow-up compared to control group 3 (Online Resource 6). The mean (standard error of the mean, SEM) change in height SDS (from baseline value) was 0.50 (0.07) compared to 0.22 (0.09) in controls (p = 0.15, two way ANOVA followed by Sidak’s multiple comparisons test) at 1-year follow-up. At 2-year follow-up, the mean (SEM) change in height SDS was 0.76 (0.10) in graft recipients receiving TWIST compared to 0.33 (0.14) in controls (p = 0.017). The mean (SEM) change in height SDS at latest follow-up was 0.72 (0.10) in graft recipients receiving TWIST.
For a boy receiving a kidney transplant at the age of 10.8 years (the mean age at transplant for the cohort), the expected difference in height between a graft recipient treated with TWIST and a graft recipient receiving a corticosteroid-based immunosuppressive regimen would be approximately 8 cm after 4.4 years (the mean length of follow-up for the cohort). In graft recipients transplanted before the age of 12 years, mean absolute height increased by 28.3% for boys and 21.4% for girls (Online Resource 7).
The mean height SDS was lower in study cohort graft recipients who were ≤ 12 years old (−2.3) compared to those who were > 12 years old (−1.5) at time of transplant (Online Resource 8). However, younger graft recipients enjoyed enhanced catch up growth with height SDS changes at latest follow-up (compared to baseline) of 1.1 in those ≤ 12 years old compared to 0.3 in those > 12 years old (Online Resource 6). A significant negative correlation was observed between the age at transplant and change in height SDS (compared to baseline) at latest follow-up (r = −0.54, p < 0.0001) (Fig. 3a). In graft recipients transplanted before attaining final adult height (less than 14 years of age) [22], there was a significant negative correlation between the age of transplant in graft recipients and height SDS change between baseline and latest follow-up (r = −0.43, p = 0.0011) (Fig. 3b).
Correlation between growth after transplant with age and allograft donor source. SDS height change at latest follow-up compared to baseline is plotted against age at transplant. a Whole cohort [Spearman’s nonparametric correlation coefficient (r) is −0.59, p < 0.0001]. b Graft recipients younger than 14 years at age of transplant (r = −0.43, p = 0.0011). c SDS change at latest follow-up in living and deceased donor recipients. Mean values are plotted with error bars representing SEM
There was no difference in the mean (SEM) SDS change between boys and girls between baseline and latest follow-up [0.83 (0.12) vs. 0.48 (0.18), p = 0.10]. A smaller change in height SDS at latest follow-up (compared to baseline) was found in deceased transplant recipients [mean 0.45 (SEM 0.130)] compared to living donor transplant recipients [0.88 (0.14)] (p = 0.037) (Fig. 3c).
BMI and weight
The proportion of graft recipients with a BMI within normal limits was 61/90 (67.8%) in the study cohort compared to 75/159 (47.2%) in control group 4 (Table 3). There were significantly more graft recipients categorised as obese in control group 4 (38/159, 23.9%) compared to the study cohort (11/90, 12.2%) (p = 0.031, Fisher’s exact test). SDS values and changes in SDS values for weight and BMI are shown in Online Resources 9–12.
Growth in graft recipients on TWIST regimen at latest follow-up
The SDS changes at 1 year, 2 years, and latest follow-up for height, weight, and BMI in graft recipients who remained on the TWIST regimen at latest follow-up are shown in Online Resources 13–15. There was no difference between the change in SDS from baseline to latest follow-up in graft recipients who received TWIST compared to those on an alternative regimen, for height [mean 0.72 (SEM, 0.12) vs. 0.71 (0.16), p = 0.91], weight [0.94 (0.19) vs. 0.77 (0.17), p = 0.53], or BMI [0.58 (0.18) vs. 0.35 (0.16), p = 0.20].
Antihypertensive treatment
At latest follow-up, a significantly lower proportion of graft recipients required antihypertensive medication in the study cohort (35/99, 35.4%) compared to control group 5 (229/336, 68.2%) (p < 0.0001) (Table 4).
A lower proportion of graft recipients treated with TWIST required antihypertensive medications at 1-year (34.3% vs. 54.7%, p = 0.046), and 2-year follow-up (32.1% vs. 54.7%, p = 0.012) compared to control group 3 (Online Resource 16). Of the 35 graft recipients requiring antihypertensive medication at latest follow-up, 11/35 (31.4%) were hypertensive at time of transplant.
Discussion
The key findings from this study investigating BPAR and growth outcomes in paediatric kidney transplant recipients treated with the TWIST immunosuppressive protocol are (i) with a mean follow-up time of 4.4 years, 72.7% grafts remained BPAR-free; (ii) 21.2% BPAR episodes occurred later than 2 years post-transplant; (iii) there was no difference in BPAR rates between graft recipients receiving TWIST and those receiving a corticosteroid-based regimen; (iv) graft recipients experiencing BPAR had worse kidney function at latest follow-up compared to those without BPAR, but only those with multiple BPAR episodes had an increased rate of graft failure; (v) graft recipients treated with TWIST showed reduced rates of obesity and hypertension, with greater height attainment; and (vi) younger children at transplant enjoyed greater catch up linear growth than older children.
Approximately half of European paediatric kidney transplant recipients have a height deficit and demonstrate only limited catch up growth following transplant [10]. Data from the North American Paediatric Renal Trials and Collaborative Studies (NAPRTCS) showed that at time of transplant the average graft recipient is shorter than the fourth percentile compared to population controls (−1.73 SDS) and following transplant, height SDS only increases 0.1 SD every 2 years [23].
Our data indicates that although graft recipients are short at time of transplant (mean −1.89 height SDS), the corticosteroid-sparing TWIST regimen is associated with larger changes in height SDS (0.72 overall), with enhanced catch up growth seen in graft recipients ≤ 12 years old at time of transplant (1.11 increase in height SDS). 71.1% of the study cohort attained a height within the normal range at time of latest follow-up compared to 55.1% in control group 2. Supporting the observation of improved linear growth in graft recipients treated with TWIST, a study involving a large cohort of paediatric transplant recipients identified an association between corticosteroid-free immunosuppression and improved post-transplant height SDS [10]. We also note that no difference in height SDS change was identified in the study cohort between graft recipients still receiving TWIST at latest follow-up compared to those who had switched to an alternative regimen. This suggests that the lack of significant corticosteroid exposure early in the post-transplant period may be particularly beneficial to improving linear growth.
Approximately one-third of paediatric transplant recipients in the UK are overweight or obese at the time of transplant, and this rises to over half by 4 years post-transplant [11]. In contrast, we found that only 27% of our cohort were overweight/obese at the end of the study period, with 68% of graft recipients recording a BMI within the normal range.
We report that 35.4% of our cohort required antihypertensive medication at latest follow-up. This figure is significantly lower than found in a study analysing data from the CERTAIN registry [19], and also lower than reported by NAPRTCS, who found 71% of deceased donor recipients and 58% of live donor recipients required antihypertensive medication at 5 years post-transplant [23].
Our overall rate of allograft survival (92.9%) is similar to data from the UK transplant registry involving 3236 paediatric transplants (performed between 1992 and 2016), which reported allograft survival rates of 89% at 1 year and 79% at 5 years [24].
European data involving 298 allografts in paediatric recipients receiving a corticosteroid-based immunosuppressive regimen demonstrated that first occurrence of BPAR > 6 months post-transplant and multiple BPAR episodes were risk factors for graft failure [21]. In this study, we also identified graft recipients experiencing multiple BPAR episodes as a group at high risk of graft failure. We did not identify late first BPAR occurrence as a risk factor for graft failure.
Our rates of BPAR are comparable to data from the corticosteroid-based regimen arm of a randomised multicentre trial investigating a corticosteroid-avoidance regimen at 1 year and 3 years post-transplant [5]. However, the corticosteroid withdrawal group in the TWIST study reported 10.2% grafts suffered BPAR at 6 months follow-up, and approximately 82% of grafts were free from BPAR at 2 years [3, 8]. Both our 6-month (15.2%) and 1-year (18.2%) BPAR rates are higher than reported in TWIST. The reasons for these differences are not entirely clear. Our cohort has similar age at transplantation, sex distribution, and mean total HLA mismatches as participants in the TWIST study [8]. However, the proportion of deceased donor recipients was much higher in the TWIST study compared to the current investigation (68.4% vs. 39.4%), and the current study recorded higher levels of deviation from TWIST protocol at 6 months (18.2% vs. 12.2%). Our observed adjusted change in height at latest follow is similar to that seen in TWIST, and we also recorded greater height attainment in younger children at time of transplant. We cannot exclude the possibility of a slightly increased risk of early BPAR, but these data suggest that use of the TWIST regimen does not impart an increased risk of BPAR in the long term.
Limitations of the current study include the retrospective approach, use of historical control groups, and relatively small number of subjects in comparison to multi-centre investigations. The mean number of HLA matches between allograft donors and recipients, and the proportion of living allograft donors, was higher in the study cohort compared to control group 1. Poorer HLA matching may have partly contributed to the increased BPAR rates observed in control group 1. Additionally, we reported the proportion of graft recipients receiving antihypertensive medication (as per the original TWIST studies) rather than blood pressure measurements [3, 8]. The threshold for prescribing antihypertensive medication may differ between centres, which may have biased the results.
In summary, this study demonstrates that corticosteroid minimisation immunosuppression protocols are not associated with an increased risk of BPAR or graft failure in the long term. Children who were initiated on this corticosteroid minimisation protocol demonstrate greater linear growth, reduced rates of obesity, and less requirement for antihypertensive medication.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Fine RN, Ho M, Tejani A (2001) The contribution of renal transplantation to final adult height: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr Nephrol 16:951–956. https://doi.org/10.1007/s004670100002
Zhang H, Zheng Y, Liu L, Fu Q, Li J, Huang Q, Liu H, Deng R, Wang C (2016) Steroid avoidance or withdrawal regimens in paediatric kidney transplantation: a meta-analysis of randomised controlled trials. PLoS One 11:e0146523. https://doi.org/10.1371/journal.pone.0146523
Webb NJA, Douglas SE, Rajai A, Roberts SA, Grenda R, Marks SD, Watson AR, Fitzpatrick M, Vondrak K, Maxwell H, Jaray J, Van Damme-Lombaerts R, Milford DV, Godefroid N, Cochat P, Ognjanovic M, Murer L, McCulloch M, Tönshoff B (2015) Corticosteroid-free kidney transplantation improves growth: 2-year follow-up of the TWIST randomized controlled trial. Transplantation 99:1178–1185. https://doi.org/10.1097/TP.0000000000000498
Mericq V, Salas P, Pinto V, Cano F, Reyes L, Brown K, Gonzalez M, Michea L, Delgado I, Delucchi A (2013) Steroid withdrawal in pediatric kidney transplant allows better growth, lipids and body composition: a randomized controlled trial. Horm Res Paediatr 79:88–96. https://doi.org/10.1159/000347024
Sarwal MM, Ettenger RB, Dharnidharka V, Benfield M, Mathias R, Portale A, McDonald R, Harmon W, Kershaw D, Vehaskari VM, Kamil E, Baluarte HJ, Warady B, Tang L, Liu J, Li L, Naesens M, Sigdel T, Waskerwitz J, Salvatierra O (2012) Complete steroid avoidance is effective and safe in children with renal transplants: a multicenter randomized trial with three-year follow-up. Am J Transplant 12:2719–2729. https://doi.org/10.1111/j.1600-6143.2012.04145.x
Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, Pohl M, Zimmering M, Fründ S, Klaus G, Wühl E, Tönshoff B (2010) Improved growth and cardiovascular risk after late steroid withdrawal: 2-year results of a prospective, randomised trial in paediatric renal transplantation. Nephrol Dial Transplant 25:617–624. https://doi.org/10.1093/ndt/gfp506
Benfield MR, Bartosh S, Ikle D, Warshaw B, Bridges N, Morrison Y, Harmon W (2010) A randomized double-blind, placebo controlled trial of steroid withdrawal after pediatric renal transplantation. Am J Transplant 10:81–88. https://doi.org/10.1111/j.1600-6143.2009.02767.x
Grenda R, Watson A, Trompeter R, Tönshoff B, Jaray J, Fitzpatrick M, Murer L, Vondrak K, Maxwell H, van Damme-Lombaerts R, Loirat C, Mor E, Cochat P, Milford DV, Brown M, Webb NJ (2010) A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant 10:828–836. https://doi.org/10.1111/j.1600-6143.2010.03047.x
Klare B, Montoya CR, Fischer DC, Stangl MJ, Haffner D (2012) Normal adult height after steroid-withdrawal within 6 months of pediatric kidney transplantation: a 20 years single center experience. Transpl Int 25:276–282. https://doi.org/10.1111/j.1432-2277.2011.01400.x
Bonthuis M, Groothoff JW, Ariceta G, Baiko S, Battelino N, Bjerre A, Cransberg K, Kolvek G, Maxwell H, Miteva P, Molchanova MS, Neuhaus TJ, Pape L, Reusz G, Rousset-Rouviere C, Sandes AR, Topaloglu R, Van Dyck M, Ylinen E, Zagozdzon I, Jager KJ, Harambat J (2019) Growth patterns after kidney transplantation in European children over the past 25 years: an ESPN/ERA-EDTA registry study. Transplantation 104:137–144. https://doi.org/10.1097/TP.0000000000002726
Plumb LA, Pitcher D, Tse Y, Shield JP, Inward C, Sinha MD, British Association for Paediatric Nephrology (2014) Longitudinal changes in body mass index following renal transplantation in UK children. Nephrol Dial Transplant 29:196–203. https://doi.org/10.1093/ndt/gft395
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. https://doi.org/10.1681/ASN.2008030287
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254. https://doi.org/10.7326/0003-4819-145-4-200608150-00004
Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee (2014) Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14:272–283. https://doi.org/10.1111/ajt.12590
Bock HA (2001) Steroid-resistant kidney transplant rejection: diagnosis and treatment. J Am Soc Nephrol 12:S48–S52
Cole TJ, Freeman JV, Preece MA (1998) British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 17:407–429. https://doi.org/10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L
Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA (1995) Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 73:17–24. https://doi.org/10.1136/adc.73.1.17
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243. https://doi.org/10.1136/bmj.320.7244.1240
Sugianto RI, Schmidt BMW, Memaran N, Duzova A, Topaloglu R, Seeman T, König S, Dello Strologo L, Murer L, Özçakar ZB, Bald M, Shenoy M, Buescher A, Hoyer PF, Pohl M, Billing H, Oh J, Staude H, Pohl M, Genc G, Klaus G, Alparslan C, Grenda R, Rubik J, Krupka K, Tönshoff B, Wühl E, Melk A (2020) Sex and age as determinants for high blood pressure in pediatric renal transplant recipients: a longitudinal analysis of the CERTAIN Registry. Pediatr Nephrol 35:415–426. https://doi.org/10.1007/s00467-019-04395-4
Baptista J, Pike MC (1977) Algorithm AS 115: exact two-sided confidence limits for the odds ratio in a 2 × 2 table. J R Stat Soc Ser C Appl Stat 26:214. https://doi.org/10.2307/2347041
Guyot C, Nguyen J-M, Cochat P, Foulard M, Bouissou F, Van Damme-Lombaerts R, Loirat C, Janssen F, Bensman A, Nivet H, Fischbach M, Guignard JP, André JL (1996) Risk factors for chronic rejection in pediatric renal allograft recipients. Pediatr Nephrol 10:723–727. https://doi.org/10.1007/s004670050199
Nissel R, Brázda I, Feneberg R, Wigger M, Greiner C, Querfeld U, Haffner D (2004) Effect of renal transplantation in childhood on longitudinal growth and adult height. Kidney Int 66:792–800. https://doi.org/10.1111/j.1523-1755.2004.00805.x
NAPRTCS (2014) Annual Transplant Report. https://naprtcs.org/system/files/2014_Annual_Transplant_Report.pdf. Accessed 19 Oct 2020
Mumford L, Maxwell H, Ahmad N, Marks SD, Tizard J (2019) The impact of changing practice on improved outcomes of paediatric renal transplantation in the United Kingdom: a 25 years review. Transpl Int 32:751–761. https://doi.org/10.1111/tri.13418
Acknowledgements
We thank Prof. Rachel Lennon and Dr. Nick Plant for their advice during the preparation of this manuscript.
Funding
The study was supported by a Jean Shanks/Pathological Society Clinical Lecturer Grant (Grant reference: JSPS CLG 2019 02) (awarded to JM).
Author information
Authors and Affiliations
Contributions
JM and MS designed the study and analysed data. Both authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was assessed by the RMCH research and development department at the time of study inception, and it was confirmed that formal ethical approval was not required for this case note review study. No patient identifiable data were recorded.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 159 KB)
Rights and permissions
About this article
Cite this article
McCaffrey, J., Shenoy, M. Acute rejection and growth outcomes in paediatric kidney allograft recipients treated with a corticosteroid minimisation immunosuppressive protocol. Pediatr Nephrol 36, 2463–2472 (2021). https://doi.org/10.1007/s00467-021-04948-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-04948-6