Abstract
Background
In its first 3 years, the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative demonstrated a statistically significant increase in the likelihood of compliance with a standardized follow-up care bundle and a significant reduction in peritonitis. We sought to determine if compliance with care bundles and low peritonitis rates could be sustained in centers continuously participating for 84 months.
Methods
Centers that participated from collaborative launch through the 84-month study period and provided pre-launch peritonitis rates were included. Children on maintenance peritoneal dialysis were eligible for enrollment. Changes in bundle compliance were assessed using a logistic regression model or a generalized linear mixed model (GLMM). Changes in average annualized peritonitis rates over time were modeled using GLMMs.
Results
Nineteen centers contributed 1055 patients with 1268 catheters and 17,247 follow-up encounters. The likelihood of follow-up compliance increased significantly over the study period (OR 1.05 95% confidence interval (CI) 1.03, 1.07; p < 0.001). Centers achieved ≥ 80% follow-up bundle compliance by 28 months and maintained a mean compliance of 84% between 28 and 84 months post-launch. Average monthly peritonitis rates decreased from 0.53 (95% CI 0.37, 0.70) infections per patient-year pre-launch to 0.30 (95% CI 0.23, 0.43) at 84 months post-launch, p < 0.001.
Conclusions
Centers participating in the SCOPE Collaborative for 84 months achieved and maintained a high level of compliance with a standardized follow-up care bundle and demonstrated a significant and continued reduction in average monthly peritonitis rates.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritonitis is a significant complication of maintenance peritoneal dialysis (PD) in children, and a leading contributor to hospitalization, PD failure, and mortality [1,2,3]. The Standardizing Care to Improve Outcomes in Pediatric End Stage Kidney Disease (SCOPE) Collaborative is a multi-center national quality transformation network that uses quality improvement methodology to increase implementation of standardized care practices in an effort to improve outcomes [4]. SCOPE previously demonstrated a significant increase in the implementation of a standardized follow-up care bundle for PD catheter care and a concomitant significant decrease in the center peritonitis rates among centers participating in the first 36 months of the collaborative, from its launch on October 1, 2011, through September 30, 2014 [5]. A sensitivity analysis revealed that across the collaborative, a threshold of 80% compliance with the follow-up bundle was associated with a significant reduction in peritonitis rates [5]. The subsequent patient-level analysis also demonstrated that compliance with the follow-up care bundle was associated with a decreased risk for peritonitis [6]. This manuscript describes the results of the first 7 years of the SCOPE Collaborative, with a focus on the rates of compliance with standardized care bundles and peritonitis rates among centers that have continuously participated in the collaborative since its launch and from which pre-launch infection rates were submitted.
Methods
Collaborative structure
The SCOPE Collaborative is a partnership among the quality improvement coaches and staff at Children’s Hospital Association (CHA), a multi-disciplinary and multi-institutional core faculty, and healthcare professionals at 52 participating pediatric dialysis centers in the USA [4]. Details of the collaborative structure and the quality improvement process utilized have been previously described [4].
Care bundles
The SCOPE PD project aims to increase the implementation of standardized bundles focused on key areas of PD catheter care: catheter insertion, PD training of the child/caregiver, and maintenance or follow-up care. The components of the care bundles are shown in Table 1 [4].
Measures and data
All children who received maintenance PD at the participating centers were eligible for enrollment in SCOPE, including neonates who initiated maintenance PD during their initial hospitalization but who had not yet established outpatient dialysis care. However, because available pre-launch baseline data was submitted only from children receiving PD in the outpatient setting, the post-launch analysis included infections and catheter months at risk for neonates on maintenance PD only after outpatient dialysis care was established. Age at enrollment for these patients was designated as the date of discharge from the initial hospitalization. For non-neonates, age at enrollment was the date of catheter insertion for incident patients and the date that the center began participating in SCOPE for prevalent patients.
Demographic data collected at enrollment included: age, race/ethnicity (Black, White, Hispanic, other), sex, and cause of kidney failure. Centers reported peritonitis episodes, including culture results, and compliance with the bundle care practices at monthly intervals following the collaborative launch. Compliance for the care bundles was assessed as all-or-none, meaning that each catheter insertion, training session, or follow-up event had to comply with all the components of the respective bundle to be considered compliant. In children who had more than one catheter inserted during the study period, each insertion was scored as a separate event. For children enrolled in SCOPE while already on PD (prevalent patients), data on catheter insertion were collected, but not scored for compliance. The collaborative staff conducted ongoing monitoring of data entry and provided biannual missing data reports to centers to encourage complete data entry. To confirm full reporting of infection events, centers were contacted prior to the data lock for the current analysis.
Baseline data collected from the centers included the number of patients, peritonitis episodes, and patient demographics for the 13 months prior to the collaborative launch. For baseline data, age at the start of the 13-month period was submitted for prevalent patients and age at dialysis initiation submitted for patients initiating dialysis during the 13-month pre-study period.
Outcomes
While the outcome measures for the SCOPE Collaborative were exit-site infection and peritonitis rates, the current analysis focuses on peritonitis rates. Centers reported peritonitis episodes as clinically diagnosed but were encouraged to follow the International Society of Peritoneal Dialysis (ISPD) guidance for the detection and diagnosis of peritonitis [7]. Per ISPD guidelines, relapsing peritonitis, defined as a recurrence of peritonitis with the same organism according to antibiotic susceptibilities as in the immediately preceding episode, or a second culture-negative infection within 4 weeks of completion of antibiotic treatment, was not counted as a new infection [7].
Statistical considerations
Categorical descriptive statistics were summarized using frequencies and percentages; continuous descriptive statistics were summarized using medians with interquartile ranges. Categorical patient demographics pre- and post-collaborative launch and distribution of peritonitis organisms were compared using a chi-square test of association; the difference in continuous variables was assessed with a Wilcoxon signed-rank test. Changes in patient demographics, distribution of causative organisms, and risk factors associated with peritonitis over time were assessed using a second-order Rao-Scott chi-square test to account for correlation of patient and catheters contributing patient months across multiple years of the collaborative. Changes in bundle compliance post-collaborative launch were assessed using a generalized linear mixed model (GLMM), assuming an underlying binomial distribution and a logit link function.
Peritonitis rates were summarized as annualized rates as previously described [5, 7]. Average monthly annualized collaborative rates were modeled as the mean of the center rates. Changes in annualized monthly peritonitis rates were assessed using an interrupted time series (ITS) approach applied to a GLMM framework. To account for overdispersion, peritonitis rates were modeled assuming a negative binomial distribution and a log link function. Initial covariates in the ITS model included a pre-launch slope effect, a post-launch slope effect, and an effect to capture any change in model intercept between the pre-launch and post-launch periods. The pre-launch slope effect and the effect capturing change in model intercept at the time of collaborative launch were highly non-significant and were removed from the model so the final ITS model included only a post-launch slope effect. All GLMM models included a random effect for the center to accommodate center-specific variability.
All analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, NC). Tests with a p value of 0.05 or less were considered statistically significant.
Ethics
All participating centers received approval from their respective institutional review boards.
Results
Fifty-two pediatric dialysis centers currently participate in the SCOPE Collaborative, 24 of which were participating at the collaborative launch in October 2011. Of the 24 centers, 19 continuously participated in SCOPE for the 84 months between launch and September 30, 2018, and were included in the analysis. Enrollments from the 19 centers included 1268 PD catheters in 1055 patients contributing 19,441 catheter months during the 84-month study period. Data from 895 incident catheter insertions, 677 initial training sessions, and 17,247 follow-up encounters were submitted during the 84-month study period. Median age at enrollment was 8 years [interquartile range (IQR): 1, 14], 54.1% of patients were male, 48.9% White, and 23.5% Hispanic (Table 2). The median age at enrollment gradually decreased over the 7-year study period with a median age of ten years (IQR 2, 15) among the 324 patients enrolled in the first year of the collaborative compared to 6 years (IQR 1, 14) among the 297 patients enrolled in the final year of the study period; however, the change was not statistically significant (p = 0.07). Similarly, the percentage of children under age two and aged two to < five years at enrollment increased over time, but the difference did not reach statistical significance (p = 0.06). Congenital anomalies of the kidney and urinary tract (CAKUT) were the cause of kidney failure in 35.2% while focal segmental glomerulosclerosis (FSGS) was present in 19.3%. Six hundred eighty-three of the 1055 patients (64.74%) terminated PD during the study period. Median (IQR) time from enrollment until termination of PD was 14 (6, 26) months, with the majority terminating PD due to kidney transplant (n = 512, 48.5%), while 52 (11.9%) transferred to hemodialysis, 18 (4.1%) regained kidney function, 99 (9.4%) transferred to a non-SCOPE center, and two (0.2%) were lost to follow-up.
Fifteen of the 19 centers provided demographic characteristics of the children receiving PD in their unit for the 13 months prior to the collaborative launch. A comparison of the demographics of the 200 patients in the pre-launch and 862 patients in the post-launch cohorts from these 15 centers revealed that the median patient age in the pre-launch cohort was significantly higher than post-launch (12 years (IQR 2, 16) versus 9 years (1.14), p < 0.001). The age distribution between the two cohorts was significantly different, with a lower proportion of patients < 2 years of age pre-launch (19.5% versus 28.3%, p = 0.008). Racial and ethnic distribution of the two cohorts also varied with a higher proportion of Black (23% versus 18%) and White (50.5% versus 47.9%) children in the pre-launch cohort and a higher proportion of Hispanic (20% versus 23.5%) patients in the post-launch period (p = 0.026). There was no significant difference in sex or cause of kidney failure between the two cohorts.
Overall mean monthly compliance with the follow-up care bundle and compliance with each of the mandatory components over the first 84 months of the collaborative is shown in Fig. 1 [4, 8]. The likelihood of overall compliance with the follow-up bundle increased significantly over the 84-month period (OR 1.05 95% confidence interval (CI) 1.03, 1.07; p < 0.001) (Fig. 1) [4, 8]. Compliance with each of the individual components increased relatively quickly, whereas delivery of a concept and demonstration test and overall compliance, which requires that all components be completed during the follow-up encounter, did not reach 80% until 28 months following collaborative launch (Fig. 1) [5]. The 19 centers subsequently maintained at least 80% overall compliance with the follow-up care bundle over the remainder of the study period, with a mean compliance of 84% between 28 and 84 months post-launch (Fig. 1).
Follow-up Care Bundle Compliance: Model estimates of aggregate monthly compliance with the Follow-up Care Bundle and each of the mandatory components of that bundle at 19 centers for 84 months following the SCOPE Collaborative launch on October 1, 2011. The likelihood of follow-up bundle compliance increased significantly over the study period (OR 1.05 95% confidence interval (CI) 1.03, 1.07; p < 0.001). Compliance with the individual bundle components was relatively high shortly after collaborative launch, but overall compliance, which requires compliance with each of the bundle components, did not achieve 80% until 28 months post-launch. Centers were able to maintain high-level compliance beyond that point and mean overall compliance was 84% between 28 and 84 months post-launch
Among the 19 centers included in this analysis, the likelihood of compliance with the training bundle also increased significantly over the 84-month study period (OR 1.04, 95% CI 1.01, 1.07, p = 0.004) (Fig. 2). The components included in this bundle are shown in Table 1 [4]. Compliance with the majority of the training bundle elements was above 80% immediately after collaborative launch except for the performance of a home visit (Fig. 2). Compliance with this element, and therefore overall bundle compliance, did not reach 80% until the spring of 2015, more than 3.5 years following collaborative launch.
Patient/Caregiver Training Bundle Compliance: Model estimates of aggregate monthly compliance with the Patient/Caregiver Training Bundle and each of the mandatory components of that bundle at 19 centers for 84 months following the SCOPE Collaborative launch on October 1, 2011. The likelihood of compliance with the training bundle increased significantly over the 84-month study period (OR 1.04, 95% CI 1.01, 1.07, p = 0.004). Compliance with the majority of the mandatory training bundle components was above 80% immediately after collaborative launch except for the performance of a home visit. Compliance with this bundle component, and therefore overall compliance, did not reach 80% until spring 2015
Figure 3 shows aggregate monthly compliance with the catheter insertion bundle and compliance with each of the mandatory components over the 84-month study period [4]. The likelihood of bundle compliance did not significantly increase over the 84-month study period (OR 1.00, 95% CI 0.99, 1.01, p = 0.687). Compliance with the majority of the bundle components was very high throughout the study period apart from avoiding the use of the catheter for PD in the first 14 days after catheter placement.
Peritoneal Dialysis Catheter Insertion Bundle Compliance: Model estimates of aggregate monthly compliance with the Peritoneal Dialysis Catheter Insertion Bundle and each of the mandatory components of that bundle at 19 centers for 84 months following the SCOPE Collaborative launch on October 1, 2011. The likelihood of compliance with this bundle did not significantly increase over the 84-month study period (OR 1.00, 95% CI 0.99, 1.01, p = 0.687). Compliance with the majority of bundle components was very high throughout the study period except for avoiding the use of the catheter for peritoneal dialysis in the first 14 days after catheter placement
Table 3 shows the distribution by study year of characteristics or factors known to be associated with an increased risk for peritonitis in children. There was no significant change in the percentage of any of these characteristics or factors among the patients enrolled in the collaborative over the 7-year study period. Note that a single catheter may contribute data to more than one study year. A review of the distribution of the number of patients enrolled, the number of catheter months, and the catheter months per enrolled patient per year revealed that each was relatively stable over the 7-year study period (data not shown).
The 19 centers reported 152 episodes of peritonitis over 3031 patient months during the 13 months pre-launch period, and 648 peritonitis episodes over 19,441 patient months during the 84 months post-launch study period. Gram-positive organisms were identified in 40% of the peritonitis episodes, while 17.7% were due to Gram-negative organisms. Both Gram-positive and Gram-negative organisms were cultured in 10.8%, with fungi and other organisms identified in 5.1% and 3.2% of episodes, respectively. Effluent cultures were negative in 150 of the reported peritonitis episodes (23.1%). Table 4 reveals the distribution of causative organisms by year. There was no statistically significant change in the percentage of any group of organisms for the 84-month study period (p = 0.88). Causative organisms were not captured in the pre-launch period.
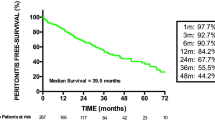
Peritonitis rates for the 19 centers, calculated for the pre-launch and post-launch periods, are shown in Fig. 4. The decrease in infection rate observed in the first 36 months persisted between 37 and 84 months, and there was a significant reduction in average peritonitis rates from 0.53 infections per patient-year (95% CI 0.37, 0.70) pre-launch to 0.30 infections per patient-year (95% CI 0.23, 0.43) at 84 months post-launch, p < 0.001.
Average monthly peritonitis rates, expressed as annual rates, among 19 SCOPE centers for whom baseline infection rates were available for the 13 months prior to the collaborative launch on October 1, 2011. Differences between the pre-launch and the post-launch peritonitis rates were modeled using generalized linear mixed models techniques assuming a negative binomial distribution with a natural log link function and a binomial distribution with a logit link function, respectively. Covariates in the model included a pre-launch slope effect, a post-launch slope effect, and an effect to capture any change in model intercept between the pre-launch and post-launch periods. A random effect for the center was included in these models to accommodate center-specific variability in peritonitis rates. This model revealed that the decrease in infection rate observed in the first 36 months persisted and there was a significant reduction in the average monthly peritonitis rates from 0.53 (95% CI 0.37, 0.70) pre-launch to 0.30 infections per patient-year (95% CI 0.23, 0.43) at 84 months post-launch, p < 0.001
Discussion
Peritonitis is a significant complication of PD and remains a leading cause of morbidity and mortality. The SCOPE Collaborative’s first project therefore focused on reducing this preventable infection among children on maintenance PD. The current analysis reveals that centers continuously participating in the SCOPE Collaborative from launch through 84 months post-launch were able to significantly increase the likelihood of compliance with a standardized follow-up care bundle. Similar to the study of centers participating in SCOPE’s first 3 years, the current analysis reveals that achieving 80% compliance with the follow-up bundle required more than 2 years of participation in the collaborative [5]. The delay in achieving this threshold may be related to the all-or-none compliance scoring. That is, every component in the care bundle had to be completed for a follow-up care episode to be considered compliant [4, 5]. Given the significant work required to achieve compliance with the care bundles, the risk for complacency or burnout increases over time. However, the current analysis reveals that once this threshold was achieved, SCOPE centers were able to maintain a high level of compliance with the follow-up bundle through 84 months of participation. Perhaps most importantly, these centers were able to demonstrate continued improvement in peritonitis rates for the entire post-launch period.
That consistent compliance with the follow-up bundle contributed to the ongoing reduction in peritonitis rates is supported by previous SCOPE analyses that demonstrated an association between compliance with this care bundle and the risk for infection at the patient level [6]. In addition, there was no significant change in other factors known to be associated with risk for infection over the 84-month study period. In fact, the median age of the post-launch cohort was lower than the pre-launch period, and young age has been associated with increased risk for peritonitis [1, 2, 6, 9].
It should be noted that the ongoing reduction in peritonitis rates occurred despite the influx of children and families new to PD during the study period, while others received kidney transplants and discontinued dialysis. Central to the quality improvement process is the creation of a culture of safety, where identification of mistakes and “near misses” is viewed as an opportunity for improvement, rather than assigning blame. Children and families new to PD following the collaborative launch were trained and received their PD care exclusively in this culture of safety, and so it is possible that this created an environment where acknowledgment of touch contaminations or breaks in the aseptic technique that require a corrective action is the norm. There may also be less resistance to the repeated review of care practices and demonstration of competency with those procedures as required by the follow-up bundle if that practice is implemented at the time of dialysis initiation.
In contrast to the analysis of SCOPE data at 3 years post-launch, the current study detected a significant increase in the likelihood of compliance with the training bundle [5]. While it is possible that increased compliance with this bundle also contributed to the ongoing reduction in peritonitis rates over time, our previous analysis did not demonstrate an association between compliance with this care bundle and risk for infection at the patient level [6]. The follow-up care bundle includes an ongoing review of the key aspects of hand hygiene, exit-site care, and aseptic technique, so children on PD and their caregivers receive ongoing training monthly, which may minimize the potential negative impact of non-compliance with the training bundle. It should be noted, however, that compliance with the home visit was the single training bundle element for which compliance did not exceed 80% in the first few months of the collaborative. As additional training sessions are submitted, further analysis to determine if compliance with a home visit is associated with a decreased risk for infection may be warranted.
While compliance with two of the care bundles improved over the course of the collaborative, there has not been significant improvement in compliance with the PD catheter insertion bundle over time, and non-compliance was largely driven by early use of the PD catheter. Previous analysis of SCOPE data has shown that the use of the PD catheter for dialysis within 14 days of insertion is associated with an increased risk of peritonitis in the first 60 days after catheter placement [10]. Barriers to compliance with this bundle component include the need to provide dialysis relatively urgently in neonates with congenital anomalies of the kidney and urinary tract, particularly those who are oligoanuric [10]. To better characterize early catheter use and its role in infection, SCOPE is now collecting additional catheter-related data regarding the specific date post-placement when dialysis is initiated and whether dialysate leakage from the catheter exit site occurred with dialysis initiation.
There are some limitations to the collaborative, in particular, that enrollment and data submission are voluntary, and so there is a risk for selection bias. However, complete data entry and data transparency are crucial to the quality improvement process, and much of the work of SCOPE’s quality improvement support team focuses on optimizing data entry. The prolonged period required to achieve compliance with the follow-up care bundle and the ongoing low compliance with specific components of the training and insertion bundles argues against the censuring of unfavorable data. Another limitation is that there was no central adjudication of infection events. However, as stated in the methods, centers were advised to use the definition of peritonitis endorsed by the ISPD [8].
In summary, SCOPE centers continuously participating in the collaborative for 84 months were able to achieve and maintain a high level of compliance with a standardized PD catheter care follow-up bundle and demonstrated a significant and continued reduction in peritonitis rates over the study period. The success of the collaborative suggests that ongoing attention to catheter care is important to minimize the risk for this preventable infection in children on PD. Further work will be required to elucidate the impact of compliance with the training bundle on the risk for infection and to address barriers to compliance with the PD catheter insertion bundle.
References
Chadha V, Schaefer FS, Warady BA (2010) Dialysis-associated peritonitis in children. Pediatr Nephrol 5:425–440. https://doi.org/10.1007/s00467-008-1113-6
NAPRTCS (2011) 2011 Annual Dialysis Report. https://naprtcs.org/. Accessed 30 September 2020
United States Renal Data System (2018) 2018 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. https://www.usrds.org/media/1737/v2_c07_esrd_pediatric_18_usrds.pdf Accessed 30 September 2020
Neu AM, Miller MR, Stuart J, Lawlor J, Richardson T, Martz K, Rosenberg C, Newland J, McAfee N, Begin B, Warady BA, SCOPE Collaborative Participants (2014) Design of the standardizing care to improve outcomes in pediatric end stage renal disease collaborative. Pediatr Nephrol 29:1477–1484. https://doi.org/10.1007/s00467-014-2891-7
Neu AM, Richardson T, Lawlor J, Stuart J, Newland J, McAfee N, Warady BA, SCOPE Collaborative Participants (2016) Implementation of standardized follow-up care significantly reduces peritonitis in children on chronic peritoneal dialysis. Kidney Int 89:1346–1354. https://doi.org/10.1016/j.kint.2016.02.015
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A (2016) Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE Collaborative. Clin J Am Soc Nephrol 11:1590–1596. https://doi.org/10.2215/cjn.02540316
Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, Chadha V, Yap HK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32(Suppl 2):S32–S86. https://doi.org/10.3747/pdi.2011.00091
Schaefer F, Klaus G, Muller-Wiefel DE, Mehls O (1999) Intermittent versus continuous intraperitoneal glycopeptide/ceftazidime treatment in children with peritoneal dialysis-associated peritonitis. The Mid-European Pediatric Peritoneal Dialysis Study Group (MEPPS). J Am Soc Nephrol 10:136–145
Zaritsky JJ, Hanevold C, Quigley R, Richardson T, Wong C, Ehrlich J, Lawlor J, Rodean J, Neu A, Warady BA, SCOPE Investigators (2017) Epidemiology of peritonitis following maintenance peritoneal dialysis catheter placement during infancy: a report of the SCOPE collaborative. Pediatr Nephrol 33:713–722. https://doi.org/10.1007/s00467-017-3839-5
Keswani M, Redpath Mahon AC, Richardson T, Rodean J, Couloures O, Martin A, Blaszak RT, Warady BA, Neu A (2019) Risk factors for early onset peritonitis: the SCOPE Collaborative. Pediatr Nephrol 34:1387–1394. https://doi.org/10.1007/s00467-019-04248-0
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr. Warady has received research funding from Baxter Healthcare. The authors otherwise declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neu, A.M., Richardson, T., De Souza, H.G. et al. Continued reduction in peritonitis rates in pediatric dialysis centers: results of the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative. Pediatr Nephrol 36, 2383–2391 (2021). https://doi.org/10.1007/s00467-021-04924-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-04924-0