Abstract
Background
Protein energy wasting (PEW) is a common cause of morbidity and mortality in patients with stage 5 chronic kidney disease (CKD 5). Intradialytic parenteral nutrition (IDPN) has been used as a therapy for preventing and treating PEW in children with CKD 5 when other conventional modalities fail. However, not enough data is available to define its effectiveness in treating malnutrition in children. This study aims to investigate potential benefits of IDPN in Egyptian children with CKD 5.
Methods
In this prospective, placebo-controlled, parallel-group single blinded study, we enrolled 50 CKD 5 patients; 25 patients (intervention group) received intravenous amino acids (KIDIMN), while 25 patients (control group) received normal saline as placebo, each given during regular dialysis 3 times a week for 9 months. Patients were subjected to nutritional assessment at baseline and 3-, 6-, and 9-month follow-up using height Z-score, hand grip strength (HGS) for muscle power assessment, body composition monitor (BCM) for assessing lean tissue mass (LTM) and adipose tissue mass (ATM), and biochemical measures including serum albumin, serum triglyceride, and serum cholesterol.
Results
When comparing baseline and 9-month follow-up values, significant improvement was recorded in height Z-score, LTM, and serum albumin in the intervention group unlike the control group where no significant changes were recorded.
Conclusion
IDPN is proposed to be an effective method for preventing and treating malnutrition in children with CKD 5. However, further multi-centric studies with larger sample size and longer duration of follow-up are still recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein energy wasting (PEW) in patients with stage 5 chronic kidney disease (CKD 5) represents a diagnostic and therapeutic dilemma [1,2,3]. PEW is highly prevalent among kidney disease patients, with up to 50% of CKD 5 patients being affected. Moreover, it represents an independent predictor of morbidity and mortality. Many different mechanisms and factors negatively impact the nutritional status in CKD 5, leading to PEW and its multiple adverse effects [4].
A range of therapies have been introduced for treatment of malnutrition in hemodialysis patients. Nutritional counseling and oral nutrition supplements are initial steps of treatment used in patients on dialysis, being simple and relatively cheap methods for preventing and treating malnutrition. Yet, progressive loss of appetite that is usually encountered in these patients makes oral nutrient supplementation challenging in most cases. Many factors contribute to anorexia in the CKD population, including abnormal hypothalamic signals, metabolic derangements caused by uremic toxin accumulation, altered levels of circulating appetite regulators (gastric mediators, adipokines, and cytokines), and dysregulation of homeostatic mechanisms of the digestive system [5].
Enteral tube feeding either by nasogastric tube (NG) or gastrostomy tube may represent a good alternative for young children. Gastrostomy is usually preferred to NG tube because of the lower risk of gastroesophageal reflux and vomiting. However, tube feeding carries additional challenges in older children as peer pressure, body image, and taste acceptance influence compliance [6, 7].
Intradialytic parenteral nutrition (IDPN) has also been proposed as a modality of nutritional supplementation for patients with CKD 5. IDPN in the form of amino acid infusion is a form of partial parenteral nutrition administered during regularly scheduled dialysis sessions that is recommended to be used only after nutritional counseling and oral and/or enteral routes have been tried [8].
Many studies have demonstrated an improvement in nutritional parameters after IDPN (body weight, arm muscle circumference, body mass index, total serum protein, serum albumin and prealbumin, normalized protein catabolic rate) [9,10,11,12]. Other studies reported significant improvements in appetite and nutritional intake after parenteral amino acid infusion [13].
However, a systematic review in 2010 indicated that there is insufficient evidence to demonstrate either a clear benefit or a clear harm associated with giving IDPN to malnourished patients on hemodialysis [14].
Our research aims to study the influence and effectiveness of IDPN on different nutritional parameters in children with CKD 5.
Methods
This is a single center, single blinded, placebo-controlled randomized controlled trial (RCT) which was conducted on fifty children with CKD 5 who were on regular hemodialysis. The study was carried out in the dialysis unit of the Mansoura University Children’s Hospital (MUCH), Mansoura, Egypt, in the period from January 2016 to September 2017.
Subjects
-
a.
Target population: The target population was children with CKD 5 attending the dialysis unit of the Mansoura University Children’s Hospital for hemodialysis.
-
b.
The inclusion criteria: Children aged from 3 to 18 years old of both gender, with CKD 5 who underwent hemodialysis for at least 6 months. All children outside of the age range and those who declined to participate were excluded from the study.
-
c.
Sample size calculation: The sample size was estimated by a software program (sample size calculator) using the following parameters: test family, t test; sample group, independent groups; number of tails, one; effect size, d = 0.8 (expected by clinical judgment); significance level (ὰ) = 0.05; study power (1-β error) = 80%.
Thus, the lowest number of subjects required was 42 (21 in each group). With the addition of an extra 20% to overcome drop out cases, the total number of children required was 50 (25 in each group).
Randomization, allocation, and blinding
Subjects were eligible for entry into the trial as long as they met the eligibility criteria and accepted to participate in the study. A computerized random number generator (2015 GraphPad software) was used to randomize the patients in a 1:1 ratio, to receive either amino acids (amino acid group) or placebo (placebo group) in addition to their ordinary treatment.
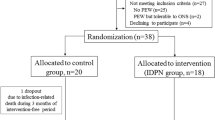
Distribution was concealed using sequentially numbered, closed, and opaque envelopes. The CONSORT flowchart describing the progress of the trial is shown in Fig. 1.
Consort 2010 flow diagram. * Four patients died during the 9-month follow-up in the intervention group. The cause of death was pneumonia in one case, pulmonary embolism in another case, cardiogenic shock out of cardiomyopathy in the third, and hypertensive crisis in the last case. Another two patients were transferred to an adult dialysis unit in another hospital and so dropped out from the study
Study protocol
The researcher met each child’s parents and explained the study in detail. Written consent was taken from parents, and patients in the adolescent age group additionally gave consent. Eligible children were randomly assigned to receive either intravenous amino acids (intervention group) or normal saline 0.9% as a placebo (control group). The intravenous amino acid used was KIDIMIN (7.2% amino acid solution), manufactured by the Egypt Otsuka pharmaceutical Co., S.A.E. Amino acids were given during each regular dialysis session 3 times a week for 9 months. The placebo was also given during each regular dialysis session 3 times a week.
Considering the cost of growth hormone (GH) therapy in Egypt, it is usually reserved for patients with no history of chronic illness, which is why none of our patients had received GH treatment. Also, none of our patients was willing to receive feeding through gastrostomy or nasogastric tube.
The intervention group (amino acid group)
All children in this group received intravenous amino acids (KIDIMN) according to the following protocol:
-
1st week of treatment: 1 ml/kg intravenous (KIDIMIN) to be commenced 30–40 min before the end of dialysis
-
2nd week of treatment: 2 ml/kg of intravenous (KIDIMN) to be commenced 30–40 min before the end of dialysis
-
3rd week until end of study: 3 ml/kg of intravenous (KIDIMN) to be commenced 30–40 min before the end of dialysis
The control group (placebo group)
All children in the control group received 3 ml/kg of normal saline commenced 40 min before the end of dialysis. Since this was a blinded study, the normal saline infusion was matched in appearance and volume to the amino acid infusion.
Study measurements
For all patients, demographic data was collected, including age and gender, primary kidney disease, and duration of dialysis. Morbidities, including suspected side effects of interventions, hospitalization, need for transfusion, and febrile episodes, were also documented.
Nutritional assessment included:
-
i)
Measurement of height in m2
-
ii)
Biochemical studies
-
Measurement of serum albumin
-
Measurement of total serum cholesterol and serum triglyceride after 12-h overnight fasting
-
-
iii)
Measurement of handgrip strength (HGS)
Handgrip strength was measured on the child’s dominant hand and on the non-fistula side of intervention group patients (before and after a hemodialysis session) using a Jamar mechanical dynamometer (Sammons Preston, Masan, Korea). Subjects were trained to self-adjust the dynamometer to fit easily into their hand size to obtain the best performance [15].
-
iv)
Body composition monitoring
-
To evaluate the lean tissue mass (LTM), electrodes were positioned close to one hand and one foot with the patient in a supine position before the patient cable was linked. Measurement was started, and results were exhibited within 2 min and documented on the patient card. Data were transferred to a particular computer for further exploration with the fluid management tool (FMT).
-
Statistical analysis
Data were processed and analyzed using the SPSS version 21. The normality of data was first tested with one-sample Kolmogorov-Smirnov tests. Qualitative data were described using number and percentage. Associations between categorical variables were analyzed using chi-square tests. Continuous variables were reported as mean ± SD (standard deviation) for parametric data and median for non-parametric data. The two groups were compared with Student t tests (parametric data) and Mann-Whitney tests (non-parametric data), while paired groups were compared by paired t test and Wilcoxon signed rank test. For all the abovementioned statistical tests, a p value ≤ 0.05 was considered significant.
Ethical considerations
Meetings with staff and nurses of the dialysis unit at MUCH were conducted to explain the nature and clarify the aims of the study. Data confidentiality and personal privacy were respected at all stages of the study. Collected data were not used for any other purpose.
Results
Characteristics of the studied patients at study enrollment are described in Table 1, which shows no statistically significant difference between the intervention group and control group regarding gender or age.
Table 2 shows baseline and 9-month follow-up values of all the studied parameters in both intervention and control groups.
As regards height Z-scores, there was a significant difference between baseline and 9-month values in the intervention group (p = 0.001), while there was no significant difference in the control group (p = 0.062). However, there was no significant difference between both arms regarding height Z-scores.
The table also shows LTM of both groups; there was significant difference between baseline and 9-month values in the intervention group (p = 0.001) and also between both groups in after 9 months (p = 0.015), while no significant change was encountered in the control group (p = 0.674). Adipose tissue of the enrolled patients was studied, and no significant difference was found between baseline and after 9 months for both intervention and control groups (p = 0.16 and p = 0.47, respectively).
Regarding HGS Z-scores, no significant difference was recorded between baseline and after 9 months in the intervention group (p = 0.084) and control group (p = 0.160). There was also no significant difference between the two groups at the end of the study.
Biochemical measurements are also shown in Table 2. Serum albumin serial measurements showed significant differences between baseline and after 9 months in the intervention group (p = 0.008), while no significant changes were encountered between baseline and 9 month values in the control group. There was also no significant difference between the two groups as regards serum albumin levels. As for serum triglycerides and serum cholesterol values, no significant differences between baseline and after 9 months were found for both the intervention group and the control group.
Serial assessment of height Z-scores, HGS Z-scores, LTM, and serum albumin of the 19 cases in the intervention group is shown respectively by line charts in Fig. 2 a, b, c, and d at baseline, 3-, 6-, and 9-month follow-up.
a Height Z-score values at baseline, 3-, 6-, and 9-month follow-up in the intervention group. b HGS Z-score values at baseline, 3-, 6-, and 9-month follow-up in the intervention group. c LTM values at baseline, 3-, 6-, and 9-month follow-up in the intervention group. d Serum albumin values at baseline, 3-, 6-, and 9-month follow-up in the intervention group
Discussion
Children with CKD 5 are at increased risk for malnutrition [1, 2]. Different nutritional modalities such as IDPN should be considered to prevent further co-morbidities and mortality associated with malnutrition when other interventions fail [16]. IDPN is a practical method for providing nutrition to malnourished hemodialysis (HD) patients during the hemodialysis sessions. Evidence on the effectiveness and long-term benefit of IDPN in children is scant. To the best of our knowledge, our research is the first to study the effectiveness of IDPN on Egyptian children with CKD 5 on chronic hemodialysis.
On comparing the anthropometric measures of the studied patients throughout the 9-month duration of the study, the height Z-score of the intervention group was noted to improve significantly between the initial value and 9-month follow-up value, in comparison with the control group that showed no significant change. However, no significant change was noted for the 9-month follow-up values between both arms, which may be attributed to the relatively short duration of the study. In this study, we did not rely upon weight or body mass index (BMI) for nutritional status assessment, as BMI in patients with CKD 5 gives little information of body composition in the setting of over hydration and altered fat distribution [17].
In Canada, a random crossover study of 6-month duration, in which intraperitoneal amino acid supplementation was given to seven children on peritoneal dialysis, aged 0.7 to 16.5 years, showed no statistical differences in weight or height increase, triceps skinfold thickness, or mean arm circumference [18]. However, another case report of a 5-year-old patient on chronic peritoneal dialysis in the USA who was treated with a balanced 1.1% amino acid solution in conjunction with standard dextrose solution for 1 year suggests that long-term therapy may be needed to obtain nutritional benefit. This child experienced significant increases in appetite, weight, and linear growth velocity [19].
Muscle status, including muscle power and muscle mass, is a crucial clinical aspect in the assessment of nutritional status in patients with CKD [1]. The impact of amino acid supplementation on the muscle power of hemodialysis patients was studied in our work using HGS. HGS had been approved as a non-invasive, practical, and dependable method for assessing muscle power in different populations, including CKD and CKD 5 patients, which in turn reflects their nutritional status [20]. Reduced values of HGS are commonly found among CKD 5 patients and show strong relationships with morbidity and mortality [21].
In our study, HGS test using a JAMAR hand dynamometer was carried out to asses muscle strength. After exclusion of a 3-year-old male child due to non-cooperation, we found that there was a slight though statistically non-significant improvement in HGS values in the intervention group throughout the 9-month duration.
In the context of muscle status assessment, muscle mass was assessed in our study by BCM. The BCM is a non-invasive device used to assess body composition in terms of LTM, ATM, and over-hydration, allowing proper assessment of each of the body compartments separately without being biased by water overload in HD patients. Results showed that there was a significant improvement in the values of LTM in the intervention group between baseline and 9-month values, while no significant change was noted in the control group.
Moreover, though being statistically insignificant, improvement in values of ATM was recorded in the intervention group after 9 months of regular amino acid infusion, in contrast to a reduction in ATM in the control group.
Serum albumin has been identified as a surrogate marker for nutritional status and morbidity/mortality in patients with CKD 5 [22]. Our results indicate that administration of IDPN was associated with a significant improvement in serum albumin levels after 9 months. Results from our study support the evidence in many similar studies. In 2017, Haskin et al. studied the effects of IDPN on fifteen pediatric hemodialysis patients. The study reported a significant rise in serum albumin levels indicating improvement of nutritional status [23].
Similarly, in a prospective study enrolling 97 adult hemodialysis patients, following intradialytic amino acid supplementation with 500 ml 10% solution per HD session over a period of 6 months, mean serum albumin concentration improved significantly [24].
As regards serum lipids (serum cholesterol and serum triglycerides), no significant change was recorded between baseline and 9-month follow-up values. In 2006, Avery-Lynch et al. studied the influence of intradialytic parenteral nutrition on nutritional status, inflammation, adipocytokines, and serum lipids in patients suffering from malnutrition-inflammation complex syndrome (MICS). The study reported that IDPN does not alter serum lipids nor induce a pro-atherogenic lipid composition enhancing the risk for atherosclerosis [25].
The data on the effect of IDPN on mortality is still limited. Mortality rate in our study was higher in the IDPN than in the control group, with reported deaths of 4 children (16%) over 9 months, while in the control group, there were no cases of mortality. Different comorbidities contributed to the mortality in the intervention arm; one patient died of pneumonia, pulmonary embolism in another case, cardiogenic shock complicating cardiomyopathy in the third, and hypertensive crisis in the last case. The higher mortality rate in the IDPN group was unrelated to the intervention.
Similarly, the French Intradialytic Nutrition Evaluation Study (FineS) RCT of 186 malnourished patients with chronic hemodialysis reported that 1 year of IDPN treatment plus oral supplements did not improve 2-year mortality, hospitalization rate, nor quality of life [26]. In another RCT of 107 patients on chronic hemodialysis, IDPN did not improve patient health outcomes in terms of mortality [27].
In summary, IDPN is proposed to be an effective method for preventing and treating malnutrition in children with CKD 5. Considering the total number of children on hemodialysis in Egypt, our sample size was relatively representative. However, further multi-centric studies with larger sample size and longer duration of follow-up are still recommended. Growth hormone therapy was not available for our patients, which should be considered in future studies. Moreover, other comorbidities and confounders, such as acid-base balance, calcium metabolism, duration of dialysis, residual kidney function, caloric intake, and efficacy of dialysis (kt/v), still need to be studied in this context for more accurate results.
References
Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C (2008) A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int 73:391–398
Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, Kuhlmann MK, Stenvinkel P, Ter Wee P, Teta D, Wang AY, Wanner C, International Society of Renal Nutrition and Metabolism (2013) Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 84:1096–1107
Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, Mitch WE, Price SR, Wanner C, Wang AYM, ter Wee P, Franch HA (2013) Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 23:77–90
Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP (2013) Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle 4:89–94
Lacson E Jr, Wang W, Zebrowski B, Wingard R, Hakim RM (2012) Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: a quality improvement report. Am J Kidney Dis 60:591–600
Rees L, Jones HJ (2013) Nutritional management and growth in children with chronic kidney disease. Pediatr Nephrol 28:527–536
Rees L, Azocar M, Borzych D, Watson AR, Büscher A, Edefonti A, Bilge I, Askenazi D, Leozappa G, Gonzales C, van Hoeck K, Secker D, Zurowska A, Rönnholm K, Bouts AH, Stewart H, Ariceta G, Ranchin B, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network (IPPN) registry (2011) Growth in very young children undergoing chronic peritoneal dialysis. J Am Soc Nephrol 22:2303–2312
Worthington P, Balint J, Bechtold M, Bingham A, Chan LN, Durfee S, Jevenn AK, Malone A, Mascarenhas M, Robinson DT, Holcombe B (2017) When is parenteral nutrition appropriate? JPEN J Parenter Enteral Nutr 41:324–377
Capelli JP, Kushner H, Camiscioli TC, Chen S-M, Torres MA (1994) Effect of intradialytic parenteral nutrition on mortality rates in end-stage renal disease care. Am J Kidney Dis 23:808–816
Chertow GM, Ling J, Lew NL, Lazarus JM, Lowrie EG (1994) The association of intradialytic parenteral nutrition administration with survival in hemodialysis patients. Am J Kidney Dis 24:912–920
Hiroshige K, Iwamoto M, Kabashima N, Mutoh Y, Yuu K, Ohtani A (1998) Prolonged use of intradialysis parenteral nutrition in elderly malnourished chronic haemodialysis patients. Nephrol Dial Transplant 13:2081–2087
Cherry N, Shalansky K (2002) Efficacy of intradialytic parenteral nutrition in malnourished hemodialysis patients. Am J Health Syst Pharm 59:1736–1741
Krause I, Shamir R, Davidovits M, Frishman S, Cleper R, Gamzo Z, Poraz I, Eisenstein B (2002) Intradialytic parenteral nutrition in malnourished children treated with hemodialysis. J Ren Nutr 12:55–59
Sigrist MK, Levin A, Tejani AM (2010) Systematic review of evidence for the use of intradialytic parenteral nutrition in malnourished hemodialysis patients. J Ren Nutr 20:1–7
Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40:423–429
Cano N, Aparicio M, Brunori G, Carrero J, Cianciaruso B, Fiaccadori E, Lindholm B, Teplan V, Fouque D, Guarnieri G, ESPEN (2009) ESPEN guidelines on parenteral nutrition: adult renal failure. Clin Nutr 28:401–414
Johansen KL, Lee C (2015) Body composition in chronic kidney disease. Curr Opin Nephrol Hypertens 24:268–275
Qamar IU, Levin L, Balfe JW, Balfe JA, Secker D, Zlotkin S (1994) Effects of 3-month amino acid dialysis compared to dextrose dialysis in children on continuous ambulatory peritoneal dialysis. Perit Dial Int 14:34–41
Brem A, Maaz D, Shemin D, Wolfson M (1996) Use of amino acid peritoneal dialysate for one year in a child on CCPD. Perit Dial Int 16:634–636
Bakr AMAEB, Hasaneen BM, Bassiouni DAH (2018) Assessment of nutritional status in children with chronic kidney disease using hand grip strength tool. J Ren Nutr 28:265–269
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis Report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Wong CS, Hingorani S, Gillen DL, Sherrard DJ, Watkins SL, Brandt JR, Ball A, Stehman-Breen CO (2002) Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int 61:630–637
Haskin O, Sutherland SM, Wong CJ (2017) The effect of intradialytic intralipid therapy in pediatric hemodialysis patients. J Ren Nutr 27:132–137
Czekalski S, Hożejowski R, Malnutrition Working Group (2004) Intradialytic amino acids supplementation in hemodialysis patients with malnutrition: results of a multicenter cohort study. J Ren Nutr 14:82–88
Joannidis M, Rauchenzauner M, Leiner B, Rosenkranz A, Ebenbichler C, Laimer M, Tatarczyk T, Meusburger E, Mayer G (2008) Effect of intradialytic parenteral nutrition in patients with malnutrition–inflammation complex syndrome on body weight, inflammation, serum lipids and adipocytokines: results from a pilot study. Eur J Clin Nutr 62:789–795
Cano NJ, Fouque D, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Laville M, Leverve XM, French Study Group for Nutrition in Dialysis (2007) Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 18:2583–2591
Marsen TA, Beer J, Mann H; German IDPN-Trial group (2017) Intradialytic parenteral nutrition in maintenance hemodialysis patients suffering from protein-energy wasting. Results of a multicenter, open, prospective, randomized trial. Clin Nutr 36:107–117
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was submitted to the institution research board of the Faculty of Medicine, Mansoura University, for approval. The protocol was granted approval in December 2016, IRB Code number: MS/16.07.46. Written consent was taken from parents, and patients in the adolescent age group additionally gave consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Serw, H.ES.S., Bassiouni, D.A.R.H., Al-Wakeil, A.A. et al. Efficacy of intradialytic amino acids on nutritional status in children with stage 5 chronic kidney disease. Pediatr Nephrol 36, 1561–1569 (2021). https://doi.org/10.1007/s00467-020-04806-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04806-x