Abstract
Background
Serum cystatin C (CysC) is a promising biomarker of kidney function, which has higher accuracy and sensitivity when compared with creatinine. To better utilize serum CysC in clinical practice, this study aimed to establish continuous paediatric reference intervals (RIs) for serum CysC.
Methods
The study subjects consisted of healthy term neonates and children aged 30 days to 18 years. Venous blood samples were collected and serum CysC levels were measured using the immunoturbidimetric measurement principle. Fractional polynomial regression model and quantile regression was applied in the statistical analysis to generate continuous RIs.
Results
A total of 378 samples with equal numbers of males and females were analysed for serum CysC. No outliers were found in this analysis. The continuous RIs are presented as equations and graphical scatterplots.
Conclusions
This study established continuous paediatric reference intervals (RIs) for serum CysC in healthy term neonates and children. The continuous RIs generated from this study show age-based dynamic changes as well as blood group and gender-specific differences for serum CysC.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystatin C (CysC), a low-molecular weight (13 kDa) basic protein, is produced throughout the body by all nucleated cells and is found in a variety of body fluids, including blood [1, 2]. Since it was first described in 1961 by Jorgen Clausen [3] in human cerebrospinal fluid, CysC has been extensively investigated and is considered as a promising biomarker in nephropathy-related diabetes [4], amyloidosis and Alzheimer’s disease [5, 6], cancer [7] and in particular in kidney diseases [8, 9].
CysC is filtered from the circulation by glomerular ultrafiltration and is enzymatically degraded at a constant rate [2], which correlates to kidney function, as measured by glomerular filtration rate (GFR) using serum creatinine clearance [10, 11]. Serum creatinine levels may be influenced by non-renal factors such as muscle mass, weight and height [8, 12], whilst serum CysC has improved accuracy and sensitivity because of its relatively constant secretion rate and exclusive renal catabolism, and it is not influenced by factors such as diet and muscle mass [2, 13]. Indeed, in 2009, serum CysC level was officially included in the GFR formula as an index aiming to improve precision of kidney function estimation [14].
To better utilize serum CysC in clinical practice, establishing well-defined reference intervals (RI) is required for an accurate interpretation of serum CysC results. According to the latest Clinical & Laboratory Standards Institute (CLSI) guidelines published in 2010 [15], the RI is defined as a range encompassing 95% of the results for apparently healthy individuals, and is normally represented by the 2.5th and 97.5th percentile of the distribution of test results. Recently, RIs for serum CysC have been established for different populations, including children, the elderly and African populations [16,17,18]. In terms of the paediatric population, dynamic age-related development is the key variable that influences the physiological changes of biomarkers and the representation of RI [19,20,21]. Thus, paediatric RIs are often established by partitioning the results into several age groups in different categories of sex and ethnicity [15, 22, 23]. However, the partitioned RIs can be problematic and can even lead to misdiagnosis, especially when a child who is being investigated just crosses an age “cutoff”, because of the large difference between the RI of the original and the next age group [20].
Therefore, continuous RIs across ages are recommended to replace current partitioned RIs for serum CysC. Compared with partitioned RIs, continuous RIs established smooth curves of reference values and reflect the dynamic relationship between age and target biomarkers. Recently, continuous RIs have been successfully implemented in several age-dynamic biomarkers, such as alkaline phosphatase, alanine aminotransferase and creatinine [19, 24, 25], showing higher accuracy and superiority. In addition, a head-to-head comparison for 30 common biochemistry analytes in the same reference population was performed and published by Hoq et al. [26], showing the feasibility and superiority of continuous RIs.
The Harmonizing Age Pathology Parameters in Kids (HAPPI Kids) study is a prospective cross-sectional study that collects paediatric blood samples for commonly requested biomarkers, aiming to address the existing gaps in reference interval studies [26, 27]. In this paper, as a part of the HAPPI Kids study, continuous paediatric RIs for serum CysC were established by prospectively collecting blood samples from healthy neonates and children, from birth until 18 years of age.
Methods
Study design
The study protocol was approved by The Royal Children’s Hospital (RCH), Melbourne, Australia, Ethics in Human Research Committee (HREC) and subsequently approved by the HRECs of all participating hospitals (HREC 34183). An outline of the study protocol is provided and full details are published elsewhere [27]. The study contributors are listed in Supplemental Table 1, which accompanies the online version of this article at: http://www.clinchem.org/content/vol65/issue10.
Study subjects and sample size
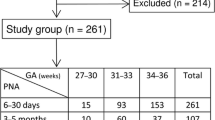
Details of participant recruitment and sample collection were described in our previous publication [27]. Briefly, the study subjects consisted of healthy term neonates (from birth up to a maximum of 72 h post-birth) and children aged 30 days to 18 years (undergoing minor day surgical procedures such as circumcision). Due to the limitation of the ethics approval, we did not collect any samples after the neonatal period up until 30 days of age, as healthy infants generally do not present to RCH for a minor day procedure within 30 days of life.
Participant recruitment occurred during the period from February 2015 to December 2016. Written consents and study questionnaires [27] were first obtained from parents or guardians of participants by the pathology collection staff to confirm study inclusion and exclusion criteria status. Participation was excluded on the presence of any systemic abnormalities, haematological diseases, immune diseases, endocrine diseases or metabolic diseases, history of liver or kidney diseases, and/or failure to thrive or a requirement for an interpreter. In addition, the child’s medical record was reviewed to assess and document the child’s general health.
A minimum of 20 samples were collected from neonates and from each age group from 30 days to 18 years with equal numbers of male and female participants, resulting in a total of 378 samples (2 samples were excluded). Specifically, neonatal blood samples were collected from healthy term neonates following routine intramuscular administration of 1 mg of vitamin K in the delivery suites, whilst venous blood samples were obtained from healthy children aged 30 days to 18 years. Only one attempt to collect blood per participant was allowed.
Sample processing and testing
Standardized laboratory procedures used are described elsewhere [27]. Briefly, venous blood samples were collected into S-Monovette serum gel tubes (Sarstedt). Samples were centrifuged at 5000 rpm (4472 g) at 6 °C for 5 min, separated into 400-μL aliquots, and stored for a maximum period of 24 months at − 80 °C. Preanalytical processing was completed within 4 h of sample collection.
All the tests were performed in the laboratory at the RCH, which is accredited to ISO15189 by the National Association of Testing Authorities at the time of testing and met required analytical performance specifications. Immunoturbidimetric measurement principle (Roche) was applied for serum CysC measurement. Roche Cobas cystatin C (Generation 2) kit was used on Roche Cobas c501. This method is standardized against ERM-DA471/IFCC reference material. Analytical performance of the cystatin C assay was assessed using two internal quality controls from Biorad Laboratories, Liquicheck unassayed chemistry Level 2 (n = 329, mean = 0.65 mg/L, SD = 0.02, CV = 3.37 and measurement of uncertainty = 6.73%) and Liquicheck Immunology Level 1 (n = 334, mean = 0.44 mg/L, SD = 0.02, CV = 3.88 and measurement of uncertainty = 7.77%). External quality assurance (QAP) for the cystatin C assay was conducted via the UKNEQAS Immunoglobulin (IG3/18) program and CV from external QAP IG3/18 (n = 191 international labs) at 2.29 mg/L = 8.24% and at 0.732 mg/L = 10.1%. For determination of blood groups (ABO), samples were collected into 500-μL S-Monovette EDTA tubes and processed in the laboratory within 24 h of collection.
Statistical methods
Test results for serum CysC were plotted to identify outliers, explore distribution normality and to assess the association between level and age. The details of the RIs establishment are fully described in previous publications [26, 27]. Briefly, for the age-specific RIs, data were first normally distributed by applying a fractional polynomial regression model of age separately for each gender. Outliers were identified and removed by calculating the residuals of the best fitting fractional polynomial model. If the residual was greater than 3 times the interquartile range above or below the third and first quartile, respectively, the result was considered an outlier [28], and removed. Afterward, 2.5th and 97.5th centiles were estimated using quantile regression and the 95% CI of the reference intervals was estimated based on the fitted model for the reference limits. Similar statistical methods were applied for the establishment of blood group-specific RIs.
All statistical analyses were performed using Stata 15.1, StataCorp LLC [29].
Results
A total of 378 samples with equal numbers of males and females from birth to 18 years were analysed for serum CysC, and no outlier was found in this analysis.
Age-specific continuous serum CysC RIs estimation
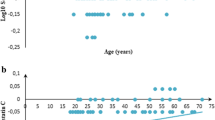
The common reference limits for the 2.5th and 97.5th percentile are reported as functions of age and sex in Table 1, and the scatterplots presented and split by gender (Fig. 1). Our results demonstrate the age- and sex-related dynamics in serum CysC activity. Starting with a peak in infancy, the percentiles show a similar-shaped pattern both in males and females.
Blood group type–specific continuous serum CysC RIs estimation
In this study, blood group types were also considered as another potential variable and were investigated using the same statistical method. Blood type data were supplied for 147 samples, and Table 2 shows the blood group frequencies. The common reference limits for the 2.5th and 97.5th percentile are reported as functions of age and blood group type in Table 1, and the scatterplot presented and split by both Rh blood group and ABO blood group, respectively (Figs. 2 and 3). Due to low group size, 95% CI could not be calculated for the AB blood. The percentiles show a similar-shaped pattern among the ABO blood groups and Rh blood groups.
Subgroup analysis
In addition, further fractional polynomial models were explored, including grouping variables (gender and blood groups) as model covariates and interaction terms. Results showed that blood groupings did not significantly contribute to modelling, with neither significant covariate nor interaction terms. However, gender was found to be a significant covariate, as well as interaction with the best-fitting fractional polynomial model [ysCysC = β1 × ln(age) + β2 × age + constant]. Consistent with Fig. 1 in the manuscript, results suggest that males show a significant linear increase in serum CysC with age.
After the establishment of RIs, the new continuous RIs for serum CysC were examined by using the same sample group used for constructing the RIs. For each gender group, the frequencies of cases lower than 2.5% or above 97.5% RI are 3.2% in males (n = 6) and 4.2% in females (n = 8). For each blood subgroup, the frequencies are 1.4%, 1.9% and 0% in blood group O (n = 1), A (n = 1) and B (n = 0) respectively, as well as 3.1% and 0% in RhD group positive (n = 4) and negative (n = 0) respectively.
Discussion
Serum CysC is a promising kidney biomarker with age dynamic features and appropriate RIs are necessary for physicians to diagnose, monitor and screen for kidney diseases [2, 13]. This study reports the first continuous RIs for serum CysC in healthy term neonates and children. Significant age-related changes of serum CysC and gender differences in the age-specific RIs were well represented by scatterplots and equations.
The study design and clinical implementation
The main strengths of this study are the representative healthy reference population, large sample size and compatibility of fitting the results into different laboratory information systems (LIS).
A proper healthy reference population is critical to establish representative RIs for serum CysC. Due to the challenges in accessing a healthy reference population [30], in previous documented RIs for serum CysC, limitations of sampling are obvious, such as unbalanced gender proportion [31, 32], samples containing both healthy and pathological subjects [33] and samples restricted to the neonatal population only [34, 35], which can cause significant bias during RI establishment. Recently, Ziegelasch et al. [36] established paediatric RIs for serum CysC in a group-based fashion at the age of 3, 6, and 12 months and thereafter up to once a year. Whilst our study was performed prospectively, careful consideration of the reference population and cross-sectional sampling method of healthy neonates and children with equal gender proportion were applied to eliminate potential variables. However, due to the difficulty of obtaining samples from children between the neonatal period and 30 days, we acknowledge that the absence of this group is a limitation of our study.
Whilst serum creatinine has been shown to be affected by ethnicity [11], previous studies including children from different ethnic backgrounds have shown similar RIs for serum CysC [23, 31,32,33,34, 36,37,38]. However, this could be due to limited precision of partitioned RIs, which were calculated to perform best near the centre of the partition; thus, any subtle differences between ethnicities could be masked. Our study utilized an ethnically heterogeneous population to eliminate potential bias [27], and as such increases the generalizability of the presented RIs. Further comprehensive investigations such as head-to-head comparison of RIs for serum CysC between ethnicities should be considered to confirm whether serum CysC is ethnicity independent.
Adequate sample size is required in establishment of RIs, which requires a balance between the study budget and reliability of gaining robust conclusions [39]. According to the CLSI [15], a sample size of 120 in each discrete age group is sufficient for establishing RIs. However, there is a lack of studies investigating the sample size for continuous RIs. In previous studies, establishment of continuous RIs was based on retrospective data only [19, 20]. In this study, detail of sample size calculation was described in a previous publication [27]. The sample size calculation is based on the publication by Royston et al. [40], which proposed that the ratio of the standard error of the estimated limits from the reference population needs to be no more than 10% of the standard deviation of the variation in the population. Therefore, recruiting 378 children across neonates and 30 days to < 18 years was considered adequate for establishing serum CysC continuous RIs with 95%CI. However, better guidance for recruiting appropriate sample size is needed to ensure reliable characterization of age-specific analyte dynamics for continuous RI establishment. The considerable variation in LISs among vendors, such as software version, programme compatibility and mathematical functions is the main obstacles for introducing the continuous RI to clinical implementation. Recently, continuous RIs for serum CysC published by Ziegelasch et al. [36] were presented by figures, but these would be difficult to utilize when identifying whether the result from a patient of a specific age is within normal range by clinical laboratory diagnosis. To overcome this difficulty, we present our continuous RIs as equations of age and sex for serum CysC to facilitate their integration into current LISs.
Comparison with documented RIs for serum CysC
We established RIs for serum CysC, which peak immediately after birth and are followed by a significant decrease within the first year of life. This is in contrast with previously published partitioned RIs, where the serum CysC levels were described as a constant value over this period [23, 31,32,33,34, 37, 38], and even in the following partitioned age groups. In these studies, the age-related dynamics of serum CysC were ignored because of the limited precision of the partitioned RIs as mentioned above. Whilst compared with the studies focusing on the neonatal population [23, 36], the serum CysC levels in those studies showed similar patterns within the first year of life, which were consistent with our results. Unlike creatinine, which may be transmitted from mothers through the placental barrier, Kristensen et al. [41] and Cataldi et al. [42] have reported that there is no correlation found between serum CysC levels of the mothers and infants, because serum CysC does not cross the placental barrier. No significant difference between serum CysC levels of the umbilical cord blood of infants and their venous blood was found by Bahar et al., which supports the hypothesis that CysC metabolism and production in infants are independent from the mother [43]. CysC is filtered from the circulation by glomerular ultrafiltration, but for newborns, the kidney is still immature, so the proportion reabsorbed is likely to be much less [37]. Therefore, all these evidences indicate that the high serum CysC levels in newborns are due to the immaturity of kidney, and the subsequent decrease of values reflects the maturation of the glomerular filtration capacity [44].
We observed gender-based differences in serum CysC levels, showing a slight increase in serum CysC levels for males whilst continuous decrease for females after the age of 6 years, which have not been shown in published partitioned RIs for serum CysC. Continuous RIs for serum CysC published by Ziegelasch et al. [36] also described a similar increase in serum CysC levels in male and a decrease in female adolescents, but the divergence occurred after the age of 12 years. In addition, studies conducted among Saudi adults and Chinese adults reported opposite RIs for serum CysC, showing lower levels in men compared with women [45, 46]. This could be due to geographical differences, but further studies need to be considered.
Apart from age, gender and ethnicity, blood type has also been shown to influence the concentration of specific biomarkers [19, 22, 47,48,49]. Therefore, in this study, we also took the participant’s blood group into consideration when establishing RIs for serum CysC. The blood group–based analysis demonstrated no statistical difference for serum CysC RIs between ABO blood groups and Rh blood groups. However, these results need to be interpreted with caution since the sample numbers for this hypothesis were too small to obtain a confident conclusion.
Conclusion
This is the first study to determine continuous age-specific RIs for serum CysC in healthy term neonates and children. Prospective sampling methods and comprehensive fractional polynomial regression modelling and quantile regression were applied in the establishment of these RIs.
Data availability
Not applicable.
References
Ferguson TW, Komenda P, Tangri N (2015) Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens 24:295–300
Mussap M, Plebani M (2004) Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci 41:467–550
Grubb A, Lofberg H (1982) Human gamma-trace, a basic microprotein: amino acid sequence and presence in the adenohypophysis. Proc Natl Acad Sci U S A 79:3024–3027
Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH (2005) Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 16:1404–1412
Kaeser SA, Herzig MC, Coomaraswamy J, Kilger E, Selenica ML, Winkler DT, Staufenbiel M, Levy E, Grubb A, Jucker M (2007) Cystatin C modulates cerebral beta-amyloidosis. Nat Genet 39:1437–1439
Levy E, Sastre M, Kumar A, Gallo G, Piccardo P, Ghetti B, Tagliavini F (2001) Codeposition of cystatin C with amyloid-beta protein in the brain of Alzheimer disease patients. J Neuropathol Exp Neurol 60:94–104
Kos J, Werle B, Lah T, Brunner N (2000) Cysteine proteinases and their inhibitors in extracellular fluids: markers for diagnosis and prognosis in cancer. Int J Biol Markers 15:84–89
Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226
Park MY, Choi SJ, Kim JK, Hwang SD, Lee YW (2013) Urinary cystatin C levels as a diagnostic and prognostic biomarker in patients with acute kidney injury. Nephrology 18:256–262
Glassock RJ, Warnock DG, Delanaye P (2017) The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol 13:104–114
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Swedko PJ, Clark HD, Paramsothy K, Akbari A (2003) Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 163:356–360
Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE (2004) Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65:1416–1421
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Horowitz GL, Altaie S, Boyd J, Ceriotti F, Garg U, Horn P, Pesce A, Harrison E, Zakowski J (2010) EP28-A3C defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. Clinical Laboratory Standards Institute
Liu C, Wen J, Xiang J, Ouyang X, Yang Y, Lu W, Wang J, Huang J, Min X (2019) Age-and sex-specific reference intervals for the serum cystatin C/creatinine ratio in healthy children (0–18 years old). J Int Med Res 47:3151–3159
Edinga-Melenge BE, Yakam AT, Nansseu JR, Bilong C, Belinga S, Minkala E, Noudjeu PA, Ondhoua M, Kokola SW, Ama Moor VJ, Ashuntantang G (2019) Reference intervals for serum cystatin C and serum creatinine in an adult sub-Saharan African population. BMC Clin Pathol 19:4
Wei L, Ye X, Pei X, Wu J, Zhao W (2014) Reference intervals for serum cystatin C and factors influencing cystatin C levels other than renal function in the elderly. PLoS One 9:e86066
Zierk J, Arzideh F, Rechenauer T, Haeckel R, Rascher W, Metzler M, Rauh M (2015) Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem 61:964–973
Loh TP, Antoniou G, Baghurst P, Metz MP (2014) Development of paediatric biochemistry centile charts as a complement to laboratory reference intervals. Pathology 46:336–343
Higgins V, Adeli K (2018) Advances in pediatric reference intervals: from discrete to continuous. J Lab Precis Med 3:3
Adeli K, Higgins V, Trajcevski K, White-Al Habeeb N (2017) The Canadian laboratory initiative on pediatric reference intervals: a CALIPER white paper. Crit Rev Clin Lab Sci 54:358–413
Harmoinen A, Ylinen E, Ala-Houhala M, Janas M, Kaila M, Kouri T (2000) Reference intervals for cystatin C in pre- and full-term infants and children. Pediatr Nephrol 15:105–108
Asgari S, Higgins V, McCudden C, Adeli K (2019) Continuous reference intervals for 38 biochemical markers in healthy children and adolescents: comparisons to traditionally partitioned reference intervals. Clin Biochem 73:82–89
Zierk J, Arzideh F, Haeckel R, Cario H, Fruhwald MC, Gross HJ, Gscheidmeier T, Hoffmann R, Krebs A, Lichtinghagen R, Neumann M, Ruf HG, Steigerwald U, Streichert T, Rascher W, Metzler M, Rauh M (2017) Pediatric reference intervals for alkaline phosphatase. Clin Chem Lab Med 55:102–110
Hoq M, Matthews S, Karlaftis V, Burgess J, Cowley J, Donath S, Carlin J, Yen T, Ignjatovic V, Monagle P, HAPPI Kids study team (2019) Reference values for 30 common biochemistry analytes across 5 different analyzers in neonates and children 30 days to 18 years of age. Clin Chem 65:1317–1326
Hoq M, Karlaftis V, Mathews S, Burgess J, Donath SM, Carlin J, Monagle P, Ignjatovic V (2019) A prospective, cross-sectional study to establish age-specific reference intervals for neonates and children in the setting of clinical biochemistry, immunology and haematology: the HAPPI Kids study protocol. BMJ Open 9:e025897
Barbato G, Barini EM, Genta G, Levi R (2011) Features and performance of some outlier detection methods. J Appl Stat 38:2133–2149
StataCorp L (2017) Stata statistical software: release 15 college station, TX
Daly CH, Liu X, Grey VL, Hamid JS (2013) A systematic review of statistical methods used in constructing pediatric reference intervals. Clin Biochem 46:1220–1227
Esezobor CI, Soriyan OO, Iroha E (2011) Serum cystatin C levels in Nigerian children: reference intervals and relationship to demographic and anthropometric variables. West Afr J Med 30:188–192
Randers E, Krue S, Erlandsen EJ, Danielsen H, Hansen LG (1999) Reference interval for serum cystatin C in children. Clin Chem 45:1856–1858
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J (1998) Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 12:125–129
Dorum S, Silfeler I, Dorum BA, Silfeler DB, Canbak Y, Say A (2012) Reference values of serum cystatin-C for full-term and preterm neonates in Istanbul. Indian J Pediatr 79:1037–1042
Nakashima T, Inoue H, Fujiyoshi J, Matsumoto N (2016) Longitudinal analysis of serum cystatin C for estimating the glomerular filtration rate in preterm infants. Pediatr Nephrol 31:983–989
Ziegelasch N, Vogel M, Muller E, Tremel N, Jurkutat A, Loffler M, Terliesner N, Thiery J, Willenberg A, Kiess W, Dittrich K (2019) Cystatin C serum levels in healthy children are related to age, gender, and pubertal stage. Pediatr Nephrol 34:449–457
Finney H, Newman DJ, Thakkar H, Fell JM, Price CP (2000) Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child 82:71–75
Uemura O, Ushijima K, Nagai T, Yamada T, Hayakawa H, Nabeta Y, Shinkai Y, Koike K, Kuwabara M (2010) Reference serum cystatin C levels in Japanese children. Clin Exp Nephrol 14:453–456
Noordzij M, Tripepi G, Dekker FW, Zoccali C, Tanck MW, Jager KJ (2010) Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant 25:1388–1393
Royston P (1991) Constructing time-specific reference ranges. Stat Med 10:675–690
Kristensen K, Strevens H, Lindström V, Grubb A, Wide-Swensson D (2008) Increased plasma levels of ß2-microglobulin, cystatin C and ß-trace protein in term pregnancy are not due to utero-placental production. Scand J Clin Lab Invest 68:649–653
Cataldi L, Mussap M, Bertelli L, Ruzzante N, Fanos V, Plebani M (1999) Cystatin C in healthy women at term pregnancy and in their infant newborns: relationship between maternal and neonatal serum levels and reference values. Am J Perinatol 16:287–295
Bahar A, Yilmaz Y, Unver S, Gocmen I, Karademir F (2003) Reference values of umbilical cord and third-day cystatin C levels for determining glomerular filtration rates in newborns. J Int Med Res 31:231–235
Filler G, Lepage N (2013) Cystatin C adaptation in the first month of life. Pediatr Nephrol 28:991–994
Li DD, Zou MN, Hu X, Zhang M, Jia CY, Tao CM, Wang LL, Ying BW (2012) Reference intervals and factors contributing to serum Cystatin C levels in a C hinese population. J Clin Lab Anal 26:49–54
Al Wakeel JS, Memon NA, Chaudhary A, Mitwalli AH, Tarif N, Isnani A, Hammad D (2008) Normal reference levels of serum cystatin C in Saudi adults. Saudi J Kidney Dis Transpl 19:361–370
Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, Pasic MD, Armbruster D, Adeli K (2012) Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 58:854–868
Ozcurumez MK, Haeckel R (2018) Biological variables influencing the estimation of reference limits. Scand J Clin Lab Inv 78:337–345
Jang JH, Seo JY, Bang SH, Park IA, Kim HJ, Kim SH (2010) Establishment of reference intervals for von Willebrand factor antigen and eight coagulation factors in a Korean population following the clinical and laboratory standards institute guidelines. Blood Coagul Fibrinolysis 21:251–255
Acknowledgements
The authors thank the staff of the Pathology Collection Department at The Royal Children’s Hospital for obtaining the consent of participants and the collection of samples. The authors thank the staff of the Anaesthetic, Surgical, and Post-Natal Departments at the Royal Children’s Hospital and collaborating laboratory.
Funding
This study was funded by the Royal Children's Hospital Foundation, with supplementary funding from Ortho Clinical Diagnostics and in-kind reagents supplied from the Medical and Scientific Affairs at Roche Diagnostics International during the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study protocol was approved by The Royal Children’s Hospital, Melbourne, Australia, Ethics in Human Research Committee (HREC) and subsequently approved by the HRECs of all participating hospitals (HREC 34183 A).
Consent to participate
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, T., Karlaftis, V., Hearps, S. et al. Reference intervals for serum cystatin C in neonates and children 30 days to 18 years old. Pediatr Nephrol 35, 1959–1966 (2020). https://doi.org/10.1007/s00467-020-04612-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04612-5