Abstract
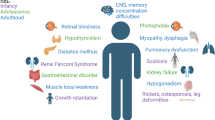
While nephropathic cystinosis is classically thought of as a childhood disease, with improved treatments, patients are more commonly living into adulthood. We performed a systematic review of the literature available on what complications this population faces as it ages. Nearly every organ system is affected in cystinosis, either from the disease itself or from sequelae of kidney transplantation. While cysteamine is known to delay the onset of end-stage kidney disease, its effects on other complications of cystinosis are less well determined. More common adult-onset complications include myopathy, diabetes, and hypothyroidism. Some less common complications, such as neurologic dysfunction, can still have a profound impact on those with cystinosis. Areas for further research in this area include additional study of the impact of cysteamine on the nonrenal manifestations of cystinosis, as well as possible avenues for new and novel treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephropathic cystinosis (OMIM #219800 and 219900) is a rare autosomal recessive disorder due to one of over a hundred known mutations in the lysosomal cystine transporter, cystinosin, and is the most frequent cause of an inherited renal Fanconi syndrome [1]. While the disorder was initially thought to result from cystine crystal deposition in organs, the biology of cystinosis has been broadened to include many disturbances in intracellular signaling from the absence of cystinosin functions [2].
Therapy for cystinosis began with the demonstration that administration of oral cysteamine bitartrate was able to lower a biomarker of the disease, white blood cell cystine levels, towards normal, and with success of such therapy, patients were able to live well beyond infancy [3].
While exact numbers of living patients are not known worldwide, estimates suggest that most affected children will live well into the adult years if treated early after diagnosis with a cystine-depleting agent. Since the outlook for living into adulthood is now more a reality than ever before, we undertook a systematic review to ascertain what is known about adult complications, and to set the stage for future studies.
Methods
In order to obtain all of the papers pertaining to adults with cystinosis, the Pubmed, Scopus, Embase, Web of Science, and CENTRAL databases were queried with the search terms “cystinosis” and “adult.” Only papers published between the years 1988 and 2018 were included. All abstracts were reviewed. Those in which the abstract had a clear indication to be excluded were not full text reviewed, while all other papers were full text reviewed. Exclusion criteria were as follows: studies that only included children (if a paper was mostly about children, but there were at least two subjects ages 18 or older, or one subject age 20 or older, then it was included), papers about patients with either ocular cystinosis or juvenile cystinosis, review articles, genetic and gene expression studies, biopsy analyses, biomarker studies, pharmacokinetic studies, papers not in English, and case reports in which the focus was that the patient had a second disease unrelated to cystinosis. Included papers were then reviewed, and key points were extracted and sorted by the relevant organ system. The level of evidence for each paper was determined using the Oxford Centre for Evidence-based Medicine’s Levels of Evidence [4] (see Supplement 1). Figure 1 shows how we arrived at the final included articles for this review.
For each organ system, a figure was then generated that included each paper relevant to that organ system, with the corresponding number of patients in each study. For studies that only included adults, the bar was made lilac. For those studies that included a mix of adults and children, but it was possible to determine the number of adults in the study, the number of adults was used as the number of patients, and the bar on the graph was also made lilac. For those studies that included both children and adults, and it was impossible to determine how many adults were in the study, the total number of patients was used, but the bar was made green rather than lilac. All case reports were placed on the right-hand side of the graph, and the number of patients for these was set to one.
Results
Cardiovascular
While the cardiovascular system is not traditionally thought to be involved in nephropathic cystinosis, some studies have shown that cardiovascular complications can and do occur (see Fig. 2a). In one cohort study, researchers found that three out of seven patients had abnormal EKGs [5]. Another cohort study demonstrated that 31% of patients in their cohort of 100 had vascular calcifications, and 22% had cerebral calcifications [6].
Studies on a cardiovascular complications of nephropathic cystinosis (8 total, with 4 case reports), b dermatologic complications of nephropathic cystinosis (2 total, with 1 case report), c hematologic complications of nephropathic cystinosis (11 total, with 10 case reports), and d urinary complications of nephropathic cystinosis (2 total, with 2 case reports)
One study looked deeper into the presence of vascular calcifications in patients with cystinosis. In this study, researchers reviewed CT scans of 41 post-transplant patients [7]. They found that 32% of patients had vascular calcifications, and of those, 85% had coronary artery calcifications. They also noted that one patient required three-vessel coronary artery bypass graft surgery at age 25. They found that those with calcifications were older and were more likely to have diabetes (a known complication; see endocrine complications herein). They found that not only did the rate of vascular calcification correlate directly with duration of life without cysteamine, and correlated inversely with duration of life with cysteamine, but that the amount of time spent on dialysis did not differ between the groups that did and did not have vascular calcifications, suggesting that cystine plays a causative role, and that this is not simply a direct result from end-stage renal disease (ESRD).
Another study looked at noninvasive measurements of atherosclerosis, such as carotid intima-media thickness, pulse-wave velocity, and pulse-wave analysis [8]. This study found normal measurement of these values once corrected for chronic kidney disease stage, despite the high level of comorbidities in this patient population. While there was no association between the carotid intima-media thickness and cysteamine treatment, the sample size was quite small.
In addition to the cohort studies noted above, there have also been a few case reports describing cardiovascular complications of cystinosis. One case report describes a patient with heart failure with biventricular hypertrophy who ultimately died from a ruptured abdominal aortic aneurysm [9]. His cardiac autopsy showed crystals with cystine in interstitial histiocytes within his heart, suggesting that cystine crystals can deposit in the heart. A second case report describes a woman with cystinosis who had a proximal aortic dissection [10]. The authors of this paper posit several possible etiologies for aortopathy in cystinosis, including malnutrition, chronic inflammation, oxidative stress, and hemodynamic stress (as a result of ESRD). It is worth noting that neither of the patients in these case reports received cysteamine.
A third case report discussed a young woman with cystinosis who was found to have isolated ventricular noncompaction, which is a congenital disorder not known to be associated with cystinosis [11]. While the authors acknowledged that the two were unlikely to be related, they reported the case so that if further cases were found, a correlation could be thought about. Finally, there has been a case of pregnancy-associated cardiomyopathy in a patient with cystinosis [12]. While her cardiomyopathy could have been only related to her pregnancy, she had also stopped taking cysteamine during her pregnancy (as it is classified as a risk for fetopathy). Her cardiac function eventually recovered, possibly because she was no longer pregnant, or perhaps because she had restarted cysteamine.
Dermatologic
While the skin can be affected in cystinosis, the effects of cystine deposition in the skin do not appear to be harmful. One study followed four patients with cystinosis after renal transplantation who had facial features mimicking premature aging, such as skin atrophy and telangiectasias [13]. On skin biopsy, intracytoplasmic cystine deposits were found within fibroblasts and macrophages. Another case report describes a patient with skin-colored dome-shaped papules on the face, who was also found to have deposition of cystine in the skin on biopsy [14]. While we focused on dermatological issues in adults, it is noteworthy that a unique vasculopathy associated with high doses of cysteamine bitartrate compounds has been described in children prior to kidney transplantation consisting of skin striae and purple or red lesions on the elbows [15], but we found no such reports in adults. The studies affecting the dermatologic system are summarized in Fig. 2b.
Endocrine
The earliest and most common endocrine complication is hypothyroidism, affecting 17–89% of individuals with cystinosis [5, 6, 16,17,18,19,20,21,22,23], with an onset at around age 13 [16]. Two studies showed a difference in hypothyroidism rate between patients treated with cysteamine and those who were not (56% vs. 87% [2] and < 10% vs. 79% [12]), suggesting that cysteamine reduces the risk of developing hypothyroidism. Multiple other studies showed a similar relationship between cysteamine use and decreased rate of hypothyroidism [16, 18, 19]. While the etiology of hypothyroidism in cystinosis has not been elucidated, a case report showed deposition of cystine crystals in macrophages of the thyroid, suggesting that this may be the mechanism. However, this patient was not reported to have hypothyroidism, so it is unclear if the deposition is in fact pathogenic [24]. This possible mechanism is consistent with the observation that the rate of hypothyroidism decreases with cysteamine use.
Diabetes is also a common manifestation of nephropathic cystinosis, though the rate of diabetes has ranged considerably in studies, from 7 to 54% [6, 16, 18,19,20, 22, 23, 25, 26]. This wide variety of rates likely reflects the effects of cysteamine, as the rates of diabetes also decrease with the use of cysteamine [6, 16, 18, 19]. It is worth noting that even those who do not develop overt diabetes are at higher risk for developing impaired glucose tolerance [25, 26]. Only one cohort study explicitly stated that they found no evidence of diabetes—in that study of 18 patients, all patients had normal hemoglobin A1C measurements [21]. The etiology of diabetes and impaired glucose tolerance in cysteamine appears to be multifactorial. One study showed an absolute reduction in insulin production in individuals with cystinosis, without evidence for antibodies against insulin and beta cells [27]. In addition, high doses of steroids given to patients to prevent transplant rejection also plays a role in the high rates of diabetes [25]. Steroid use is unlikely to be the sole mechanism, as patients with cystinosis have higher rates of diabetes than those with renal transplants for other reasons [25].
Short stature is almost universal for patients with cystinosis, accompanied by delayed bone age [5, 17,18,19,20,21, 26, 28, 29]. Often, patients with cystinosis are 2–3 standard deviations below average height [18]. Cysteamine treatment has been correlated with improved growth [17, 19], as has renal transplant [29], which suggests that the etiology of impaired growth is likely from renal disease as well as cystinosis itself. Some patients are treated with recombinant growth hormone, which has been shown to increase growth velocity [19, 30, 31].
In males, cystinosis can cause hypergonadotropic hypogonadism, characterized by elevated LH and FSH, with normal testosterone [6, 16, 28, 29, 31, 32] (though significantly lower on average than those without cystinosis [28]) and decreased sperm production. Some have speculated that this may be a side effect of cysteamine [32]; however, testicular biopsies have shown cystine deposition [28, 29, 32], suggesting that this may be caused by cystinosis itself. Only one case report of a man with cystinosis fathering a child was found. In that instance, he had normal hormone levels, but had azoospermia, and underwent percutaneous epididymal sperm aspiration, followed by in vitro fertilization [33].
Both men and women experience pubertal delay, with the average age of onset of puberty at 15.5 years for men and 15 for women [20, 26, 28, 29, 34]. However, it seems that women are less likely to experience reproductive effects of cystinosis. Four case reports of women with cystinosis who gave birth were found in this review [12, 35,36,37]. One of these four patients developed cardiomyopathy following a stillbirth (described above) [12], but the other three either had normal, healthy pregnancies, or experienced very common complications of pregnancy (i.e., pre-eclampsia [35] and cervical insufficiency [37]). In one case, cystine deposits in the placenta were found [35].
Other case reports pertaining to endocrine complications of cystinosis include one that followed a patient with cystinosis through age 28 which noted he developed hypothyroidism at age nine [38], a patient with a painless scrotal swelling, which was found to be due to extensive deposits of cystine in testicular interstitial histiocytes [39], and a patient who developed several endocrine complications, including adrenal insufficiency, hypothyroidism, hypogonadism, and hyperprolactinemia [30]. These endocrine complications and their sources are summarized in Fig. 3.
Gastrointestinal
Gastrointestinal manifestations of nephropathic cystinosis are universal (see Fig. 4a). The most common reported symptom is periods of nausea and vomiting [40], which is often transient—sometimes appearing as a new symptom, and sometimes disappearing with time. Many patients with cystinosis report poor appetite, which is consistent with their below average height and weights below the 5th percentile [40]. Other reported symptoms include constipation, diarrhea, and abdominal pain. Swallowing dysfunction and gastroesophageal reflux are also common [23, 40]. In one survey of 200 patients, 50% underwent a GI evaluation, and 43% of those who underwent endoscopy were found to have GERD [40]. About 25% had swallowing dysfunction, and 20% had dysmotility. Another study looking specifically at swallowing dysfunction found that 74% of patients reported a swallowing abnormality, while oral and esophageal abnormalities on barium swallow study were 24% and 73%, respectively [41]. An earlier, smaller study by the same author goes through the specific swallowing abnormalities that can be seen in these patients in each phase of swallowing [42]. This swallowing dysfunction is primarily thought to be musculoskeletal in origin, so it will be discussed later. Overall, these gastrointestinal symptoms can be so severe that in some surveys between 14 and 78% of patients had PEG tubes placed to help with nutrition [5, 21, 40].
Liver enlargement, splenomegaly, and portal hypertension can also occur, with rates that range from 3 to 5% [6, 19, 22]. In case reports and small case series, liver biopsies have shown cystine deposition in Kupffer cells in the liver, which is thought to be the etiology of liver enlargement and liver-protein synthetic dysfunction in cystinosis [24, 30, 43,44,45,46]. In one set of two case reports, the liver biopsies were described as having “sclerosing cholangitis,” meaning bile ducts surrounded by a prominent basement membrane [44]. In this same case report, the patients’ liver tests stabilized or improved with initiation of UDCA. It is notable that the liver enlargement and dysfunction in cystinosis is typically characterized by nodular regenerating hyperplasia and does not cause cirrhosis—the only case report found of a patient with cystinosis and cirrhosis had developed cirrhosis from a chronic hepatitis C infection [24].
The medications that patients take for cystinosis can also cause gastrointestinal complications. One side effect of cysteamine is halitosis, caused by DMS, a breakdown product of cysteamine [47]. A study comparing the amount of DMS in the breath of patients on the immediate release versus the delayed release cysteamine found that the breath of patients on the delayed release version had less DMS [48]. While this difference was not statistically significant, there was a very small sample size (four patients). As the source of halitosis is exogenous, good oral hygiene is not sufficient to prevent it, and patients instead try different methods to mask the smell. One such method, the use of chlorophyllin, may have a beneficial side effect. One study looked at patients who experienced symptoms of cysteamine toxicity (including skin abnormalities, musculoskeletal pain, and bone deformities), and found that all of these patients were also copper deficient, and all lived in Europe [49]. The authors note that chlorophyllin contains elemental copper and is more accessible in the USA than it is in Europe, suggesting that those symptoms of cysteamine toxicity could be mediated through copper deficiency.
Patients with cystinosis typically have had renal transplants as children or as young adults, and are therefore on chronic anti-rejection medications, which come with their own set of side effects. One case report describes a patient who was on cyclosporine. When he developed gingival overgrowth, it was assumed to have been a result of the cyclosporine. However, when it was biopsied, the gingival tissue showed cystine crystals, which could have been incidental, but could also have been contributory. In addition, his medical complexity contributed to the surgical complexity of the correction [50].
Hematologic
Overall, hematologic complications of cystinosis are rare, and the studies found were primarily case reports (see Fig. 2c). The most common hematologic complication is hypersplenism [16], which could be due to portal hypertension (noted above) or due to deposition of cystine crystals in the red pulp of the spleen [30]. One cohort study reported the overall rate of splenectomy due to hypersplenism was 21%, but noted that no splenectomies were performed in that cohort after 1997 (this paper was published much later, in 2012), suggesting that cysteamine may prevent deposition of cystine in the spleen, preventing splenomegaly [16]. There is a case report in 2015, however, in which a patient who was thought to be compliant with oral cysteamine required a splenectomy due to hypersplenism, but this patient also had portal hypertension [30].
Numerous case reports describe the deposition of cystine into the bone marrow, causing anemia, thrombocytopenia, or pancytopenia [51,52,53,54,55,56,57,58]. While this is a rare complication, it is important for physicians to have a high index of suspicion for this complication when patients present with anemia, as this can easily be mistaken for anemia due to ESRD. Unlike anemia secondary to ESRD, this anemia will not be responsive to erythropoietin treatment [51]. The last case report describing a hematologic complication was of a patient who developed posttransplant lymphoproliferative disorder, thought to be due to the chronic immunosuppressive medications that he took for his kidney transplant [59].
Musculoskeletal
While musculoskeletal complications of cystinosis outside of rickets rarely occur in children, they are quite common in adults (see Fig. 5). In a cohort study, the overall rate of neuromuscular disorders in adult patients with cystinosis was 37.2% [16]. One of the more common and well-described musculoskeletal complications is myopathy, occurring at rates ranging from 24 to 69% [6, 16, 20, 60]. This form of myopathy typically begins in the distal muscles of the upper extremities and can progress to involve the more proximal muscles, as well as the lower extremities [60]. Individuals with this complication often have normal or brisk reflexes, normal sensory function, and a normal or elevated CK level [60,61,62,63]. EMG findings include reduced amplitude of the motor unit action, brief duration, polyphasic potentials, and spontaneous activity [60,61,62,63,64]. The cause of this myopathy is thought to be cystine crystal deposition into muscles, as biopsies in patients with myopathy have shown crystals [61, 64, 65], and cysteamine use is associated with lower rates of myopathy [6, 16]. The amount of cystine can be extremely high in muscle—in one case report, a patient had 1000× the normal amount of cystine in his muscles [64].
Treatments for this myopathy are severely limited. Cysteamine can prevent it, and some case reports have also suggested that the initiation of cysteamine after its development can stabilize or slightly improve it [41, 60, 65]. One author reported that oxandrolone and L-carnitine have been effective in his clinical practice [66].
In addition to affecting muscles in the extremities, the myopathy in cystinosis can also affect the muscles of respiration, causing pulmonary insufficiency [6, 23, 67, 68]. This complication is surprisingly common, affecting up to 69% of patients [6]. The pulmonary insufficiency in cystinosis is characterized by a restrictive pattern on pulmonary function testing with a normal appearing chest X-ray and CT [18, 67]. The etiology of this complication is likely multifactorial. Myopathy affecting the respiratory muscles, including the diaphragm, is thought to be a major contributing factor, especially since the severity of pulmonary disease in patients typically correlates with the severity of their myopathy [67]. In addition, patients with cystinosis have anatomic abnormalities that may contribute (thought to arise from childhood rickets and growth impairment), including reduced posterior airway space, causing restriction of airway dimension [69]. Ultimately, some patients require the use of noninvasive ventilation at night [63, 68].
Myopathy is also thought to contribute to swallowing dysfunction. There are various rates of swallowing abnormalities in different studies. One study from the Netherlands reported a prevalence of 20% [18], and one study of both adults and children with cystinosis from Brazil reported 2% [19], but most studies report rates ranging from 40 to 75% [6, 16, 20, 40, 41, 70]. This can result in various symptoms, including gagging and vomiting, slow eating, choking, pain or difficulty with swallowing, and voice changes [20, 40, 41]. Dysphagia associated with cystinosis is a major contributor to mortality [6, 30, 65]. Abnormalities can exist in any phase of swallowing, which can all be diagnosed on barium swallow [41, 42].
Bone complications are less common than muscular complications and can be subtle. Patients with cystinosis have a high prevalence of fractures, both vertebral and long bone, as well as bone deformities and scoliosis [71], with mixed findings regarding bone mineral density [71, 72]. In one cohort of seven patients, one had osteopenia [5]. One case report describes sclerotic bone lesions found incidentally, which were shown on biopsy to be made up of histiocytes with cystine accumulation [73]. While skeletal abnormalities occur, they do not appear to interfere with quality of life in the way that other types of complications can. For example, while in one cohort study, 56% of patients developed orthopedic problems, 29% of patients participated in sports [23].
Finally, a case report described a patient who developed an odontogenic cyst with deposition of particles that were consistent with cystine crystals [74].
Neurologic
Neurologic complications of cystinosis are uncommon, but heterogeneous and sometimes debilitating (see Fig. 6). In cohort studies, the prevalence of central nervous system involvement is between 3 and 27% [19, 20, 22, 75, 76]. The main neurologic complication is a syndrome called “cystinosis-associated encephalopathy” [75, 76]. This syndrome appears to have two forms: an encephalopathic form and a stroke-like form. The encephalopathic form describes a syndrome in which patients develop cerebellar and pyramidal signs with cognitive deterioration and pseudobulbar palsy [20, 30, 75,76,77]. The stroke-like form involves symptoms such as hemiplegia or coma—symptoms more similar to a stroke [19, 75, 76, 78, 79]. Associated imaging findings include discrete calcifications, either periventricular or in the basal ganglia, or generalized atrophy [18,19,20, 30, 76,77,78,79]. It has been suggested that the central nervous system effects of cystinosis arise from deposition of cystine in the brain, but there have been mixed responses of these symptoms to cysteamine treatment [75, 77, 78].
There have also been case reports of neurologic symptoms from differing etiologies. One such case report describes a patient who began developing neck pain and finger numbness and was found to have an edematous cord along the cervical spine [80]. A subsequent biopsy showed chronic active demyelinating myelitis. When her symptoms progressed, she was started on steroids and cysteamine, and her condition improved. Another case report describes a patient who developed seizures and respiratory failure, thought to be due to posterior reversible encephalopathy syndrome [81]. Cysteamine was thought to be the culprit, so this was stopped, and he was started on steroids and carbamazepine. After clinical improvement, he resumed cysteamine at a lower dose. This has been the only case reported of PRES attributed to cysteamine. A third case report describes a patient who developed recurrent strokes at the age of 32 [82]. On brain autopsy, generalized atrophy was found with a perivascular inflammatory infiltrate with vascular calcifications. Rare crystals, consistent with cystine crystals, were also found.
While the complications described above are quite severe, a more mild and common neurologic manifestation of cystinosis is elevated intracranial pressure [83, 84]. Small case series have placed the estimated frequency at around 5% [83]. It is worth noting that patients with cystinosis have several risk factors for elevated intracranial pressure, including having a kidney transplant, and taking growth hormone replacement [84]. The authors of these two studies also suggest that cystine deposition in the meninges and arachnoid granulations, obstructing CSF outflow could be an additional mechanism.
As the vast majority of adult patients with cystinosis have kidney transplants, neurologic sequelae of transplants must also be considered in this patient population. As having a renal transplant requires lifelong immunosuppression, patients with cystinosis are also at risk for developing opportunistic infections, including cryptococcal meningitis [85].
It appears that the peripheral nervous system is unaffected in cystinosis. A study of patients without known neurologic symptoms (though some did have hypotonia) showed normal peripheral nerve velocities and amplitudes [86]. In addition, studies of the myopathy that can occur with cystinosis have shown that it is primarily a muscular disease [63].
A few studies have also looked at learning and neurologic processing in cystinosis. Individuals with cystinosis have difficulties with image processing compared to those without cystinosis, though those differences disappear when they are given additional time to process images [87, 88]. Other studies have found deficits in arithmetic and tactile recognition [89, 90]. These differences did not worsen with age, which is inconsistent with a mechanism of chronic cystine deposition in the brain.
Ophthalmologic
Like the renal complications of cystinosis, ophthalmologic complications are well known, and they occur early (see Fig. 7). The most common complications are decreased visual acuity and photophobia, which arise from the deposition of cystine crystals in the eye, mainly in the cornea [5, 6, 18, 20, 22, 23, 34, 36, 91,92,93,94,95,96,97,98,99,100]. Oral cysteamine is not effective in treating this, but there are topical cysteamine eyedrops available that are effective in removing the cystine crystals, improving symptoms [91, 101,102,103,104,105,106,107]. Many of the studies found in this literature review were comparison trials of different formulations of eyedrops [101, 103, 106,107,108]. While all patients have ocular involvement, with complete progression of symptoms as early as age 12 without treatment [5, 6, 22, 34, 91, 109], rates of photophobia and decreased vision are lower [18, 23]. Photophobia rates range considerably in different studies, which may be a function of whether or not patients in those studies were taking topical cysteamine [23, 103]. Many patients have decreased vision, but this can be severe enough that in one study, around 12% of patients were legally blind [110].
While photophobia and decreased visual acuity are more common, there are several other possible complications. Foreign body sensation in the eye is not uncommon [92, 100, 110]. Retinopathy and band keratopathy can also occur [6, 18, 93, 100, 102, 109, 111]. Rates of retinopathy vary between 32 and 52% [6, 100]. Decreased peripheral vision and abnormalities in color vision are also quite common [100, 110, 111]. In addition to the cornea, cystine can also deposit in the retina, in approximately 16% of patients [111]. Patients are more likely than the general population to develop cataracts. In one study of 172 patients, only 10 had cataracts, but the oldest patient was only 42, which is a high prevalence for this age group [109]. There have also been case reports of patients who have experienced macular edema [96], recurrent spontaneous hyphema and hemorrhages [93], choroidal neovascular membrane (though this patient also had bilateral diabetic retinopathy) [112], and enucleation due to phthisis [20].
Finally, there are anatomic ocular differences between individuals with cystinosis and those without, including thicker corneas, a narrower angle, a shallower anterior chamber, and a closed or narrow ciliary sulcus [113, 114].
Interestingly, there is a unique population of individuals with cystinosis living in Canada with relatively mild ocular complications. In one study of 18 individuals with cystinosis in Canada, there were two adults with good visual acuity and color vision. While they both had peripheral corneal deposits and one had superficial keratopathy, their visual acuity and relatively mild photophobia was achieved without the use of cysteamine eyedrops [99].
As the ocular complications of cystinosis are prevented and treated through a different modality than the other systemic manifestations, compliance with this particular component of therapy is challenging. Previously, this topical solution had to be applied every hour and required refrigeration, making compliance very difficult for patients [102, 105, 106, 108]. More recently, formulations of these eyedrops have been produced that do not require refrigeration, are dosed less frequently, and are just as effective as the older formulation. As one might expect, patients prefer the newer formulation with improved adherence [106].
Psychosocial
Though the medical complications of cystinosis are substantial, the psychosocial impact of the disease should be considered as well (see Fig. 4b). Cystinosis has a major impact on quality of life, especially in adulthood. Many individuals with cystinosis say that they “feel different” from other people, and that cystinosis affects both their personal and professional lives, through mechanisms such as job absenteeism, short stature, and photophobia [115, 116]. A study looking at performance on several school subjects found that those with cystinosis may demonstrate academic difficulties, particularly in arithmetic and spelling [89].
One aspect of the disease that contributes significantly to quality of life is the strict dosing regimen of cysteamine. Originally, this medication required strict every 6-h dosing, requiring patients to wake up during the night to take medication, resulting in poor compliance, especially in patients who were old enough to be responsible for taking their own medication [115, 117,118,119]. Newer cysteamine medications requiring only every 12-h administration may lead to enhanced adherence from the absence of having to awake during the sleep cycle.
Halitosis caused by cysteamine can also be a significant contributing factor to psychological distress [115, 117]. As noted previously, this is thought to be from DMS, which is produced as a breakdown product of cysteamine [47, 48, 118].
An interesting aspect of cystinosis is that in the subset of patients who are homozygous for the 57-kb deletion, it may cause a decrease in temperature regulation and sensation of spicy foods due to interruption of the TRPV1 gene [120]. The study that looked into this also found that many of these patients report a preference for spicy foods, and some report difficulties in temperature regulation.
Some structured interviews and focus groups have also looked into some of the more subjective experiences of those with cystinosis. Many individuals find strength in the cystinosis community, through mentoring, exchanging information, and by simply having a group of people with whom they feel a connection [117, 121]. Another theme noted was the anxiety and difficulty not only of transitioning from the pediatrics medical world to the adult one but also of children being able to gain independence in managing their disease as they become adults [117, 122].
Overall, there is considerable heterogeneity in independence and quality of life for those with cystinosis [18, 23].
Renal
Perhaps the most well-known consequence of cystinosis is its impact on kidney function (see Fig. 8). Without treatment, all progress to ESRD, resulting in the need for either a kidney transplant or maintenance dialysis [20, 23, 36, 38]. The administration of cysteamine slows the progression to ESRD [6, 16, 19, 21, 123]. A study looking at the association between medication compliance and renal outcomes found that while glomerular function was preserved by the use of cysteamine, tubular function was not, and hypothesized that this was because the renal tubular disease was already severe at the time of diagnosis [123]. Another study looked at the differences in renal outcomes across different countries. It found that in developed countries, fewer patients had reached ESRD, median renal survival was twice as long, and more patients had transplants rather than dialysis [124]. Adults with cystinosis today typically are a mix of patients on dialysis, those with functioning transplants, as well as those with earlier-stage kidney disease [5].
The outcomes of renal transplants in cystinosis patients have also been studied, with mixed results. One study showed that graft survival was better in patients with cystinosis than in their control population [125]. The authors of this paper suggest that downregulation of the mTORC1 pathway in cystinosis causes better immune tolerance of the transplant. However, another study showed a lower graft survival rate, although this study lacked a control group [20]. At that time, there was a concern of cysteamine adversely impacting renal grafts, but they found that only one kidney failed for every 22 years of cysteamine therapy. A third study found no difference in death-censored graft survival between those with cystinosis and those without cystinosis [126]. They did find a significantly increased risk for patient death, which likely reflects the systemic manifestations of cystinosis. It also appears that as time has gone on, kidney transplants in those with cystinosis are more frequently performed pre-emptively, with better outcomes [127]. Renal graft loss is typically unrelated to cystinosis—they have the same types of complications that anyone with kidney transplants is at risk of developing [18, 30]. In terms of other renal complications, one paper noted that a patient had signs of severe renal osteodystrophy [34].
Multiple biopsies of transplanted kidneys of those with cystinosis have shown cystine crystal deposition, but it is thought that the level at which cystine deposits is so low that it does not affect renal function [24, 125]. However, there was one case report in which the deposition of cystine was thought to play a role in declining kidney function after a kidney transplant. The patient had a renal biopsy which showed recurrent cystinosis, though there was also a component of calcineurin-inhibitor toxicity [128]. The authors posit that since she got her transplant from her mother, who would have been heterozygous for cystinosis, she was at higher risk for recurrent cystinosis in the transplanted kidney, although this has not been proven outside of this case report.
Three other cases reports describe other renal complications that occurred in patients with cystinosis. These included a patient who developed renal papillary necrosis due to posttransplant hypotension [129], a patient who had class III lupus nephritis [130], and one who had developed a renal cell carcinoma [131].
Urinary
There have been two case reports describing urinary complications of cystinosis (see Fig. 2d). One describes a woman with a bladder wall rupture. Since there was no definite cause found, the authors speculate that cystine deposition in the bladder wall could have predisposed it to rupture [132]. The other describes a patient who had a renal transplant, and developed encrusted myelitis from Coynebacterium urealyticum, requiring nephrectomy [133].
Discussion
In the course of performing this literature review, we found very few high-quality studies, likely due to the rarity of this disease. Most of the data in several organ systems (most notably, hematologic and cardiovascular) was comprised of case reports and small cohort studies. A few larger cohort studies have been performed, but as most of their adult patients did not receive cysteamine as young children, it is unclear how applicable these studies are to the current population of adults with cystinosis.
The impact of cysteamine on the extra-renal complications of cystinosis is incompletely characterized. While there are data on cysteamine preventing endocrine complications (e.g., hypothyroidism and diabetes), it is confounded by changing clinical practice over time (those who did not receive cysteamine as children were often treated decades before those who were). Two studies have suggested that cysteamine may prevent myopathy, but they are subject to the same limitation. There are also mixed data regarding the use of cysteamine as a treatment for the neurologic and musculoskeletal manifestations of cystinosis, likely because these interventions were mostly performed in the context of case reports.
There is currently a push to develop ongoing patient databases for nephropathic cystinosis. As the large cohort studies we found in this review are older and may not reflect the current population of adults with cystinosis, gathering this repository of data is needed.
Much of the data gathered on the extra-renal complications of cystinosis focus on the prevalence of these conditions, and some comment on the impact of cysteamine on these rates. While this is important information, there is a notable lack of data regarding effective treatments. Some case reports have suggested possible avenues for treatments, but these have yet to be studied in any systematic way. The rarity of this disease makes the prospect of a randomized clinical trial daunting if not impractical, but these interventions could be studied in the form of longitudinal cohort studies or well-designed single-arm trials.
Overall, as more patients with cystinosis live into adulthood, more attention is needed regarding the complications that they face. Specifically, as myopathy is a prevalent complication in adults resulting in significant morbidity and mortality, more research is needed to better prevent and treat this complication.
References
Langman CB, Barshop BA, Deschênes G, Emma F, Goodyer P, Lipkin G, Midgley JP, Ottolenghi C, Servias A, Soliman NA, Thoene JG, Levtchenko EN (2016) Controversies and research agenda in nephropathic cystinosis: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 89:1192–1203
Langman CB (2019) Oh cystinosin: let me count the ways! Kidney Int 96:275–277
Emma F, Nesterova G, Langman C, Labbé A, Chergui S, Goodyer P, Janssen MC, Greco M, Topaloglu R, Elenberg E, Dohil, Trauner D, Antignac C, Cochat P, Kaskel F, Servais A, Wühl E, Niaudet P, Van’t Hoff W, Gahl W, Levtchenko E (2014) Nephropathic cystinosis: an international consensus document. Nephrol Dial Transplant 29(Suppl 4):iv87–iv94
OCEBM Levels of Evidence Working Group. “The Oxford Levels of Evidence 1”. Oxford Centre for Evidence-Based Medicine
Sharma R (2015) Cystinosis in an adult metabolic clinic – a truly multi-systemic disease requiring multi-professional and multidisciplinary management. J Inherit Metab Dis 38:S289
Gahl WA, Balog JZ, Kleta R (2007) Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med 147:242–250
Ueda M, O’Brien K, Rosing DR, Ling A, Kleta R, McAreavey D, Bernardini I, Gahl WA (2006) Coronary artery and other vascular calcifications in patients with cystinosis after kidney transplantation. Clin J Am Soc Nephrol 1:555–562
Besouw MT, Holewijn S, Levtchenko EN, Janssen MC (2011) Non-invasive measurements of atherosclerosis in adult cystinosis patients. J Inherit Metab Dis 34:811–818
Dixit MP, Greifer I (2002) Nephropathic cystinosis associated with cardiomyopathy: a 27-year clinical follow-up. BMC Nephrol 3:8
Tajdini M, Bayati M, Vasheghani-Farahani A (2017) Aortic dissection and cystinosis: is there any relationship? Cardiol Young 27:1434–1436
Ahmed I, Phan TT, Lipkin GW, Frenneaux M (2009) Ventricular noncompaction in a female patient with nephropathic cystinosis: a case report. J Med Case Rep 3:31
Ramappa AJ, Pyatt JR (2010) Pregnancy-associated cardiomyopathy occurring in a young patient with nephropathic cystinosis. Cardiol Young 20:220–222
Guillet G, Sassolas B, Fromentoux S, Gobin E, Leroy JP (1998) Skin storage of cystine and premature skin ageing in cystinosis. Lancet 352:1444–1445
Stevens MS, Sade S, Walsh S (2016) Facial papules in a patient with long-term cystinosis. JAMA Dermatol 152:108–109
Besouw MTP, Bowker R, Dutertre J-P, Emma F, Gahl WA, Greco M, Lilien MR, McKiernan J, Nobili F, Schneider JA, Skovby F, van den Heuval LP, Van’t Hoff WG, Letchenko EN (2011) Cysteamine toxicity in patients with cystinosis. J Pediatr 159:1004–1011
Brodin-Sartorius A, Tête MJ, Niaudet P, Antignac C, Guest G, Ottolenghi C, Charbit M, Moyse D, Legendre C, Lesavre P, Cochat P, Servais A (2012) Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int 81:179–189
Kimonis VE, Troendle J, Rose SR, Yang ML, Markello TC, Gahl WA (1995) Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis. J Clin Endocrinol Metab 80:3257–3261
Geelen JM, Monnens LA, Levtchenko EN (2002) Follow-up and treatment of adults with cystinosis in the Netherlands. Nephrol Dial Transplant 17:1766–1770
Vaisbich MH, Koch VH (2010) Report of a Brazilian multicenter study on nephropathic cystinosis. Nephron Clin Pract 114:c12–c18
Theodoropoulos DS, Krasnewich D, Kaiser-Kupfer MI, Gahl WA (1993) Classic nephropathic cystinosis as an adult disease. JAMA 270:2200–2204
Pittendrigh L, Hagerty C, Ramage I (2017) Nephropathic cystinosis: 25 year multicentre follow up. Pediatr Nephrol 32:1813–1814
Cohen C, Charbit M, Chadefaux B, Giral M, Garrigue V, Kessler M, Antoine C, De Lonlay P, Kreis H, Legendre C, Servais A (2014) Outcome of renal transplantation in adult cystinosis patients. J Inherit Metab Dis 37(Suppl 1):S42
Latta K, Latta A, Selbsthilfe C (2010) Clinical situation of patients with cystinosis in Germany. Pediatr Nephrol 25:1821–1822
Singh JK, Kusior MF (1999) Cystine crystals in Fanconi’s syndrome. N Engl J Med 341:1807
Robert JJ, Tête MJ, Guest G, Gagnadoux MF, Niaudet P, Broyer M (1999) Diabetes mellitus in patients with infantile cystinosis after renal transplantation. Pediatr Nephrol 13:524–529
Gultekingil Keser A, Topaloglu R, Bilginer Y, Besbas N (2014) Long-term endocrinologic complications of cystinosis. Minerva Pediatr 66:123–130
Filler G, Amendt P, von Bredow MA, Rohde W, Ehrich JH (1998) Slowly deteriorating insulin secretion and C-peptide production characterizes diabetes mellitus in infantile cystinosis. Eur J Pediatr 157:738–742
Chik CL, Friedman A, Merriam GR, Gahl WA (1993) Pituitary-testicular function in nephropathic cystinosis. Ann Intern Med 119:568–575
Winkler L, Offner G, Krull F, Brodehl J (1993) Growth and pubertal development in nephropathic cystinosis. Eur J Pediatr 152:244–249
Besouw MT, Van Dyck M, Cassiman D, Claes KJ, Levtchenko EN (2015) Management dilemmas in pediatric nephrology: Cystinosis. Pediatr Nephrol 30:1349–1360
Ahn MB, Kim SE, Cho WK, Jung MH, Suh BK (2016) Endocrine complications during and after adolescence in a patient with cystinosis. Ann Pediatr Endocrinol Metab 21:174–178
Besouw MT, Kremer JA, Janssen MC, Levtchenko EN (2010) Fertility status in male cystinosis patients treated with cysteamine. Fertil Steril 93:1880–1883
Veys KR, D’Hauwers KW, van Dongen AJCM, Janssen MC, Besouw MTP, Goossens E, van den Heuvel LP, Wetzels AAMM, Levtchenko EN (2018) First successful conception induced by a male cystinosis patient. JIMD Rep 38:1–6
Peco-Antić A, Kostić M, Bogdanović R, Spasojević B, Djordjević M, Paripović D, Kovacević D (2011) Infantile nephropathic cystinosis. Srp Arh Celok Lek 139:486–490
Reiss RE, Kuwabara T, Smith ML, Gahl WA (1988) Successful pregnancy despite placental cystine crystals in a woman with nephropathic cystinosis. N Engl J Med 319:223–226
Chuang YW, Wen MC, Wu MJ, Shu KH, Cheng CH, Yu TM, Huang ST, Chen CH (2012) Follow-up and treatment of renal transplantation with nephropathic cystinosis in central Taiwan. Transplant Proc 44:80–82
Haase M, Morgera S, Bamberg C, Halle H, Martini S, Dragun D, Neumayer HH, Budde K (2006) Successful pregnancies in dialysis patients including those suffering from cystinosis and familial Mediterranean fever. J Nephrol 19:677–681
Jellouli M, Ferjani M, Abidi K, Zarrouk C, Abdelmoula J, Gargah T (2017) Infantile cystinosis: from dialysis to renal transplantation. Saudi J Kidney Dis Transpl 28:1180–1183
Hillenbrand M, Stropahl G, Seiter H (1998) Massive tumour-like testicular cystine accumulation in a patient with infantile nephropathic cystinosis. Br J Urol 81:331–332
Elenberg E, Norling LL, Kleinman RE, Ingelfinger JR (1998) Feeding problems in cystinosis. Pediatr Nephrol 12:365–370
Sonies BC, Almajid P, Kleta R, Bernardini I, Gahl WA (2005) Swallowing dysfunction in 101 patients with nephropathic cystinosis: benefit of long-term cysteamine therapy. Medicine (Baltimore) 84:137–146
Sonies BC, Ekman EF, Andersson HC, Adamson MD, Kaler SG, Markello TC, Gahl WA (1990) Swallowing dysfunction in nephropathic cystinosis. N Engl J Med 323:565–570
Rossi S, Herrine SK, Navarro VJ (2005) Cystinosis as a cause of noncirrhotic portal hypertension. Dig Dis Sci 50:1372–1375
Cornelis T, Claes K, Gillard P, Nijs E, Roskams T, Lombaerts R, Nevens F, Cassiman D (2008) Cholestatic liver disease in long-term infantile nephropathic cystinosis. J Gastroenterol Hepatol 23:e428–e431
O’Brien K, Hussain N, Warady BA, Kleiner DE, Kleta R, Bernardini I, Heller T, Gahl WA (2006) Nodular regenerative hyperplasia and severe portal hypertension in cystinosis. Clin Gastroenterol Hepatol 4:387–394
Klenn PJ, Rubin R (1994) Hepatic fibrosis associated with hereditary cystinosis: a novel form of noncirrhotic portal hypertension. Mod Pathol 7:879–882
Besouw M, Blom H, Tangerman A, de Graaf-Hess A, Levtchenko E (2007) The origin of halitosis in cystinotic patients due to cysteamine treatment. Mol Genet Metab 91:228–233
Besouw M, Tangerman A, Cornelissen E, Rioux P, Levtchenko E (2012) Halitosis in cystinosis patients after administration of immediate-release cysteamine bitartrate compared to delayed-release cysteamine bitartrate. Mol Genet Metab 107:234–236
Besouw MT, Schneider J, Janssen MC, Greco M, Emma F, Cornelissen EA, Desmet K, Skovby F, Nobili F, Lilien MR, De Paepe A, Malfait F, Symoens S, van den Heuvel LP, Levtchenko EN (2013) Copper deficiency in patients with cystinosis with cysteamine toxicity. J Pediatr 163:754–760
Heller AN, Heller DS, Schwimmer A, Gordon RE, Cambria RJ (1994) Cystinosis and gingival hyperplasia: demonstration of cystine crystals in gingival tissue and unusual aspects of management. J Periodontol 65:1139–1141
Busuttil DP, Liu Yin JA (2000) The bone marrow in hereditary cystinosis. Br J Haematol 111:385
Lyou Y, Zhao X, Nangia CS (2015) Pancytopenia in a patient with cystinosis secondary to myelosuppression from cystine crystal deposition: a case report. J Med Case Rep 9:205
Gebrail F, Knapp M, Perotta G, Cualing H (2002) Crystalline histiocytosis in hereditary cystinosis. Arch Pathol Lab Med 126:1135
Cornet E, Repesse Y (2015) Haematological complications of cystinosis. Eur J Haematol 94:187
Bigley V, Bhartia S, Wood A (2007) Nephropathic cystinosis with bone marrow involvement. Br J Haematol 136:180
Quinn JP, Royston D, Murphy PT (2004) Bone marrow findings in hereditary cystinosis with renal failure. Am J Hematol 76:79
Monier L, Mauvieux L (2015) Cystine crystals in bone marrow aspirate. Blood 126:1515
Emadi A, Burns KH, Confer B, Borowitz MJ, Streiff MB (2008) Hematological manifestations of nephropathic cystinosis. Acta Haematol 119:169–172
Rozenberg G (2009) Post transplant lymphoproliferative disease following renal transplantation in a young male. Aust J Med Sci 30:19–20
Charnas LR, Luciano CA, Dalakas M, Gilliatt RW, Bernardini I, Ishak K, Cwik VA, Fraker D, Brushart TA, Gahl WA (1994) Distal vacuolar myopathy in nephropathic cystinosis. Ann Neurol 35:181–188
Kastrup O, Koeppen S, Schwechheimer K, Keidel M, Diener HC (1998) Myopathy in two siblings with nephropathic cystinosis. Eur J Neurol 5:609–612
Vester U, Schubert M, Offner G, Brodehl J (2000) Distal myopathy in nephropathic cystinosis. Pediatr Nephrol 14:36–38
Müller-Felber W, Schröder M, Hirschmann M, Kastrup K, Töpfer M, Pongratz D (1999) Neurophysiological testing in long-standing cystinosis. Electromyogr Clin Neurophsiol 39:67–70
Cabrera-Serrano M, Junckerstorff RC, Alisheri A, Pestronk A, Laing NG, Weihl CC, Lamont PJ (2017) Cystinosis distal myopathy, novel clinical, pathological and genetic features. Neuromuscul Disord 27:873–878
Gahl WA, Dalakas MC, Charnas L, Chen KT, Pezeshkpour GH, Kuwabara T, Davis SL, Chesney RW, Fink J, Hutchison HT (1988) Myopathy and cystine storage in muscles in a patient with nephropathic cystinosis. N Engl J Med 319:1461–1464
Vargus-Adams J (2002) Correspondence. Pediatr Neurol 26:244
Anikster Y, Lacbawan F, Brantly M, Gochuico BL, Avila NA, Travis W, Gahl WA (2001) Pulmonary dysfunction in adults with nephropathic cystinosis. Chest 119:394–401
Edens MA, van Son WJ, de Greef MH, Levtchenko EN, Blijham T, Wijkstra PJ (2006) Successful treatment of respiratory dysfunction in cystinosis by nocturnal non-invasive positive pressure ventilation. Clin Nephrol 66:306–309
Bassim CW, Gautam P, Domingo DL, Balog JZ, Guadagnini JP, Gahl WA, Hart TC (2010) Craniofacial and dental findings in cystinosis. Oral Dis 16:488–495
Sadjadi R, Duong R, Corre C, Sullivan S, Eichler F (2018) Pervasive neuromuscular deficits in adults with nephropathic cystinosis. Muscle Nerve 58:S6
Florenzano P, Ferreira C, Nesterova G, Roberts MS, Tella SH, de Castro LF, Brown SM, Whitaker A, Pereira RC, Bulas D, Gafni RI, Salusky IB, Gahl WA, Collins MT (2018) Skeletal consequences of nephropathic cystinosis. J Bone Miner Res 33:1870–1880
Bertholet-Thomas A, Claramunt-Taberner D, Gaillard S, Deschênes G, Sornay-Rendu E, Szulc P, Cohen-Solal M, Pelletier S, Carlier MC, Cochat P, Bacchetta J (2018) Teenagers and young adults with nephropathic cystinosis display significant bone disease and cortical impairment. Pediatr Nephrol 33:1165–1172
Sirrs S, Munk P, Mallinson PI, Ouellette H, Horvath G, Cooper S, Da Roza G, Rosenbaum D, O’Riley M, Nussbaumer G, Hoang LN, Lee CH (2014) Cystinosis with sclerotic bone lesions. JIMD Rep 13:27–31
DeVilliers P, Gutta R, Szymela VF (2008) Cystinosis, fanconi syndrome, and odontogenic cysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:866–871
Broyer M, Tête MJ, Guest G, Berthélémé JP, Labrousse F, Poisson M (1996) Clinical polymorphism of cystinosis encephalopathy. Results of treatment with cysteamine. J Inherit Metab Dis 19:65–75
Fink JK, Brouwers P, Barton N, Malekzadeh MH, Sato S, Hill S, Cohen WE, Fivush B, Gahl WA (1989) Neurologic complications in long-standing nephropathic cystinosis. Arch Neurol 46:543–548
Muller M, Baumeier A, Ringelstein EB, Husstedt IW (2008) Long-term tracking of neurological complications of encephalopathy and myopathy in a patient with nephropathic cystinosis: a case report and review of the literature. J Med Case Rep 2:235
Vogel DG, Malekzadeh MH, Cornford ME, Schneider JA, Shields WD, Vinters HV (1990) Central nervous system involvement in nephropathic cystinosis. J Neuropathol Exp Neurol 49:591–599
Dongo E, Magyar M, Aradi G, Szőcs I, Csillik A, Resch M, Rimely E, Varallyay G, Bereczki D, Vastagh I (2015) Neurological complications of nephropathic cystinosis in a young female adult – case report. Eur J Neurol 22(Suppl 1):553
Berger JR, Dillon DA, Young BA, Goldstein SJ, Nelson P (2009) Cystinosis of the brain and spinal cord with associated vasculopathy. J Neurol Sci 284:182–185
Marguardt L, Kuramatsu JB, Roesch J, Engelhorn T, Huttner HB (2013) Posterior reversible encephalopathy syndrome in cystinosis. Clin Neurol Neurosurg 115:644–645
Neutel D, Geraldes R, Pereira P, Gomes da Costa A, Pimentel J, E Melo TP (2013) Recurrent ischemic stroke in an adult with cystinosis: a clinical-pathological case. J Stroke Cerebrovasc Dis 22:e674–e675
Dogulu CF, Tsilou E, Rubin B, Fitzgibbon EJ, Kaiser-Kupper MI, Rennert OM, Gahl WA (2004) Idiopathic intracranial hypertension in cystinosis. J Pediatr 145:673–678
Rogers DL, McGregor ML (2010) Increased intracranial pressure in patients with cystinosis. J Pediatr Ophthalmol Strabismus 47:e1–e3
Dahdal S, Kalicki R, Von Steiger N, Sendi P (2017) Disseminated cryptococcal infection in a patient who had kidney transplant: discrepancy between clinical symptoms and microbiological findings. BMJ Case Rep. https://doi.org/10.1136/bcr-2017-219234
Swenson MR, Rimmer S, Schneider JA, Melles RB, Trauner DA, Katz B (1991) Neurophysiologic studies of the peripheral nervous system in nephropathic cystinosis. Arch Neurol 48:528–529
Spilkin AM, Ballantyne AO, Trauner DA (2009) Visual and verbal learning in a genetic metabolic disorder. Neuropsychologia 47:1883–1892
Sathappan AV, Trauner D (2016) Hierarchical processing of visual stimuli in individuals with nephropathic cystinosis and obligate heterozygotes. J Inv Med 64:344
Ballantyne AO, Scarvie KM, Trauner DA (1997) Academic achievement in individuals with infantile nephropathic cystinosis. Am J Med Genet 74:157–161
Colah S, Trauner DA (1997) Tactile recognition in infantile nephropathic cystinosis. Dev Med Child Neurol 39:409–413
Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI (2000) Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab 71:100–120
Fung AT, Fraser-Bell S, Ojaimi E, Sutton G (2007) In vivo confocal microscopy and polarizing microscopy of the cornea in a patient with nephropathic cystinosis. Clin Exp Ophthalmol 35:292–293
Tsilou E, Zhou M, Gahl W, Sieving PC, Chan CC (2007) Ophthalmic manifestations and histopathology of infantile nephropathic cystinosis: report of a case and review of the literature. Surv Ophthalmol 52:97–105
Grupcheva CN, Ormonde SE, McGhee C (2002) In vivo confocal microscopy of the cornea in nephropathic cystinosis. Arch Ophthalmol 120:1742–1745
Fahey DK, Fenton S, Mohamed Q, Logan P (2001) Cystinosis, cataract surgery, and corneal erosions. J Cataract Refract Surg 27:2041–2043
Flockerzi E, Daas L, Schlötzer-Schrehardt U, Zimpfer A, Bohle R, Seitz B (2018) Ocular changes in nephropathic cystinosis: the course of the gold-dust. Int Ophthalmol. https://doi.org/10.1007/s10792-018-0954-7
Won JY, Hwang HB, Chung SK (2015) A case of corneal cystinosis in a patient with rickets and chronic renal failure. Indian J Ophthalmol 63:785–787
Liang H, Baudouin C, Tahiri Joulei Hassani R, Brignole-Baudouin F, Labbe A (2015) Photophobia and corneal crystal density in nephropathic cystinosis: an in vivo confocal microscopy and anterior-segment optical coherence tomography study. Invest Ophthalmol Vis Sci 56:3218–3225
Richler M, Milot J, Quigley M, O’Regan S (1991) Ocular manifestations of nephropathic cystinosis: the French-Canadian experience in a genetically homogeneous population. Arch Ophthalmol 109:359–362
Dureau P, Broyer M, Dufier JL (2003) Evolution of ocular manifestations in nephropathic cystinosis: a long-term study of a population treated with cysteamine. J Pediatr Ophthalmol Strabismus 40:142–146
Kaiser-Kupfer MI, Gazzo MA, Datiles MB, Caruso RC, Kuehl EM, Gahl WA (1990) A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis. Arch Opthalmol 108:689–693
Tavares R, Coelho D, Macário MC, Torres A, Quadrado MJ, Murta J (2009) Evaluation of treatment with cysteamine eyedrops for cystinosis with confocal microscopy. Cornea 28:938–940
Iwata F, Kuehl EM, Reed GF, McCain LM, Gahl WA, Kaiser-Kupfer MI (1998) A randomized clinical trial of topical cysteamine disulfide (cystamine) versus free thiol (cysteamine) in the treatment of corneal cystine crystals in cystinosis. Mol Genet Metab 64:237–242
Blanksma LJ, Jansonius NM, Reitsma-Bierens WC (1996) Cysteamin eyedrops in three patients with nephropathic cystinosis. Doc Ophthalmol 92:51–53
Macário MC, Torres A, Tavares R, Cunha L (2011) Successful use of topical cysteamine in cystinosis. J Inherit Metab Dis 34(Suppl 3):S87
Liang H, Labbé A, Le Mouhaër J, Plisson C, Baudouin C (2017) A new viscous cysteamine eye drops treatment for ophthalmic cystinosis: an open-label randomized comparative phase III pivotal study. Invest Ophthalmol Vis Sci 58:2275–2283
Labbé A, Baudouin C, Deschênes G, Loirat C, Charbit M, Guest G, Niaudet P (2014) A new gel formulation of topical cysteamine for the treatment of corneal cystine crystals in cystinosis: the Cystadrops OCT-1 study. Mol Genet Metab 111:314–320
MacDonald IM, Noel LP, Mintsioulis G, Clarke WN (1990) The effect of topical cysteamine drops on reducing crystal formation within the cornea of patients affected by nephropathic cystinosis. J Pediatr Ophthalmol Strabismus 27:272–274
Tsilou ET, Rubin BI, Reed GF, Iwata F, Gahl W, Kaiser-Kupfer MI (2002) Age-related prevalence of anterior segment complications in patients with infantile nephropathic cystinosis. Cornea 21:173–176
Iwata F, Caruso RC, McCain LM, Gahl WA, Kaiser-Kupfer MI (1996) Visual function assessment in adults with nephropathic cystinosis. Invest Ophthalmol Vis Sci 37:S102
Tsilou ET, Rubin BI, Reed G, Caruso RC, Iwata F, Balog J, Gahl WA, Kaiser-Kupfer MI (2006) Nephropathic cystinosis: posterior segment manifestations and effects of cysteamine therapy. Ophthalmology 113:1002–1009
Tsilou E, Csaky K, Rubin BI, Gahl W, Kaiser-Kupfer M (2002) Retinal visualization in an eye with corneal crystals using indocyanine green videoangiography. Am J Opthalmol 134:123–125
Katz B, Melles RB, Schneider JA, Rao NA (1989) Corneal thickness in nephropathic cystinosis. Br J Ophthalmol 73:665–668
Mungan N, Nischal KK, Héon E, MacKeen L, Balfe JW, Levin AV (2000) Ultrasound biomicroscopy of the eye in cystinosis. Arch Ophthalmol 118:1329–1333
Ariceta G, Lara E, Camacho JA, Oppenheimer F, Vara J, Santos F, Muñoz MA, Cantarell C, Gil Calvo M, Romero R, Valenciano B, García-Nieto V, Sanahuja MJ, Crespo J, Justa ML, Urisarri A, Bedoya R, Bueno A, Daza A, Bravo J, Llamas F, Jiménez Del Cerro LA (2015) Cysteamine (Cystagon) adherence in patients with cystinosis in Spain: successful in children and a challenge in adolescents and adults. Nephrol Dial Transplant 30:475–480
Beinart N, Hackett RA, Graham CD, Weinman J, Ostermann M (2015) Mood and illness experiences of adults with cystinosis. Ren Fail 37:835–839
Doyle M, Werner-Lin A (2015) That eagle covering me: transitioning and connected autonomy for emerging adults with cystinosis. Pediatr Nephrol 30:281–291
Dohil R, Cabrera BL (2013) Treatment of cystinosis with delayed-release cysteamine: 6-year follow-up. Pediatr Nephrol 28:507–510
Levtchenko EN, van Dael CM, de Graaf-Hess AC, Wilmer MJ, van den Heuvel LP, Monnens LA, Blom HJ (2006) Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr Nephrol 21:110–113
Buntinx L, Voets T, Morlion B, Vangeel L, Janssen M, Cornelissen E, Vriens J, de Hoon J, Levtchenko E (2016) TRPV1 dysfunction in cystinosis patients harboring the homozygous 57kb deletion. Sci Rep 6:35395
Doyle M (2015) Peer support and mentorship in a US rare disease community: findings from the cystinosis in emerging adulthood study. Patient 8:65–73
Doyle M, Werner-Lin A (2016) Family strategies for living with rare disease: the experience of cystinosis. J Soc Social Work Res 7:547–567
Nesterova G, Williams C, Bernardini I, Gahl WA (2015) Cystinosis: renal glomerular and renal tubular function in relation to compliance with cystine-depleting therapy. Pediatr Nephrol 30:945–951
Bertholet-Thomas A, Berthiller J, Tasic V, Kassai B, Otukesh H, Greco M, Ehrich J, de Paula BR, Deschênes G, Hulton SA, Fischbach M, Soulami K, Saeed B, Valavi E, Cobenas CJ, Hacihamdioglu B, Weiler G, Cochat P, Bacchetta J (2017) Worldwide view of nephropathic cystinosis: results from a survey from 30 countries. BMC Nephrol 18:210
Cohen C, Charbit M, Chadefaux-Vekemans B, Giral M, Garrigue V, Kessler M, Antoine C, Snanoudj R, Niaudet P, Kreis H, Legendre C, Servais A (2015) Excellent long-term outcome of renal transplantation in cystinosis patients. Orphanet J Rare Dis 10:90
Mannon R, Reed R, Locke J (2014) Kidney transplantation in nephropathic cystinosis: outcomes in the 21st century. Transplantation 98(Suppl 1):496
Spicer RA, Clayton PA, McTaggart SJ, Zhang GY, Alexander SI (2015) Patient and graft survival following kidney transplantation in recipients with cystinosis: a cohort study. Am J Kidney Dis 65:172–173
Schaefer HM, Helderman JH, Fogo AB (2006) Slow decline in allograft function in a renal transplant patient. Am J Kidney Dis 48:335–338
Raza MN, Tomson CRV, AshMiles J (2007) Papillary necrosis in a kidney transplant following profound hypotension. Dial Transplant 36:611–615
Rocha S, Martins LS, Vizcaíno R, Dias L, Almeida M, Pedroso S, Vidinha J, Rocha M, Rocha G, Mota C, Henriques A, Cabrita A (2011) New-onset lupus nephritis in a kidney transplant recipient with cystinosis-differential diagnosis with cysteamine-induced lupus: case report. Transplant Proc 43:2265–2268
Cameron C, Greenbaum L, Sato T, Trost B, Lundeen B, Kelly ME (2008) Renal cell carcinoma in a patient with cystinosis and inflammatory bowel disease: a case report. Pediatr Nephrol 23:1167–1170
Gündüz D, Windpessl M, Wallner M (2011) Reverse PD – a rare cause of acute renal failure in a transplanted patient. NDT Plus 4:144–145
Fontana I, Bertocchi M, Rossi AM, Gasloli G, Santori G, Ferro C, Patti V, Rossini A, Valente U (2010) Corynebacterium urealyticum infection in a pediatric kidney transplant recipient: case report. Transplant Proc 42:1367–1368
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 271 kb)
Rights and permissions
About this article
Cite this article
Kasimer, R.N., Langman, C.B. Adult complications of nephropathic cystinosis: a systematic review. Pediatr Nephrol 36, 223–236 (2021). https://doi.org/10.1007/s00467-020-04487-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04487-6