Abstract
Background
JC polyomavirus (JCPyV)-associated nephropathy (JCPyVAN) is a severe, but rare complication in adult renal transplant (RTx) recipients. Related data in pediatric patients are scarce.
Methods
Based on the CERTAIN Registry, we therefore performed a multi-center, retrospective study on the JCPyV antibody status, prevalence of JCPyV replication, and its associated disease in 139 pediatric RTx recipients (mean age, 8.5 ± 5.3 years). JCPyV DNA in plasma and/or urine was measured by quantitative PCR at a median time of 3.2 (IQR, 0.3–8.1) years post-transplant.
Results
53.2% of patients were JCPyV-seronegative prior to transplantation; younger age was associated with JCPyV seronegativity. 34/139 (24.5%) patients post-transplant showed active JCPyV replication in either urine (22.0%), plasma (13.4%), or both (7.6%). JCPyV viremia occurred significantly (p < 0.001) more often in patients with viruria (34.6%) than in those without (7.6%), but 7/118 (5.9%) had isolated viremia. High-level viruria (> 107 copies/mL) was found in 29.6% of viruric patients. A higher net state of immunosuppression constituted an independent risk factor for JCPyV replication both in urine and plasma (OR 1.2, p < 0.02). Male patients tended to have a higher risk of JCPyV viremia than females (OR 4.3, p = 0.057). There was one male patient (0.7%) with JCPyVAN 7 years post-transplant, which resolved after reduction of immunosuppressive therapy. No patient exhibited progressive multifocal leukoencephalopathy.
Conclusions
This first multi-center study on JCPyV in pediatric renal transplant recipients shows that JCPyV replication is common (24.5%), with strong immunosuppression being a significant risk factor, but associated nephropathy is rare.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human neurotropic JC polyomavirus (JCPyV) belongs to the family of polyomaviruses, also comprising more than 10 human polyomaviruses, including BK polyomavirus (BKPyV) [1]. JCPyV was discovered in the 1960s through electron microscopy and eventual isolation from post-mortem brain tissue of a patient (J.C.) succumbing to progressive multifocal leukoencephalopathy (PML) [2,3,4]. Seroprevalence studies indicated at least two phases of JCPyV exposure: an early phase in childhood in approximately 25%, and a second phase in adult life eventually reaching 70% by the sixth decade of life [4]. Various seroprevalence studies detected JCPyV-specific antibodies in 30–70% of healthy individuals [5,6,7]. Unlike BKPyV, JCPyV is difficult to culture and requires specific glial progenitor cells or cell lines that express the SV40 large T-antigen [4]. After primary infection, JCPyV, similarly as BKPyV, causes a persistent infection in the renourinary tract. Urinary shedding of JCPyV in healthy individuals rises from about 10% in the third decade to 30% in the sixth decade of life [6].
JC polyomavirus-attributed pathologies have been reported in diverse immunocompromised hosts, including human immunodeficiency virus (HIV)-infected individuals and patients under immunosuppressive medication, e.g., transplant recipients and patients suffering from autoimmune disease, treated with natalizumab [8] or rituximab [9]. Progressive multifocal leukoencephalopathy is the most severe and best-known complication of JCPyV replication in immunosuppressed patients. Other relevant entities include JCPyV-associated nephropathy (JCPyVAN), which has been systematically studied and contrasted with BKPyV replication and nephropathy in a large prospective study of adult kidney transplant patients [10]. Additional information about JCPyVAN comes from small case series [11] or case reports [10, 12,13,14,15], but data on the epidemiology of JCPyV infection and associated disease in kidney transplant patients have largely been obtained only in adults [16,17,18].
Here, we report on the first multi-center study investigating the prevalence of JCPyV infection and disease in a cohort of 139 pediatric renal transplant recipients. To identify the prevalence of ongoing JCPyV replication, we determined the presence of JCPyV DNA in urine (viruria) and in plasma (viremia). To analyze the pre-transplant exposure and infection status, we determined the presence of JCPyV antibody. To identify the occurrence of JCPyV-associated diseases, we assessed the rate of JCPyVAN and PML in pediatric kidney transplant recipients.
Materials and methods
Study design and patient population
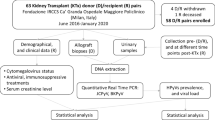
This is a multi-center, retrospective study of data provided to the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) Registry (www.certain-registry.eu) [19, 20]. The CERTAIN Registry is a voluntary registry used by 60 pediatric renal transplant centers from 16 European countries, that provide data from pediatric kidney allograft recipients aged ≤ 21 years at transplantation. The registry’s dataset offers essential information on generic kidney transplantation-related topics and also captures pediatric-specific topics, such as growth, physical and psychosocial development, and adherence [19, 20]. JCPyV-specific data collection for this analysis followed a defined protocol (see Supporting information in the online version of this article). Eligible patients were pediatric kidney allograft recipients (i) aged ≤ 21 years at time of engraftment; (ii) having a complete and validated dataset; and (iii) with JCPyV surveillance at least once in plasma and/or urine using quantitative nucleic acid testing (QNAT). All diagnostic investigations for JCPyV events had to be documented, even those found to be negative. Written informed consent to participate in the registry was obtained from all parents/guardians, with assent from patients, if appropriate for their age. The CERTAIN Registry has been approved by the ethics committee of each contributing center and is maintained in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was designed, evaluated, and reported in agreement with the STROBE guidelines (https://www.strobe-statement.org) [21]. In addition, written informed consent was obtained by the patient who developed JCPyVAN and by his parents, to describe his clinical course in detail.
Pediatric Vasudev score
To assess the net state of immunosuppressive therapy, a pediatric immunosuppressive score [22, 23] modified according to Vasudev et al. [24], named “pediatric Vasudev score”, was calculated for each patient (Table 1). Whereas an independent, well-validated measure of “over-immunosuppression” is still the holy grail of transplantation medicine, the pediatric Vasudev score has been repeatedly published by our group [22, 23, 25,26,27] and others [28, 29] to estimate the net state of immunosuppression. It is therefore proposed as a valuable scale applicable by other transplant researchers who compare pediatric renal allograft recipients on different immunosuppressive regimens [28].
Virological assessments
Quantitative nucleic acid testing in urine and/or plasma was performed according to the manufacturers’ instructions, including RealStar® JCV PCR Kit (Altona Diagnostics) in six contributing centers, and TIB LightMix® JC kit (TIB Molbiol) and GeneProof JC Virus (JCV) PCR Kit (Medac Diagnostika) in two further contributing centers, respectively. All PCR assays are CE-certified tests and conform to proficiency panels; quantitative results are comparable among different assays performed at different sites. High-level JCPyV viruria was defined as > 107 copies/mL [16], and high-level JCPyV viremia was arbitrarily defined as > 104 copies/mL, in analogy to BKPyV. JCPyVAN was defined as JCPyV replication, absence of BKPyV replication, and histopathological changes characteristic of PyVAN, together with a positive immunohistochemistry for the simian virus 40 large T-antigen (SV40LTag) staining, as proposed by the Banff 2013 meeting report [30, 31]. Quantitative nucleic acid testing was performed routinely at different study sites shortly after sample collection for all but the one patient who developed JCPyVAN. Samples of this patient, which were stored at − 80 °C (Department of Infectious Diseases, Virology, Heidelberg, Germany,) were analyzed retrospectively. Seroreactivity against the JCPyV major capsid protein VP1 was determined by multiplex serology, as previously described [32], and a mean fluorescence intensity of > 400 was defined as positive. Serum samples used for multiplex serology were also stored at − 80 °C (Department of Infectious Diseases, Virology, Heidelberg, Germany) before analysis. In addition, BKPyV replication (QNAT) in plasma and/or urine was determined routinely shortly after sample collection by means of the RealStar® BKV PCR Kit (Altona Diagnostics) in a subset of patients.
Statistical analysis
Data were analyzed with the aid of PASW (SPSS) Statistics 24.0. Results for continuous variables are expressed as mean ± standard deviation, unless stated otherwise. Categorical parameters are given as number and percentage of patients. The Shapiro-Wilk test was used to assess normality of data distribution. Rates in two groups were compared by means of the Fisher’s exact or chi-square test. Differences between two groups were analyzed by means of the two-tailed Student’s t test or, if normality failed, with the Mann-Whitney rank-sum test. Univariate and multivariable analyses (logistic regression) were performed to examine the effect of factors having a potential impact on the development of JCPyV replication and JCPyVAN. Factors with a p value < 0.20 according to univariate analysis were included in the multivariable model. Two-sided p values < 0.05 were considered statistically significant. All analyses were of an explorative nature.
Results
Patient characteristics and virological assessments
A total of 139 patients, having received a renal allograft between January 20, 1999 and July 28, 2017, were available for this analysis. Patient and transplant characteristics are given in Table 2. JCPyV QNAT was analyzed in 12 plasma samples per patient on average (range, 0–55 samples per patient; median 10 (IQR, 2–20) samples per patient). In addition, JCPyV QNAT was analyzed in eight urine samples per patient on average (range, 0–33 samples per patient; median 5 (IQR, 1–13) samples per patient). Plasma QNAT was performed in 132 (95.0%) patients, urine QNAT in 125 (89.9%), providing both plasma, and urine results in 118 (84.9%) patients. The median time-to-measurement of JCPyV QNAT post-transplant was 3.2 years (IQR, 0.3–8.1 years). The pre-transplant JCPyV serostatus (IgG) was available for a subset of patients (47/139 [33.8%]). The mean age (8.9 ± 5.6 years) and gender distribution (61.7% male gender) as well as the immunosuppressive regimen (data not shown) of this subgroup were comparable to those of the entire patient population. BKPyV replication (QNAT) in plasma and/or urine was determined in 113/139 patients (81.3%).
JCPyV viruria and viremia
JCPyV-viruria was detected in 27 (21.6%) of 125 pediatric renal transplant recipients; JCPyV viremia was found in 16 (12.1%) of 132 patients. In 118 (84.9%) patients, JCPyV viral load was analyzed in both urine and plasma: 26 (22.0%) patients had JCPyV viruria, 16 (13.4%) JCPyV viremia, and 9 (7.6%) patients had both. The rates of JCPyV replication remained similar over the first 3 years, before declining slightly (Fig. 1). High-level JCPyV viruria (> 107 copies/mL) was observed in 8/27 (29.6%) patients with JCPyV viruria, while only one viremic patient (6.3%) developed high-level JCPyV viremia (> 104 copies/mL). JCPyV viremia was seen significantly (p < 0.001) more often in patients with JCPyV viruria (9/26, 34.6%) than in those without viruria (7/92, 7.6%). Interestingly, 7/118 (5.9%) patients had JCPyV viremia without concomitant JCPyV viruria; these patients had median viral loads (4.0 × 102 copies/mL) patients with concomitant viruria (6.0 × 102 copies/mL; p = 0.750). Table 3 summarizes the JCPyV viral load values in urine and plasma. Altogether, 34 of 139 (24.5%) patients exhibited JCPyV viruria and/or JCPyV viremia.
Prevalence of JC polyomavirus (JCPyV) viruria and JCPyV viremia in pediatric renal transplant recipients. Gray bars, JCPyV viruria; black bars, JCPyV viremia. Rates are expressed as number of patients with positive result per number of patients with quantitative nucleic acid testing (QNAT) in the respective time period. QNAT in urine was performed in 125 patients (year 1, n = 40; year 2, n = 8; year 3, n = 9; > year 3, n = 68). QNAT in plasma was conducted in 132 patients (year 1, n = 41; year 2, n = 10; year 3, n = 11; > year 3, n = 70)
JCPyV serology
Pre-transplant sera for JCPyV IgG antibody analysis were available from 47/139 (33.8%) patients; 25 (53.2%) of whom were JCPyV-naïve prior to transplantation. Seronegative patients were significantly younger than seropositive recipients (mean age at transplantation: 7.5 ± 4.7 vs. 12.7 ± 5.2 years, p = 0.001). Primary JCPyV infection was observed in 8/25 (32.0%) seronegative patients after renal transplantation, while only 3/22 (13.6%) seropositive patients developed post-transplant JCPyV reactivation or reinfection (p = 0.179). Of the 25 seronegative patients, six (24.0%) developed JCPyV viruria (median peak viral load, 1.9 × 106 copies/mL), and three (12.0%) showed JCPyV viremia (median peak viral load, 3.0 × 103 copies/mL), including one patient with both JCPyV viruria and viremia. Of the 22 seropositive patients, three (13.6%) developed JCPyV viruria (median peak viral load, 4.7 × 107 copies/mL), and one (4.5%) of them exhibited concomitant JCPyV viremia with a viral load of 7.8 × 102 copies/mL. Hence, JCPyV-seronegative patients tended to show JCPyV replication more frequently (p = 0.179).
BKPyV viruria and viremia
BKPyV QNAT data were available in 113/139 (81.3%) of patients. Out of these 113 patients, 99 were tested for JCPyV DNA in urine: ten of 23 (43.5%) patients with JCPyV viruria had also BKPyV viruria, whereas 45/76 (59.2%) patients without JCPyV viruria developed BKPyV viruria (p = 0.233). In addition, 106/113 patients with BKPyV QNAT were tested for JCPyV DNA in plasma: 4/13 (30.8%) patients with JCPyV viremia showed concomitant BKPyV viremia, while 33/93 (35.5%) without JCPyV viremia developed BKPyV viremia (p = 0.738). None of the seven patients with BKPyV-associated nephropathy (BKPyVAN) showed JCPyV viruria or viremia.
Risk factors associated with JCPyV viruria and viremia
According to uni- and multivariate analysis, a higher net state of immunosuppression was an independent risk factor, significantly associated with the development of JCPyV viruria (univariate analysis: odds ratio (OR) 1.17 per unit, p = 0.028, multivariate analysis: OR 1.18 per unit, p = 0.019) and viremia (univariate analysis: OR 1.26 per unit, p = 0.004, multivariate analysis: OR 1.24 per unit, p = 0.016) (Table 4). It was also associated with any evidence of JCPyV replication (OR 1.29 per unit, p = 0.001). A trend towards an increased risk of JCPyV viremia was observed in male patients (OR 4.32; 95% confidence interval, 0.96–1.16; p = 0.057). Other potential factors, such as patient and transplant characteristics or specific immunosuppressive agents, did not reveal a significant association with a higher JCPyV viruria or viremia risk (Table 4). Also, no significant association was found between concomitant JCPyV and BKPyV viruria, on the one hand, and the development of JCPyV viremia (OR 2.7, p = 0.264), on the other.
JCPyV-associated disease
No case of PML was reported over 6.8 ± 4.0 years post-transplant. One patient (0.7%) developed JCPyVAN. His primary renal disease was congenital anomalies of the kidney and urinary tract (CAKUT). He received a living kidney donation from his mother at the age of 10 years. Initial immunosuppressive therapy consisted of tacrolimus (TAC), mycophenolate mofetil (MMF), and methylprednisolone, with steroid withdrawal 4 years post-transplant. Because of declining graft function 7 years post-transplant (Fig. 2a), he underwent a kidney allograft biopsy, which exhibited borderline rejection according to Banff criteria [30]. Immunohistochemistry showed an absence of viral antigens, especially no signs of SV40Ag. He did not respond to methylprednisolone pulse therapy, and estimated glomerular filtration rate (eGFR) declined further (Fig. 2a). He therefore had a second renal allograft biopsy, which showed interstitial nephritis suggestive of acute cellular rejection, again graded as borderline changes. But this time, immunoperoxidase staining with a monoclonal antibody against SV40 LTag, which reacts also with the LTag of BKPyV and JCPyV, was positive, indicating PyVAN grade A [30] (Fig. 2b). PyV NAT in the renal allograft biopsy sample showed JCPyV DNA, but no BKPyV DNA. Both urine and plasma had been analyzed 3 months since transplantation and were repeatedly found negative for BKPyV DNA. Retrospective evaluation of urine and plasma samples revealed that JCPyV viruria and viremia were already positive 1 year before biopsy-proven JCPyVAN. After establishing this diagnosis, immunosuppressive treatment was reduced: TAC dosage was lowered by 42% to achieve target trough concentrations of 3–5 μg/L, and MMF withdrawn. This intervention was associated with graft function stabilization (Fig. 2a). JCPyV viruria declined from a peak viral load of 1.1 × 1010 to 4.5 × 103 copies/mL at last observation (Fig. 2a). Peak JCPyV viral load in plasma was 5.6 × 104 copies/mL, decreased in response to reduction of the immunosuppressive medication to 1.0 × 102 copies/mL 8 months later, and became undetectable thereafter (Fig. 2a).
a Course of JC polyomavirus (JCPyV) replication and transplant function in the patient with JCPyV-associated nephropathy. Blue squares, estimated glomerular filtration rate (eGFR); yellow triangles, JCPyV viral load in urine; red circles, JCPyV viral load in plasma. MPR, methylprednisolone. Stars indicate the time point of biopsies. b Histopathological findings of the second kidney allograft biopsy in the patient with JCPyV-associated nephropathy. I. Masson trichrome stain with nuclear enlargement and nuclear inclusions. II. Immunohistochemistry for SV40 T antigen with focal nuclear staining in tubular epithelial cells
Discussion
This is the first multi-center study investigating JCPyV replication in plasma and urine and JCPyV-associated disease in more than 100 pediatric renal transplant recipients. The main findings are that, first, the prevalence of JCPyV viruria is 21.6% and that of JCPyV viremia 12.1%. Second, JCPyV replication was associated with a higher net state of immunosuppression according to the modified Vasudev score. Third, despite the fact that one half of tested patients had no detectable JCPyV-Vp1 IgG prior to transplantation, JCPyV-associated nephropathy remained rare, though associated with a significant impact on graft function.
One previous study reported a higher prevalence of JCPyV viremia (21%) in the first year post-transplant in pediatric renal transplant recipients [34], which may be explained by differences in the patient population (higher mean age in the study of Ettenger et al. [34]) and/or differences in the immunosuppressive regimen (induction therapy in 35.3% of patients in the present study vs. 100% in the study of Ettenger et al. [34]). A recent single-center study among 46 pediatric renal transplant recipients [35] identified JCPyV viruria in 17%, which is markedly higher than the reported rate of 2–7% in healthy children and adolescents [36,37,38]. An obvious explanation for this difference could be that pediatric renal transplant recipients are frequently JCPyV seronegative prior to transplantation and acquire primary JCPyV infection via the transplant organ. Our observations that more than 50% of patients were JCPyV-seronegative pre-transplant and that post-transplant JCPyV replication occurred more frequently (28.0%) in JCPyV seronegative than in seropositive (13.6%) patients are consistent with this line of reasoning. Patients with JCPyV viruria developed viremia significantly more often, as has been shown for BKPyV [39]. Interestingly, however, a few patients exhibited JCPyV viremia without concomitant viruria. This supports the hypothesis that JCPyV replicates also at sites other than the urogenital tract. It has been reported that JCPyV virions persist in B cells [40]. Resident B cells in the renal allograft could therefore be the source of transmission to the recipient. However, these data are discussed controversially and need still to be reproduced by other researchers [4].
Our study is the first reporting that a higher net state of immunosuppression represents an independent risk factor for the development of both JCPyV viruria and viremia. The association between immunosuppressive treatment and JCPyV replication has been discussed controversially in adult patients. It has even been proposed that, once JCPyVAN has been established, the reduction of immunosuppression has a contentious impact on the clinical course [41]. Although no specific immunosuppressive agent was significantly associated with a higher risk of JCPyV replication, reducing immunosuppressive medication in kidney transplant recipients with proven JCPyVAN was followed by stabilization of graft function in 75% of cases [10,11,12,13,14,15]. Similarly, in the patient developing biopsy-proven JCPyVAN reported here, the reduction of immunosuppressive therapy was associated with clearance of JCPyV viremia, decline of urine viral load, and improvement of graft function. Together, these observations are in line with the notion that a higher net state of immunosuppression is a risk factor for JCPyV replication progressing to organ-invasive disease.
We report on a pediatric case of late JCPyVAN, in which a careful stepwise reduction of immunosuppressive therapy was associated with transplant function improvement over a long period of time, and stabilization still continues. Another case report on an adolescent patient with early JCPyVAN has been published previously [12]. In that case, however, reduction of immunosuppression merely led to an intermittent graft function stabilization, followed by graft loss due to antibody-mediated rejection [12].
The fact that SV40Ag was absent in the first allograft biopsy of the patient with JCPyVAN deserves a comment, especially since only one core had been taken during this first biopsy. European and international guidelines recommend a minimum of two kidney biopsy cores, preferably containing medulla tissues, for a histological diagnosis of human polyomavirus-associated nephropathy, in order to reduce the rate of false negative results [42]. Our observations underline the importance of these recommendations.
A previous, prospective study systematically analyzed BKPyV and JCPyV replication and disease in adult kidney allograft recipients [10]. In contrast to BKPyV, JCPyV shedding was more often asymptomatic, and decoy cells were detectable more frequently despite immunosuppression reduction. In addition, JCPyV viremia was rare and lower than BKPyV viremia. In case of biopsy-proven JCPyVAN, the number of infected cells was lower than in BKPyVAN, and graft loss was seldom [10]. Inhibitory interactions between JCPyV and BKPyV have been proposed in a study among 200 adult kidney allograft recipients [43]. Our data do not confirm this hypothesis, since concomitant BKPyV replication was observed in 48.1% of patients with and in 54.7% of patients without JCPyV replication. In particular, no differences were found between BKPyV viruria or viremia rates in JCPyV viruric or viremic patients. The discrepancy between these observations may be related to differences in patient populations and particularly between adult and pediatric kidney transplant patients. Prospective studies in larger patient cohorts are needed to clarify this aspect.
The limitations of our study are that data on JCPyV replication were obtained in a retrospective manner and that JCPyV antibody levels were only available in a subset of patients. Another limitation is the uneven number of samples per patient available for urine and plasma QNAT as well as the broad range of the post-transplant follow-up period. Moreover, differences between GST-Vp1-based and VLP-based assays have been reported [44, 45]. A relatively small number of patients were investigated, but this is an inherent problem for all studies in the pediatric renal transplant population (only 5% of all renal transplantations are performed in children and adolescents).
In conclusion, this first multi-center study on JCPyV replication and associated disease in pediatric renal transplant recipients shows that JCPyV replication is common and that a higher net state of immunosuppression constitutes a significant risk factor. Though a rare complication of approximately 1% in our study, JCPyVAN is a serious cause of graft function deterioration. Timely reduction of the immunosuppressive medication is currently the only viable treatment option for JCPyVAN, as long as specific antiviral agents are lacking. While our data do not support a routine screening of JCPyV replication in pediatric renal transplant recipients, JCPyV plasma viral load determination is advisable in the event of unclear graft function deterioration in the presence of SV40Ag positivity and in the absence of BKPyV replication.
References
Calvignac-Spencer S, Feltkamp MC, Daugherty MD, Moens U, Ramqvist T, Johne R, Ehlers B, Polyomaviridae Study Group of the International Committee on Taxonomy of V (2016) A taxonomy update for the family Polyomaviridae. Arch Virol 161:1739–1750
Zurhein G, Chou SM (1965) Particles resembling papova viruses in human cerebral demyelinating disease. Science 148:1477–1479
Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH (1971) Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1:1257–1260
Hirsch HH, Kardas P, Kranz D, Leboeuf C (2013) The human JC polyomavirus (JCPyV): virological background and clinical implications. APMIS 121:685–727
Padgett BL, Rogers CM, Walker DL (1977) JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun 15:656–662
Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH (2009) Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 199:837–846
Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, Miller E (2003) Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 71:115–123
Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D (2005) Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 353:375–381
Berger JR (2007) Progressive multifocal leukoencephalopathy. Curr Neurol Neurosci Rep 7:461–469
Drachenberg CB, Hirsch HH, Papadimitriou JC, Gosert R, Wali RK, Munivenkatappa R, Nogueira J, Cangro CB, Haririan A, Mendley S, Ramos E (2007) Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation 84:323–330
Kantarci G, Eren Z, Demirag A, Dogan I, Cakalagaoglu F, Yilmaz G (2011) JC virus-associated nephropathy in a renal transplant recipient and comparative analysis of previous cases. Transpl Infect Dis 13:89–92
Lautenschlager I, Jahnukainen T, Kardas P, Lohi J, Auvinen E, Mannonen L, Dumoulin A, Hirsch HH, Jalanko H (2014) A case of primary JC polyomavirus infection-associated nephropathy. Am J Transplant 14:2887–2892
Kazory A, Ducloux D, Chalopin JM, Angonin R, Fontaniere B, Moret H (2003) The first case of JC virus allograft nephropathy. Transplantation 76:1653–1655
Wen MC, Wang CL, Wang M, Cheng CH, Wu MJ, Chen CH, Shu KH, Chang D (2004) Association of JC virus with tubulointerstitial nephritis in a renal allograft recipient. J Med Virol 72:675–678
Aubert O, Galmiche L, Rozenberg F, Duquesne A, Scemla A, Rabant M, Leruez M, Laude H, Legendre C, Sberro-Soussan R (2013) The case | post-tranplant allograft dysfunction. Kidney Int 83:765–767
Helantera I, Hirsch HH, Auvinen E, Mannonen L, Nummi M, Wernli M, Ortiz F, Raisanen-Sokolowski A, Lempinen M, Lautenschlager I (2016) High-level JCPyV viruria after kidney transplantation-clinical and histopathological findings. J Clin Virol 85:75–79
Helantera I, Ortiz F, Auvinen E, Raisanen-Sokolowski A, Lappalainen M, Lautenschlager I, Koskinen P (2009) Polyomavirus BK and JC infections in well matched Finnish kidney transplant recipients. Transpl Int 22:688–693
Saundh BK, Baker R, Harris M, Hale A (2016) A prospective study of renal transplant recipients reveals an absence of primary JC polyomavirus infections. J Clin Virol 77:101–105
Koster L, Krupka K, Hocker B, Rahmel A, Samuel U, Zanen W, Opelz G, Susal C, Dohler B, Plotnicki L, Kohl CD, Knaup P, Tonshoff B (2015) Integrating data from multiple sources for data completeness in a web-based registry for pediatric renal transplantation—the CERTAIN Registry. Stud Health Technol Inform 216:1049
Plotnicki L, Kohl CD, Hocker B, Krupka K, Rahmel A, Pape L, Hoyer P, Marks SD, Webb NJ, Soylemezoglu O, Topaloglu R, Szabo AJ, Seeman T, Marlies Cornelissen EA, Knops N, Grenda R, Tonshoff B (2013) The CERTAIN Registry: a novel, web-based registry and research platform for pediatric renal transplantation in Europe. Transplant Proc 45:1414–1417
Chisolm JJHH, Eberlein WR, Harrison HE (1955) Aminoaciduria, hypophosphatemia, and rickets in lead poisoning. Am J Dis Child 89:159–168
Hocker B, Bohm S, Fickenscher H, Kusters U, Schnitzler P, Pohl M, John U, Kemper MJ, Fehrenbach H, Wigger M, Holder M, Schroder M, Feneberg R, Kopf-Shakib S, Tonshoff B (2012) (Val-)Ganciclovir prophylaxis reduces Epstein-Barr virus primary infection in pediatric renal transplantation. Transpl Int 25:723–731
Hocker B, Fickenscher H, Delecluse HJ, Bohm S, Kusters U, Schnitzler P, Pohl M, John U, Kemper MJ, Fehrenbach H, Wigger M, Holder M, Schroder M, Billing H, Fichtner A, Feneberg R, Sander A, Kopf-Shakib S, Susal C, Tonshoff B (2013) Epidemiology and morbidity of Epstein-Barr virus infection in pediatric renal transplant recipients: a multicenter, prospective study. Clin Infect Dis 56:84–92
Vasudev B, Hariharan S, Hussain SA, Zhu YR, Bresnahan BA, Cohen EP (2005) BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int 68:1834–1839
Cordts SE, Schneble L, Schnitzler P, Wenzel JJ, Vinke T, Rieger S, Fichtner A, Tonshoff B, Hocker B (2018) Prevalence, morbidity, and therapy of hepatitis E virus infection in pediatric renal allograft recipients. Pediatr Nephrol 33:1215–1225
Hocker B, Aguilar M, Schnitzler P, Pape L, Bald M, Konig J, Marks SD, Genc G, Buscher A, Kemper MJ, Billing H, Pohl M, Dello Strologo L, Webb NJA, Rieger S, Mankertz A, Krupka K, Bruckner T, Fichtner A, Tonshoff B (2018) Vaccination titres pre- and post-transplant in paediatric renal transplant recipients and the impact of immunosuppressive therapy. Pediatr Nephrol 33:897–910
Hocker B, Zencke S, Krupka K, Fichtner A, Pape L, Dello Strologo L, Guzzo I, Topaloglu R, Kranz B, Konig J, Bald M, Webb NJ, Noyan A, Dursun H, Marks S, Yalcinkaya F, Thiel F, Billing H, Pohl M, Fehrenbach H, Bruckner T, Tonshoff B (2016) Cytomegalovirus infection in pediatric renal transplantation and the impact of chemoprophylaxis with (val-)ganciclovir. Transplantation 100:862–870
Green M (2017) Preventing CMV and EBV in children undergoing organ transplantation: retrospective studies can only teach us so much. Pediatr Transplant. https://doi.org/10.1111/petr.12866
Cameron BM, Kennedy SE, Rawlinson WD, Mackie FE (2017) The efficacy of valganciclovir for prevention of infections with cytomegalovirus and Epstein-Barr virus after kidney transplant in children. Pediatr Transplant. https://doi.org/10.1111/petr.12816
Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing c (2014) Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14:272–283
Adam B, Randhawa P, Chan S, Zeng G, Regele H, Kushner YB, Colvin RB, Reeve J, Mengel M (2014) Banff initiative for quality assurance in transplantation (BIFQUIT): reproducibility of polyomavirus immunohistochemistry in kidney allografts. Am J Transplant 14:2137–2147
Kjaerheim K, Roe OD, Waterboer T, Sehr P, Rizk R, Dai HY, Sandeck H, Larsson E, Andersen A, Boffetta P, Pawlita M (2007) Absence of SV40 antibodies or DNA fragments in prediagnostic mesothelioma serum samples. Int J Cancer 120:2459–2465
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Ettenger R, Chin H, Kesler K, Bridges N, Grimm P, Reed EF, Sarwal M, Sibley R, Tsai E, Warshaw B, Kirk AD (2017) Relationship among viremia/viral infection, alloimmunity, and nutritional parameters in the first year after pediatric kidney transplantation. Am J Transplant 17:1549–1562
Herman J, Van Ranst M, Snoeck R, Beuselinck K, Lerut E, Van Damme-Lombaerts R (2004) Polyomavirus infection in pediatric renal transplant recipients: evaluation using a quantitative real-time PCR technique. Pediatr Transplant 8:485–492
Vanchiere JA, White ZS, Butel JS (2005) Detection of BK virus and simian virus 40 in the urine of healthy children. J Med Virol 75:447–454
Chang H, Wang M, Tsai RT, Lin HS, Huan JS, Wang WC, Chang D (2002) High incidence of JC viruria in JC-seropositive older individuals. J Neuro-Oncol 8:447–451
Van Loy T, Thys K, Tritsmans L, Stuyver LJ (2013) Quasispecies analysis of JC virus DNA present in urine of healthy subjects. PLoS One 8:e70950
Hirsch HH, Randhawa P, Practice ASTIDCo (2013) BK polyomavirus in solid organ transplantation. Am J Transplant 13(Suppl 4):179–188
Chapagain ML, Nerurkar VR (2010) Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier. J Infect Dis 202:184–191
Delbue S, Ferraresso M, Ghio L, Carloni C, Carluccio S, Belingheri M, Edefonti A, Ferrante P (2013) A review on JC virus infection in kidney transplant recipients. Clin Dev Immunol 2013:926391
Hirsch HH, Babel N, Comoli P, Friman V, Ginevri F, Jardine A, Lautenschlager I, Legendre C, Midtvedt K, Munoz P, Randhawa P, Rinaldo CH, Wieszek A, Hosts ESGoIiC (2014) European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect 20(Suppl 7):74–88
Cheng XS, Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Randhawa P, Hardinger KL, Brennan DC (2011) Inhibitory interactions between BK and JC virus among kidney transplant recipients. J Am Soc Nephrol 22:825–831
Bodaghi S, Comoli P, Bosch R, Azzi A, Gosert R, Leuenberger D, Ginevri F, Hirsch HH (2009) Antibody responses to recombinant polyomavirus BK large T and VP1 proteins in young kidney transplant patients. J Clin Microbiol 47:2577–2585
Wunderink HF, van der Meijden E, van der Blij-de Brouwer CS, Mallat MJ, Haasnoot GW, van Zwet EW, Claas EC, de Fijter JW, Kroes AC, Arnold F, Touze A, Claas FH, Rotmans JI, Feltkamp MC (2017) Pretransplantation donor-recipient pair seroreactivity against BK polyomavirus predicts viremia and nephropathy after kidney transplantation. Am J Transplant 17:161–172
Acknowledgements
The authors wish to thank study nurse Annette Mechler for her continuous excellent contributions to the CERTAIN Registry. BH is an awardee of the “DZIF Clinical Leave Stipend” from the German Center for Infection Research (DZIF).
Funding
The authors acknowledge their gratitude to the Dietmar Hopp Stiftung as well as to the pharmaceutical companies Astellas, Novartis, and Roche for their grants in support of the CERTAIN Registry.
Author information
Authors and Affiliations
Contributions
BH: study design, data collection and analysis, preparation of manuscript, principal investigator. JT and LS: study design, data collection and analysis. JO, FT, LP, KR, RT, BK, GK, NP, OY, JT, AF, and KK: data collection. TB: statistical analysis. RW: histopathological evaluation. MP and PS: virological methodology and analyses, data collection. HHH: study design, preparation of the manuscript. BT: study design, data analyses, preparation of manuscript.
Corresponding author
Ethics declarations
Conflict of interests
B.H. received travel grants and participated in advisory boards of Astellas, Novartis, and Roche. H.H.H. reported consultant and speaker honoraria by Novartis, and Chimerix. B.T. received research grants, travel grants, lecture fees, and participated in advisory boards of Astellas, Bristol-Myers Squibb, Novartis, and Roche. J.T., L.S., J.O., F.T., L.P., K.R., R.T. B.K., G.K. N.P., O.Y., A.F., K.K., T.B., R.W., M.P., and P.S. declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Höcker, B., Tabatabai, J., Schneble, L. et al. JC polyomavirus replication and associated disease in pediatric renal transplantation: an international CERTAIN Registry study. Pediatr Nephrol 33, 2343–2352 (2018). https://doi.org/10.1007/s00467-018-4029-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4029-9