Abstract
Background
Two previous randomized controlled trials showed that treatment of severe childhood immunoglobulin A (IgA) nephropathy using prednisolone with azathioprine, heparin-warfarin, or dipyridamole prevented the increase of sclerosed glomeruli. Prednisolone alone, however, did not prevent further increase. These studies indicated the importance of immunosuppressants in the treatment. An additional pilot study using mizoribine instead of azathioprine enabled us to complete 2 years of combined regimen. It showed non-numerical inferior effectiveness compared with the azathioprine regimen. Further examination of the additional efficacy of warfarin and dipyridamole was required.
Methods
A randomized control trial of prednisolone and mizoribine with (group 1) or without (group 2) warfarin and dipyridamole was administered for treatment of 71 children with severe IgA nephropathy to evaluate the efficacy of additional warfarin and dipyridamole.
Results
Thirty of 34 patients (88.2%) in group 1, and 27 of 36 patients (75.0%) showed the disappearance of proteinuria as defined by early morning urinary protein to creatinine ratio of < 0.2 during the 2-year treatment period. The cumulative disappearance rate of proteinuria determined by the Kaplan-Meier method showed that the disappearance rate of proteinuria was significantly higher in group 1 than in group 2 (log-rank P = 0.04). There was no significant difference in pathological findings, but there was a tendency of increase of global sclerosis in group1 which might be related to warfarin. Most of the adverse effects were related to prednisolone, but fortunately transient.
Conclusions
The balance between minimal benefits of warfarin/dipyridamole and potential adverse effects may be in favor of avoiding them in children with IgA nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common cause of primary glomerulonephritis worldwide. It was originally considered to be a benign disease with favorable outcomes, but a long-time follow-up study revealed that 11% of 241 Japanese pediatric patients with IgAN developed ESRD within 15 years [1]. Factors for poor prognosis of childhood IgAN include heavy proteinuria and severe pathological changes, such as diffuse mesangial proliferation [2].
On the basis of our two multicenter randomized controlled trials (RCTs), treatment of childhood IgAN with diffuse mesangial proliferation using prednisolone, azathioprine, warfarin, and dipyridamole for 2 years early in the course of disease reportedly reduced proteinuria. There was also reduction in the severity of immunologic renal injury and that prevented any increase of sclerosed glomeruli [3, 4]. Follow-up study of these RCTs clarified that the 2-year combination therapy not only ameliorated the activity of the acute phase of nephritis, but also improved the long-term outcome of severe childhood IgAN [5]. In these two studies of RCTs, the immunosuppressant, i.e., azathioprine, is considered to play an important role. It was often necessary, however, to withdraw azathioprine due to the problems such as anemia, leukopenia, and alopecia in about 10% of patients. An additional pilot study showed acceptable efficacy and safety of combination therapy including prednisolone and mizoribine instead of azathioprine, warfarin, and dipyridamole for severe childhood IgAN [6].
In our first RCT study [3], heparin-warfarin and dipyridamole treatment for 2 years did not reduce urinary protein excretion, serum IgA concentration, and mesangial IgA deposition, and did not prevent increase of sclerosed glomeruli. It is therefore necessary for us to evaluate the efficacy of additional warfarin and dipyridamole. This control study was undertaken to determine whether warfarin and dipyridamole are essential components of the combination therapy.
Materials and methods
This study was a prospective non-double-blinded controlled randomized clinical trial involving Japanese pediatric renal centers (The Japanese Pediatric IgA Nephropathy Treatment Study Group). Study protocol followed the Declaration of Helsinki and was in accordance with the standards of the ethics committee of Wakayama Medical University. All patients’ parents or legal guardians gave informed consent for inclusion. This study has been registered in a public trial registry, UMIN (University Hospital Medical Information Network, ID C000000363, https://www.umin.ac.jp/).
Patients were eligible for the study if they were newly diagnosed with severe IgA nephropathy with diffuse mesangial proliferation by renal biopsy and if the following criteria were satisfied: (1) aged 2 to 18 years at study entry; (2) excluded secondary IgAN such as IgA vasculitis, systemic lupus erythematosus, accompanied by liver disease; (3) no previous treatment with corticosteroids or immunosuppressive drugs; and (4) sufficient renal biopsy specimens available for pathological evaluation (minimum of ten glomeruli). In addition, it was necessary for patients to show heavy proteinuria (early morning urinary protein > 0.3 g/dl) and hypoproteinemia (serum total protein ≤ 6.0 g/dl) on at least one occasion between onset and entry to the study due to the Japanese health insurance system regulating the use of mizoribine. Renal biopsies were performed on children with persistent proteinuria (early morning urinary protein to creatinine ratio of ≥ 0.2) with or without hematuria as our clinical routine.
The pathologist of each center examined each renal biopsy specimen by light and immunofluorescence microscopy. An independent investigator (N.Y.) at the coordinating center also reviewed the histopathological findings. Diagnosis of IgAN was based on the presence of IgA as the sole or predominant immunoglobulin present in the glomerular mesangium and the absence of systemic disease. Diffuse mesangial proliferation was defined by the World Health Organization (WHO) criteria (more than 80% of glomeruli showing moderate or severe mesangial cell proliferation, i.e., more than three cells per peripheral mesangial area). Mesangial cell proliferation was always accompanied by an increased mesangial matrix. The intensity of mesangial IgA deposits was graded semiquantitatively on a scale of 0 to 3+: 0 none; 1+ slight; 2+ moderate; and 3+ intense.
After study eligibility was examined and consent obtained, participants were randomly assigned to one of two treatment groups. Randomization was by sealed envelope technique.
Patients assigned to group 1 received prednisolone, mizoribine, warfarin, and dipyridamole treatment for 24 months. Prednisolone was given orally at a dose of 2 mg/kg body weight per day in three divided doses for a total dose of not more than 80 mg/day for 4 weeks. This was followed by 2 mg/kg every 2 days, given as a single dose in the morning every other day for 4 weeks, 1.5 mg/kg per 2 days for 4 weeks, and 1 mg/kg per 2 days for 21 months. Mizoribine was given at a dose of 4 mg/kg body weight per day in two divided doses for a total dose of no more than 150 mg/day for 24 months. Warfarin was given in a single morning dose to maintain the thrombotest at 20–50% for 24 months. Dipyridamole was given orally at a dose of 6 mg/kg body weight per day in three divided doses for a total dose of up to 300 mg/day for 24 months. Participants assigned to group 2 received prednisolone and mizoribine for 24 months, as same way as those for group 1.
On entry into this study, all participants underwent a physical examination and their complete medical histories were obtained. Initial clinical and laboratory results were forwarded to the coordinating center. Participants were followed up once a month during this study. At each follow-up visit, they were asked about their symptoms and were monitored for any side effects of the therapy. Tests and measurements carried out at each visit comprised of blood counts (including hemoglobin, white blood cells, and platelets), thrombotest, serum creatinine, blood urea nitrogen, serum IgA concentration, urinary protein excretion, hemostix test, blood pressure, body weight, and body height. Hypertension was defined as diastolic pressure exceeding the upper normal limit of healthy Japanese children (mean + 2SD).
At the time of study enrollment, all participants were asked to undergo repeat renal biopsies at the end of treatment. An independent investigator (N.Y.) at the coordinating center reviewed the second renal biopsy pathological specimens.
Based on our previous randomized controlled studies and pilot study, the primary endpoint was decided as the disappearance of proteinuria, as defined by early morning urinary protein to creatinine ratio of < 0.2. Secondary endpoints were urinary protein excretion at the end of treatment, change in the percentage of sclerosed glomeruli during the trial, and side effects.
The sample size was calculated from the data of our previous randomized control study. We predicted that the disappearance rate of proteinuria would be 65% in the group treated with prednisolone, mizoribine, warfarin, and dipyridamole and 50% in the group treated with prednisolone, and mizoribine with a decision threshold 8%. Thirty-six patients were required for each study group, based on a selection design in which the probability of correctly selecting the better treatment is 0.9 when it is superior by an absolute difference of 15% in the disappearance rate of proteinuria [7]. The decision threshold of 8% was set before the start of the study based on a consensus reached by pediatric nephrologists in the Japanese Pediatric IgA Nephropathy Treatment Study Group.
Results were analyzed with the JMP 12.2 software package (SAS Institute Japan, Tokyo, Japan). The distribution of clinical and pathological attributes between the treatment groups was examined by Fisher’s exact test. Continuous characteristics at the start of treatment were compared by Mann-Whitney U test. Differences between study entry and study end in each treatment group were tested by Wilcoxon signed rank test. The disappearance rate of proteinuria was analyzed by the Kaplan-Meier method, and the two groups were compared by intension-to-treat analysis by log-rank test. A two-tailed P < 0.05 was taken as the level of significance.
Results
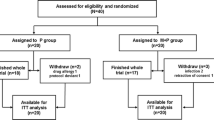
Between August 2001 and March 2009, 71 children newly diagnosed as IgAN showing diffuse mesangial proliferation were registered. Initially, we planned the registration of 72 patients based on the calculated sample size, but only 71 patients were enrolled. Finally, we decided to analyze in the 71 patients, because the sample size of 70 patients was enough for a selection design in which the probability of correctly selecting the better treatment is 0.89. All 71 children met the criteria for inclusion in the trial. All were willing to enter the study, but after allocation, one patient in group 1 withdrew consent (Fig. 1). Finally, 34 patients were assigned to group 1 (prednisolone, mizoribine, warfarin, and dipyridamole) and 36 patients were assigned to group 2 (prednisolone and mizoribine).
All 34 patients in group 1 completed the trial and 33 of 34 patients had repeated renal biopsies at the end of 2 years of treatment. All 36 patients in group 2 completed the study, including repeated renal biopsies. However, one patient had a protocol violation (use of an angiotensin converting enzyme inhibitor before the start of the study treatment and was treated with group 1 treatment), one patient canceled group 2 treatment at the 12th month, one patient stopped prednisolone in the 4th month due to a side effect (symptom of psychosis), and one patient stopped mizoribine in the 10th month due to side effect (hyperbilirubinemia). Data from these patients were included for analyses managed in an intention-to treat manner.
Clinical and laboratory findings of the patients in the two groups at the start of treatment are shown in Table 1. These characteristics of the patients in the two groups were similar except male sex ratio was significantly lower (P = 0.002) and duration from biopsy to treatment was significantly shorter (P = 0.04) in group 1 than in group 2. Twenty-six (76.5%) patients in group 1 and 25 (69.5%) patients in group 2 with asymptomatic hematuria and proteinuria were found by the school screening program.
At the end of the 2-year treatment, 30 (88.2%) of the 34 patients in group 1 and 27 (75.0%) of the 36 patients in group 2 reached the primary endpoint (early morning urinary protein to creatinine ratio of < 0.2). The cumulative disappearance rate of proteinuria was 88.2% (95% CI, 72.5 to 95.5%) in group 1 and 75.9% (95 CI, 59.2 to 87.3%) in group 2, respectively. The cumulative disappearance rate of proteinuria in group 1 was 13.2% higher than that in group 2, which was larger than the decision threshold of 8%. Kaplan-Meier analysis demonstrated that the disappearance rate of proteinuria was significantly higher in group 1 than in group 2 during the 2-year follow-up period (log-rank P = 0.04, Fig. 2).
Median urinary protein excretion in group 1 was reduced from 0.88 g/m2/day at the start of treatment to 0.09 g/m2/day at the end (P < 0.0001), and that in group 2 was also reduced from 0.81 g/m2/day to 0.12 g/m2/day (P < 0.0001, Table 2). The presence of blood in morning urine, quantified using dipsticks, a colorimetric test for hemoglobin, showed significant reduction in both groups (Table 2). The median serum IgA concentration significantly decreased from 211 mg/dl at the start of treatment to 184 mg/dl at the end in group 1 (P < 0.0001), and that in group 2 also decreased from 218 to 192 mg/dl (P = 0.0074). Estimated creatinine clearance and mean body mass index z score were unchanged in both groups (Table 2).
As to the changes in pathological data, 33 of 34 patients in group 1 and all of 36 patients in group 2 underwent repeat renal biopsies at the end of treatment (Table 2). Duration from start of treatment to repeat biopsy was 24.1 months (23.2–25.1) in group 1 and 24.5 months (23.9–25.8) in group 2. The median percentage of glomeruli showing segmental sclerosis was not increased in both groups. The percentage of glomeruli showing global sclerosis was increased in group1 without significance (P = 0.06). The percentage of glomeruli showing crescents was significantly decreased in both groups (P < 0.0001, respectively). The percentage of glomeruli showing capsular adhesions was not changed in either group. In examinations by the Oxford classification of IgAN, ratios of M1, E1, and C1/C2 as acute lesions were significantly decreased in both groups. On the contrary, ratios of S1 and T1 as chronic lesions were unchanged in both groups (Table 2).
Mesangial deposits of IgA in the initial renal biopsy of all patients were intense or moderate. Mesangial IgA deposits became significantly less intense in both groups at the end of treatment (P < 0.0001 in group 1 and P = 0.04 in group 2, Table 2). And the intensity of fibrinogen became less intense in group 1 (P = 0.03, Table 2).
At the end of 2 years of treatments, there was no significant difference between the two groups in clinical and pathological findings (Table 3).
Adverse events of both treatment groups are shown in Table 4. Adverse events were shown in 17 of 34 patients in group 1 and 22 of 36 patients in group 2. The total number of cases of side effects observed was 54.
Two patients needed changes in group 2 treatment due to severe side effects. One needed to stop prednisolone because of the symptom of psychosis in consideration of steroid psychosis; another needed to stop mizoribine due to elevation of serum bilirubin.
Obesity as a result of prednisolone was the most frequent side effect in both groups (six patients in group 1 and seven patients in group 2). Hyperuricemia caused by mizoribine was observed in both groups (two patients in group 1, and five patients in group 2). Other adverse effects were hypertension, steroid-induced gastric ulcer, glaucoma, steroid acne, stretch marks, decreased bone mineral density, and cataracts, which were all possibly related to prednisolone use. Headaches and bleeding were observed in group 1, which were considered to be in association with dipyridamole and warfarin which were used only in group 1. All adverse effects except for cataracts subsided after the treatment.
Discussion
In our two previous RCTs and one pilot study, the combination therapy of prednisolone, azathioprine/mizoribine, warfarin, and dipyridamole for 2 years significantly reduced the level of urinary protein excretion, serum IgA concentration, and mesangial IgA deposition and prevented any sclerosed glomeruli in children with newly diagnosed IgAN showing diffuse mesangial proliferation [3, 4, 6]. In contrast, treatment with only warfarin and dipyridamole for 2 years showed no effectiveness in reduction of urinary protein excretion, serum IgA concentration, or mesangial IgA deposition. It also showed no effectiveness in prevention of increased sclerosed glomeruli for patients with severe childhood IgA nephropathy in our first RCT [3]. Accordingly, prednisolone and immunosuppressant are considered to play a major, important role in the combination therapy including prednisolone, azathioprine/mizoribine, warfarin, and dipyridamole. We therefore performed this study to determine the effect of warfarin and dipyridamole in the combination therapy.
Disappearance of proteinuria (urine protein/creatinine ration < 0.2, primary end point) was observed in 30 (88.2%) of the 34 patients in group 1 and in 27 (75.0%) of the 36 patients in group 2. The cumulative disappearance rate of proteinuria determined by the Kaplan-Meier method showed that the disappearance rate of proteinuria was significantly higher in group 1 than in group 2 (log-rank P = 0.04). Regarding secondary endpoints, urinary protein excretion at the end of treatment was significantly decreased in both groups. And as to the change in the percentage of sclerosed glomeruli during the trial, the change in the percentage pf global sclerosis in group 1 increased though they had no significance (P = 0.06). The results in group 1 of the present RCT show the similar efficacy compared with our previous RCTs, suggesting a stable treatment effect of the combination therapy.
Considering a selection design adopted in the present RCT, we select the group 1 treatment including prednisolone, mizoribine, warfarin, and dipyridamole as a standard treatment for severe IgAN in our study group from the point of the proteinuria remission.
When considering the some increase of the percentage of global sclerosis in group1 without significance, we might have to re-consider the use of additional warfarin and dipyridamole in the combination therapy. At first, the warfarin and dipyridamole were used for the suppression of cell proliferation through cytokine inhibition in treating IgA nephropathy [8]. Subsequently, patients with unexplained acute kidney injury (AKI) during the warfarin therapy have been successively reported, so-called warfarin-related nephropathy (WRN) [9,10,11]. Though the mechanism of these risks is still unclear, warfarin prevents the activation of Matrix Gla Protein (MGP) and Growth Arrest specific Gene 6 (GAS-6) which prevents vascular calcification and induces vascular calcification [12].
Most of the side effects observed in this study were commonly associated with prednisolone and mizoribine (Table 4). The reasons for hypertension being more frequently observed in the patients of group 2, and headaches being found in the patients of group 1, are thought to depend on use or no-use of dipyridamole, which has a vasodilator effect.
Corticosteroids have been widely used to treat moderate to severe pediatric IgAN patients, and their usefulness for retarding disease progression is mostly accepted among immunosuppressive agents [13]. Their adverse effects, however, especially in patients with earlier stages of IgAN, are not fully investigated [14]. In our study, body mass index z score was unchanged after 2 years of treatment in both groups. About one fifth of patients showed obesity as a side effect of the 2 years of oral prednisolone use. Such side effects related to prednisolone were transient, however, except for cataracts. Although fortunately the severe irreversible side effect was not seen in this study, since we have already known the severe adverse effects such as aseptic necrosis of femur related to the use of prednisolone, we may have to consider modification of prednisolone administration or combination of drugs which provides similar benefits.
Though therapeutic evidence of the use of immunosuppressive agents other than prednisolone is weak and scarce in both adults and children [15,16,17,18], additional value of immunosuppressants is supported by experimental insights into IgAN pathogenesis. The proposed “multi-hit” theory comprises multiple steps, from the start with defective glycosylation of IgA subclass IgA1 resulting in overproduction of galactose-deficient IgA1, to the mesangial deposition of nephritogenic immune complexes [19,20,21,22,23].
In the retrospective VALIGA study, the histological severity of renal progression was attenuated by corticosteroids/immunosuppressive treatment, and clear benefits of corticosteroids in addition to renin-angiotensin system blockers (RASBs) were shown particularly in patients with proteinuria > 1 g/day [24]. On the other hand, in the STOP-IgAN clinical trials, the addition of immunosuppression to ongoing comprehensive supportive care was not beneficial for patients with IgA nephropathy that was characterized by moderate proteinuria and chronic kidney disease stages 1 through 3 [25]. However, the authors address several concerns raised on the design and interpretation of the trial [26]. These studies were clinical trials mainly focused on adult-IgAN patients, and were not stratified for clinical and histologic data at renal biopsy [27]. The situation may be different in countries without routine school urine examinations, for example.
Although the use of RASBs has been shown to be clearly effective even against childhood IgA nephropathy [28], the use of them was restricted in the present study period, so information for efficacy of combination with our protocols and RASBs was unavailable. Taking into the consideration of the superiority of combination therapy using prednisolone, mizoribine, warfarin and dipyridamole compared with the combination therapy using only prednisolone and mizoribine from the point of proteinuria remission, and insignificant increase of glomerulosclerosis which was thought to be related with warfarin use in group1, combination therapy with prednisolone, warfarin, and RASBs will be an important issue to be addressed in the future.
Some of patients with IgAN show proteinuria < 0.5 g/day/1.73m2 which is the threshold given by KDIGO [13] even with diffuse mesangial proliferation. Therefore, we may have to consider the balance risk/benefits in low proteinuria children when we start our combination therapy in the patients.
Although the condition improved, all participants were asked to undergo repeat renal biopsies at the end of treatment in the present study. However, based on the results of previous studies including this study, in clinical practice at present we perform repeat renal biopsies only when the patients show exacerbation or no improvement to determine the next appropriate treatments. In this study, the patients were given a chance to deny a repeat renal biopsy.
This study was not a double-blinded controlled randomized clinical trial. Therefore, the situation may influence results.
In conclusion, combination treatment including prednisolone and mizoribine with warfarin and dipyridamole may be better for severe childhood IgAN than the combination treatment including prednisolone and mizoribine without warfarin or dipyridamole from the point of proteinuria remission. But the use of warfarin might lead to the increase of global glomerulosclerosis due to the vascular calcification of warfarin; we should consider combination therapy such as prednisolone, mizoribine, and RASBs. Also, we may have to consider modification of prednisolone administration due to the high cumulative dosage used.
References
Yoshikawa N, Tanaka R, Iijima K (2001) Pathophysiology and treatment of IgA nephropathy in children. Pediatr Nephrol 16:446–457
Yoshikawa N, Ito H, Nakamura (1992) Prognostic indicators in childhood IgA nephropathy. Nephron 60:60–67
Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu M, Ito K, Iitaka K, Koitabashi Y, Yamaoka K, Nakagawa K, Nakamura H, Matsuyama S, Seino Y, Takeda N, Hattori S, Ninomiya M, The Japanese Pediatric IgA Nephropathy Treatment Study Group (1999) A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. J Am Soc Nephrol 10:101–109
Yoshikawa N, Honda M, Iijima K, Awazu M, Hattori S, Nakanishi K, Ito H, Japanese Pediatric IgA Nephropathy Treatment Study Group (2006) Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol 1:511–517
Kamei K, Nakanishi K, Ito S, Saito M, Sako M, Ishikura K, Hataya H, Honda M, Iijima K, Yoshikawa N, Japanese Pediatric IgA Nephropathy Treatment Study Group (2011) Long-term results of a randomized controlled trial in childhood IgA nephropathy. Clin J Am Soc Nephrol 6:1301–1307
Yoshikawa N, Nakanishi K, Ishikura K, Hataya H, Iijima K, Honda M, Japanese Pediatric IgA Nephropathy Treatment Study Group (2008) Combination therapy with mizoribine for severe childhood IgA nephropathy: a pilot study. Pediatr Nephrol 23:757–763
Iijima K, Sako M, Oba MS, Ito S, Hataya H, Tanaka R, Ohwada Y, Kamei K, Ishikura K, Yata N, Nozu K, Honda M, Nakamura H, Nagata M, Ohashi Y, Nakanishi K, Yoshikawa N, Japanese Study Group of Kidney Disease in Children (2014) Cyclosporine C2 monitoring for the treatment of frequently relapsing nephrotic syndrome in children: a multicenter randomized phase II trial. Clin J Am Soc Nephrol 9:271–278
Liem LK, Choong HL, Woo KT (2001) Action of dipyridamole and warfarin on growth of human endothelial cells cultured in serum free media. Clin Biochem 34:141–147
Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T (2009) Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis 54(6):1121–1126
Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA (2011) Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 80(2):181–189
Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, Wu H, Bhatt U, Nadasdy T, Hebert LA (2010) Warfarin therapy that results in an international normalization ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract 115(2):c142–c146
Danziger J (2008) Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol 3(5):1504–1510
Radhakrishnan J, Cattran DC (2012) The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines--application to the individual patient. Kidney Int 82:840–856 2012
Vecchio M, Bonerba B, Palmer SC, Craig JC, Ruospo M, Samuels JA, Molony DA, Schena FP, Strippoli GF (2015) Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev 3(8):CD003965
Ballardie FW, Roberts IS (2002) Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 13:142–148
Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, Ho YW, Lai KN (2005) Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 68:802–812
Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN (2010) Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int 77:543–549
Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, Appel G, Cattran D, Fischer D, Hurley RM, Cerda J, Carter B, Jung B, Hernandez G, Gipson D, Wyatt RJ (2015) Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis 66(5):783–791
Mestecky J, Tomana M, Moldoveanu Z, Julian BA, Suzuki H, Matousovic K, Renfrow MB, Novak L, Wyatt RJ, Novak J (2008) Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res 31(1):29–37
Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119(6):1668–1677
Suzuki Y, Suzuki H, Nakata J, Sato D, Kajiyama T, Watanabe T, Tomino Y (2011) Pathological role of tonsillar B cells in IgA nephropathy. Clin Dev Immunol 2011:639074. https://doi.org/10.1155/2011/639074
Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J (2012) Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23(9):1579–1587
Rauen T, Floege J (2017) Inflammation in IgA nephropathy. Pediatr Nephrol. https://doi.org/10.1007/s00467-017-3628-3631
Tesar V, Troyanov S, Bellur S (2015) Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. J Am Soc Nephrol 26(9):2248–2258
Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J, STOP-IgAN Investigators (2015) Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373(23):2225–2236
Rauen T, Eitner F, Fitzner C, Floege J (2016) Con: STOP immunosuppression in IgA nephropathy. Nephrol Dial Transplant 31(11):1771–1774
Coppo R (2017) Corticosteroids in IgA nephropathy: lessons from recent studies. J Am Soc Nephrol 28(1):25–33
Nakanishi K, Iijima K, Ishikura K, Hataya H, Awazu M, Sako M, Honda M, Yoshikawa N, The Japanese Pediatric IgA Nephropathy Treatment Study Group (2009) Efficacy and safety of lisinopril for mild childhood IgA nephropathy: a pilot study. Pediatr Nephrol 24(4):845–849
Acknowledgments
This study was supported by Health and Labor Sciences Research Grants (Research on Children and Families) from the Japanese Ministry of Health Labor and Welfare, and in part by a research grant from the Kidney Foundation, Japan. The authors wish to thank all of the participants and attending physicians for their contributions.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The study protocol followed the Declaration of Helsinki and was in accordance with the standards of the ethics committee of Wakayama Medical University. All patients’ parents or legal guardians gave informed consent for inclusion. This study has been registered in a public trial registry, UMIN (University Hospital Medical Information Network, ID C000000363, https://www.umin.ac.jp/).
Disclosures
KN received lecture fees from Asahi Kasei Pharma Corp. YK received consulting fees from Asahi Kasei Pharma Corp. KI received grants from Asahi Kasei Pharma Corp. and Eizai Co. Ltd. and lecture fees from Asahi Kasei Pharma Corp. HH received lecture fees from Asahi Kasei Pharma Corp. MH received lecture fees from Asahi Kasei Pharma Corp. and Takeda Pharmaceutical Co. Ltd. MS received grants from Eizai Co. Ltd. KN received lecture fees from Asahi Kasei Pharma Corp. KI received grants from Takeda Pharmaceutical Co. Ltd. and Eizai Co. Ltd., and lecture fees and/or consulting fees from Asahi Kasei Pharma Corp., Boehringer Ingelheim Japan Co. Ltd. and Takeda Pharmaceutical Co. Ltd. NY received lecture fees from Boehringer Ingelheim and Asahi Kasei Pharma Corp. The other authors had no disclosure to declare.
Rights and permissions
About this article
Cite this article
Shima, Y., Nakanishi, K., Kaku, Y. et al. Combination therapy with or without warfarin and dipyridamole for severe childhood IgA nephropathy: an RCT. Pediatr Nephrol 33, 2103–2112 (2018). https://doi.org/10.1007/s00467-018-4011-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4011-6