Abstract
Background
Rhabdomyolysis is a clinical emergency that may cause acute kidney injury (AKI). It can be acquired or due to monogenic mutations. Around 60 different rare monogenic forms of rhabdomyolysis have been reported to date. In the clinical setting, identifying the underlying molecular diagnosis is challenging due to nonspecific presentation, the high number of causative genes, and current lack of data on the prevalence of monogenic forms.
Methods
We employed whole exome sequencing (WES) to reveal the percentage of rhabdomyolysis cases explained by single-gene (monogenic) mutations in one of 58 candidate genes. We investigated a cohort of 21 unrelated families with rhabdomyolysis, in whom no underlying etiology had been previously established.
Results
Using WES, we identified causative mutations in candidate genes in nine of the 21 families (43%). We detected disease-causing mutations in eight of 58 candidate genes, grouped into the following categories: (1) disorders of fatty acid metabolism (CPT2), (2) disorders of glycogen metabolism (PFKM and PGAM2), (3) disorders of abnormal skeletal muscle relaxation and contraction (CACNA1S, MYH3, RYR1 and SCN4A), and (4) disorders of purine metabolism (AHCY).
Conclusions
Our findings demonstrate a very high detection rate for monogenic etiologies using WES and reveal broad genetic heterogeneity for rhabdomyolysis. These results highlight the importance of molecular genetic diagnostics for establishing an etiologic diagnosis. Because these patients are at risk for recurrent episodes of rhabdomyolysis and subsequent risk for AKI, WES allows adequate prophylaxis and treatment for these patients and their family members and enables a personalized medicine approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
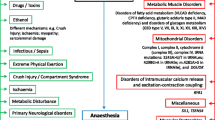
Rhabdomyolysis is a clinical emergency encountered by nephrologists. It is characterized by acute skeletal muscle damage, subsequent marked serum elevations in muscle enzymes [particularly creatine kinase (CK)] and increased risk for acute kidney injury (AKI). The differential diagnosis of rhabdomyolysis is heterogeneous [1]. In general, two types of underlying etiologic conditions have been reported. The first consists of acquired conditions, such as trauma, infections and exposure to drugs or toxins; the second encompasses a high number of rare metabolic and neuromuscular monogenic disorders. In some cases, rhabdomyolysis may be secondary to a combination of genetic predisposition factors and environmental factors [1]. In those cases, the environmental factor may erroneously be considered as the sole etiology, with the risk that the primary genetic etiologic diagnosis is missed and rhabdomyolysis episodes may recur [1].
In the clinical setting, identification of the exact underlying monogenic diagnosis is challenging because more than 60 monogenic etiologies have already been implicated in rhabdomyolysis (Table 1) [1]. In addition, in most cases of rhabdomyolysis the clinical presentation is nonspecific, including elevated CK levels, dark urine secondary to myoglobinuria, and, in some cases, AKI. To date, most of the available data on the genetic basis of rhabdomyolysis are limited to individual gene-specific case reports. Therefore, no accurate estimates of monogenic etiology in patients presenting with rhabdomyolysis are available.
To address this issue, we investigated the frequency of disease-causing mutations in 21 unrelated individuals from different families with rhabdomyolysis, who were evaluated in a tertiary children’s hospital center in Israel between January 2012 and December 2015 by means of whole exome sequencing (WES). We show that mutations in known rhabdomyolysis-causing genes are present in 43% of these families, and we outline important clinical implications generated from knowledge of the molecular genetic diagnosis.
Methods
Study participants
Following informed consent, we obtained clinical data, pedigree data, and blood samples from individuals with rhabdomyolysis from several medical centers in Israel. Approval for human subjects’ research was obtained from the Institutional Review Boards of Sheba Medical Center, Boston Children’s Hospital, and other relevant local Ethics Review Boards. Informed consent was obtained from the individuals and/or parents, as appropriate. Included in the study were individuals with the diagnosis of rhabdomyolysis which had been made by pediatric nephrologists and/or pediatric metabolists based on clinical presentation and elevated levels of CK. All patients presented at or were referred for second opinion to Sheba Medical Center between January 2012 and December 2015. Of the 25 patients who presented during this time period, 21 were recruited to the study. All except one of these 21 patients were children and/or adolescents at presentation (<21 years of age). There was only one adult who presented with rhabdomyolysis; in this case following general anesthesia, which he had had for the first time in his life. He was referred to Safra Children’s Hospital (at Sheba Medical Center) as he was initially suspected to have glycogen storage disease for which the pediatric metabolic clinic in Safra Children’s Hospital provides genetic analysis. This analysis was negative and, given that he met the inclusion criteria for the study, this case was also included for analysis.
Whole exome sequencing
We investigated 21 unrelated index patients. DNA samples from affected individuals were subjected to WES as established previously [2, 3] using Agilent SureSelect™ human exome capture arrays (Life Technologies Corp., Carlsbad, CA) with next generation sequencing (NGS) on an Illumina™ sequencing platform (Illumina, Inc., San Diego, CA). Sequence reads were mapped against the human reference genome (NCBI build 37/hg19) using CLC Genomics Workbench (version 6.5.1) software (CLC bio A/S, Aarhus, Denmark). For homozygosity mapping, aligned BAM files were processed according to Picard and Samtools [4]. Single nucleotide variant (SNV) calling was performed using GATK [5], and the generated VCF file was subsequently used in HomozygosityMapper [6]. Mutation calling was performed by geneticists who had knowledge of clinical phenotypes, pedigree structure, genetic mapping, and WES evaluation, and was in line with proposed guidelines [7]. Sequence variants that remained after the WES evaluation process were examined for segregation.
WES analysis
Following WES, all regions of homozygosity in all individuals were identified. Genetic regions of homozygosity by descent (“homozygosity peaks”) were plotted across the genome as candidate regions for recessive genes as previously described [3, 8]. Consanguinity was determined on the basis of long (>5 Mb) stretches of homozygosity. When homozygosity was present (three cases), we used homozygosity mapping in combination with WES as previously described [3, 8] to detect recessive homozygous disease-causing mutations. In one case in which trio WES data were available to us, we performed trio analysis for homozygous as well as for compound heterozygous mutations. Finally, for all other individuals with no evidence of homozygosity by descent, we generated a list of previously reported rhabdomyolysis-causing genes and performed a targeted analysis for this predefined rhabdomyolysis gene panel.
Rhabdomyolysis gene panel
Previously reported genes which if mutated cause rhabdomyolysis were selected through a literature search in the OMIM database, NCBI-gene, Pubmed database, Human Gene Mutation Database Genetics home reference site using the search terms “rhabdomyolysis” and/or “myoglobinuria”. Following review of the relevant reports, we included genes that have been implicated as an underlying monogenic cause for rhabdomyolysis. Altogether, 58 genes were selected (Table 1).
Variant calling
Following WES, genetic variants were first filtered to retain only non-synonymous and splice variants. Second, filtering was performed to retain only alleles with a minor allele frequency (MAF) of <0.1% for dominant genes and <1% for recessive genes. These are widely accepted cutoffs for autosomal dominant and recessive disorders, respectively [2, 8]. MAF was estimated using combined datasets incorporating all available data from the 1000 Genomes Project, the Exome Variant Server project, dbSNP142 and the Exome Aggregation Consortium (ExAC). Third, observed sequence variants were analyzed using the UCSC Human Genome Bioinformatics Browser for the presence of paralogous genes, pseudogenes or misalignments. Fourth, we scrutinized all variants with MAF of <1% or <0.1%, respectively, within the sequence alignments of the CLC Genomic Workbench™ software program for poor sequence quality and for the presence of mismatches that indicate potential false alignments. Fifth, we employed web-based programs to assess variants for evolutionary conservation, to predict the impact of disease candidate variants on the encoded protein and to determine whether these variants represented known disease-causing mutations. Sanger sequencing was performed to confirm the remaining variants in the original DNA samples and, when available, to test for familial segregation of phenotype with genotype.
Results
Our cohort included 21 unrelated individuals with rhabdomyolysis in whom the underlying etiology had not been established prior to this study. Most patients had a negative family history and presented without additional symptoms or signs and without syndromic features (Table 2). Exceptions were four individuals with a positive family history for rhabdomyolysis, and one individual who exhibited syndromic features in addition to rhabdomyolysis (Table 2).
Monogenic causes of rhabdomyolysis have been described for 58 genes (45 recessive, 7 dominant, 3 recessive and dominant, and 3 X-linked) and can be grouped into seven categories (Table 1 ). We detected disease-causing mutations in nine of the 21 cases analyzed. The eight genes in which we identified causative mutations were AHCY, CPT2, MYH3, PFKM, SCN4A, RYR1, CACNA1S and PGAM2. In six cases the disease-causing mutations that we identified in five genes had been previously reported as pathogenic (AHCY, CPT2, MYH3, PFKM and SCN4A) [9,10,11,12,13]. In one case we found a novel mutation that affects a residue that was previously shown to cause disease if altered (RYR1) [14, 15]. In two cases we identified novel mutations in highly conserved amino acid residues (Table 2).
In two patients with rhabdomyolysis (patients ANX and AN3; Table 2), we identified homozygous disease-causing mutations in CPT2. Mutations in CPT2 cause carnitine palmitoyltransferase II (CPT2) deficiency [10].
In two other individuals with rhabdomyolysis (patients AN2 and AN5), we identified mutations in genes that cause two different forms of glycogen storage disease, PFKM and PGAM2. In PFKM, we identified a previously reported splice mutation [9] that causes Tarui Disease, also known as glycogen storage disease type VII. In PGAM2 [phosphoglycerate mutase 2 (muscle)] we identified a novel homozygous missense mutation in a highly conserved amino acid residue (Table 2). Mutations in this gene are responsible for an extremely rare autosomal recessive type of glycogen storage disease, also known as Glycogenosis type X [16].
In four individuals with rhabdomyolysis, we identified autosomal dominant mutations in four different genes that lead to rare disorders of abnormal skeletal muscle relaxation and contraction (RYR1, CACNA1S, SCN4A and MYH3). Importantly, in patients AN7 and AN11 we identified mutations in RYR1 and CACNA1S (Table 2) that can lead to life-threatening malignant hyperthermia in the setting of certain environmental triggers, such as exercise or drugs. Patient AN13, in whom we identified a previously reported SCN4A pathogenic mutation (Table 2), had an autosomal dominant family history of rhabdomyolysis. Segregation analysis confirmed the presence of the pathogenic mutation in the patient’s affected father. Mutations of the gene SCN4A lead to a skeletal muscle channelopathy with variable clinical presentation, including myotonia, episodic hypokalemic paralysis and, in rare cases, rhabdomyolysis [17]. In patient AN21, we identified a previously reported pathogenic mutation in MYH3 (Table 2) [12]. Mutations in this gene cause Freeman–Sheldon/Sheldon–Hall syndrome [12].
Finally, in one infant (AN20), who presented in the neonatal period with hypotonia, poor sucking reflex, apnea episodes and rhabdomyolysis, we identified two previously reported compound heterozygous pathogenic mutations in the gene AHCY (adenosylhomocysteinase) (Table 2) [13].
Discussion
In the current study, we examined a group of 21 unrelated individuals with rhabdomyolysis for the presence of disease-causing mutations. We identified causative mutations in different genes in nine of the 21 individuals (43%) studied. None of the above disease-causing mutations were suspected on clinical grounds prior to the current study, and affected patients were not clinically distinguishable from other rhabdomyolysis patients.
Our study has several limitations: First, in 11 individuals we did not identify the underlying genetic etiology despite the fact that they presented similarly to the individuals whose genetic state was resolved. This, in part, can be explained by the known limitations of WES, which does not detect copy number variations and mutations in non-coding regions. It is also possible that there are additional rhabdomyolysis genes that have not yet been characterized. In addition, it is possible that the rhabdomyolysis cases we analyzed in this study may represent patients with relatively more severe disease and, as such, may lead to an overestimation of our disease causing mutation detection rate.
The causative mutated genes found in our cohort have been grouped into four main categories (see Tables 1, 2): (1) disorders of fatty acid metabolism; (2) disorders of glycogen metabolism; (3) disorders of abnormal skeletal muscle relaxation and contraction; (4) disorders of purine metabolism. These categories are discussed here in detail.
Disorders of fatty acid metabolism
Mutations in CPT2 are considered to be the most common monogenic cause for rhabdomyolysis [10, 18]. Mutations in CPT2 lead to carnitine palmitoyltransferase II (CPT2) deficiency, a metabolic disorder of long-chain fatty-acid oxidation [18]. This syndrome has variable clinical presentations, which typically consist of one of the following three forms: (1) lethal neonatal form; (2) severe infantile hepato-cardio-muscular form; (3) myopathic form. The myopathic form is characterized by exercise-induced muscle pain, rhabdomyolysis, myoglobinuria and, as a result, predisposition to AKI. Other triggers include prolonged exercise (especially following fasting), cold exposure, infection, stress, sleep deprivation or general anesthesia. Patients usually will have no signs of myopathy or elevation of serum CK between attacks. Age at presentation can be variable, ranging from the first to the sixth decade of life. While most individuals are mildly affected, severe renal failure requiring dialysis has been reported [19]. A definitive diagnosis of this syndrome can be made by the detection of reduced CPT enzyme activity or by genetic testing of the CPT2 gene. To date, >70 CPT2 mutations have been reported. The p.Ser113Leu missense mutation that we identified in both patients accounts for 60% of cases of the myopathic CPT2 deficiency form and is considered to cause a milder phenotype within this group [20]. On the other hand, CPT2 pathogenic null variants are often associated with the severe lethal neonatal form. Identification of the causative mutation will have consequences for the management of these patients, such as provision of a high-carbohydrate and low-fat diet in order to provide fuel for glycolysis. In addition, further episodes of rhabdomyolysis may be prevented by the provision of glucose during infections to prevent catabolism, avoidance of extended fasting, avoidance of certain medications and refraining from prolonged exercise activity. Finally, providing adequate hydration during rhabdomyolysis episodes in order to prevent AKI is important in this context.
Disorders of glycogen metabolism
The gene PFKM (phosphofructokinase, muscle) encodes phosphofructokinase (PFK), which is the rate-limiting enzyme in the glycolytic pathway. It catalyzes the phosphorylation of fructose 6-phosphate to fructose-1,6-bisphosphate [21]. Mutations in this gene lead to glycogen storage disease, type VII. Phenotypically, four different clinical presentations have been reported, including: (1) exercise intolerance, myalgias and rhabdomyolysis; (2) a severe infantile form, with hypotonia, progressive myopathy, cardiomyopathy and respiratory failure; (3) a late-onset form, with myopathy, usually appearing in the fifth decade of life; (4) a hemolytic form, characterized by hemolytic anemia without muscle symptoms [22]. PFK-deficient patients usually show a severe reduction of the enzyme activity in skeletal muscle and a partial deficiency in erythrocytes. Although the diagnosis can be made by testing the enzymatic activity, this can be misleading, leaving the genetic diagnosis as the gold standard for definitive diagnosis [23]. To date, around 26 different disease-causing mutations have been described in the PFKM gene.
Deficiency of the terminal glycolysis enzyme, muscle phosphoglycerate mutase (PGAM), causes glycogenosis type X, a metabolic myopathy characterized by exercise-induced cramps, rhabdomyolysis and myoglobinuria [16]. To date only eight different disease-causing mutations have been reported, mostly due to biallelic mutations. However, two independent reports have also implicated a possible late-onset symptomatic disease among heterozygote carriers [24, 25]. In the current study, we identified a PGAM mutation in individual AN5, who presented at 20 years of age for evaluation of chronic symptoms of muscle cramps following exercise. Symptoms began at the age of 10 years and were reported to appear even following moderate daily activities, such as writing, climbing stairs, lifting objects, walking uphill or running. No similar symptoms were reported following fever or triggers other than physical exertion, and no changes were noted in urine color. The patient underwent extensive metabolic screening tests, including urine organic acids, serum acylcarnitine and very long chain fatty acids profiles, which were all within normal limits. Echocardiogram, abdominal ultrasound, electromyography and nerve conduction tests were normal. In addition, a muscle biopsy (for light and electron microscopy) was performed elsewhere at the age of 12 years and was reported as normal, including normal respiratory chain enzymes. A forearm ischemic test showed elevation of serum ammonia (from 46.3 mcg/dl at baseline to 651.4 mcg/dl; normal range 31–123 mcg/dl) and lactate levels (from 3.9 mg/dl to 13.5 mg/dl; normal range 6–18 mg/dl). Past genetic testing included targeted sequencing for known mutations in CPT2 for which the patient was negative. Family history was notable for a paternal uncle and grandfather who both had experienced occasional muscle cramps without related functional disability. The PGAM2 mutation we identified in this patient has never been reported in the homozygous state in any available public database, was predicted to be deleterious by three different prediction programs (SIFT, Mutation Taster and Polyphen), was located in one of the protein’s domains (PGAM 1) and affected a highly conserved residue. Continuous evolutionary conservation of the amino acid residue was evident across the following species: Mus musculus, Gallus gallus, Xenopus tropicalis, Danio rerio, Ciona intestinalis, Caenorhabditis elegans and Drosophila.
Disorders of abnormal skeletal muscle relaxation and contraction
Malignant hyperthermia could be caused by mutations in RYR1 and CACNA1S. More than 500 different mutations in RYR1, which encodes the ryanodine receptor 1, have emerged as the underlying cause of a wide clinical spectrum encompassing periodic exertional rhabdomyolysis and severe life-threatening malignant hyperthermia [26]. Ryanodine receptor 1 is an ion channel responsible for the release of calcium from the sarco/endoplasmic reticulum [26]. It functions in concert with the α-subunit of the voltage-dependent L-type calcium channel, encoded by CACNA1S [26]. CACNA1S is important for the activation of RYR1, for which approximately 30 mutations have been reported to date. In the current study we identified a RYR1 mutation in individual AN7 and a CACNA1S mutation in individual AN11. AN7, a 46-year-old male, presented for evaluation following elevated CK levels (reaching 93,000 U/L) obtained due to dark-colored urine following general anesthesia for an elective inguinal hernia repair. The patient was born to non-consanguineous parents of Ashkenazi-Jewish descent, and his family history was non-contributory. His father and two brothers were reported to be asymptomatic and had never required general anesthesia. Of note, the patient reported performing regular exercise as a volleyball player, after which he remains asymptomatic. He did not undergo a muscle biopsy. He was found to harbor a novel missense mutation in a highly conserved amino acid residue in RYR1 (c.179A > G; p.Asp60Gly). This mutation affects an amino acid residue that was previously shown to cause disease if altered [14, 15, 27]. As parental DNA was not available to us, the question of whether this mutation was de novo remains open.
Patient AN11, currently 23 years old, presented at 20 years of age for evaluation due to myalgia and abdominal pain accompanied by elevation of serum CK levels to 17,974 U/L. Symptoms were preceded by an episode of extreme exercise as part of his military training. He was afebrile, and no change was noted in urine color. His family history was reportedly negative; however his two older siblings have never undergone similar strenuous physical exertion. Previous medical history included a single episode of mild CK elevation to 800 U/L following pneumonia. Genetic evaluation prior to the WES included Sanger sequencing for known CPT2 mutations common in the Ashkenazi-Jewish population. The sequencing results excluded the presence of any of those mutations. This patient was found to harbor a novel mutation in a highly conserved amino acid residue in CACNA1S which has never been reported in any available databases, including the ExAC database, and is predicted to be deleterious [maximum PolyPhen-2 (PP2) humvar score of functional “deleteriousness” of 1.0]. In addition, the mutation is located in an ion transport domain of the protein and affects a highly conserved residue. Continuous evolutionary conservation of the amino acid residue was evident across the following species: Mus musculus, Gallus gallus, Xenopus tropicalis, Danio rerio, Ciona intestinalis, Caenorhabditis elegans and Drosophila.
The gene SCN4A encodes the α-subunit of the skeletal muscle voltage-dependent sodium channel type 4, and mutations in SCN4A lead to a skeletal muscle channelopathy with variable clinical presentations, including myotonia, episodic hypokalemic paralysis, and rhabdomyolysis in rare cases [17].
Interestingly, mutations in MYH3 usually lead to a rare and severe form of arthrogryposis and myopathy also known as Freeman–Sheldon/Sheldon–Hall syndrome [12]. Mild clinical presentations, as well as rhabdomyolysis, have been reported in this syndrome [28].
Disorders of purine metabolism
Only a few families have been reported to harbor mutations in the gene AHCY, which encodes the enzyme S-adenosylhomocysteine (SAH) hydrolase. This enzyme catalyzes the final step in the conversion of methionine to homocysteine. Disease severity and phenotype can be variable; however, most affected individuals exhibit delayed psychomotor development and hypotonia at birth, accompanied by elevated serum aminotransferase levels [29]. Elevated levels of muscle enzymes, such as CK, lactate dehydrogenase and aspartate aminotransferase, have also been reported in a few cases [30]. Importantly, dietary methionine restriction and dietary supplements of creatine and phosphatidylcholine can substantially lower circulating levels of methionine in SAH hydrolase-deficient patients [29, 31], although the long-term clinical benefits of this therapy are still unknown.
Our study highlights several important insights for the diagnosis of rhabdomyolysis: First, it demonstrates a high prevalence of monogenic etiologies for individuals with rhabdomyolysis, which in most part has been underrecognized to date. The fact that most cases presented sporadically without a positive family history led to a low index of suspicion for the genetic nature of this condition. As a result, several cases were falsely assigned only to be secondary to environmental exposures, such as exertion or drug intake. As both exertion and monogenic mutations play a role, this disease group is an example of monogenic disorders in the context of a gene–environment interaction. Second, individuals with mutations in genes that cause rhabdomyolysis can initially present with kidney-related symptoms and signs of AKI and/or dark urine. As a result, nephrologists, who may be the first clinicians to be approached, should be aware that there are over 60 different individual genes that, if mutated, may cause rhabdomyolysis in those patients. Third, our results suggest broad genetic heterogeneity for rhabdomyolysis. To the best of our knowledge, only a few rhabdomyolysis cohorts have been systematically analyzed by means of NGS and/or WES [32,33,34,35]. Given the rarity and the high number of genes that can cause rhabdomyolysis if mutated, WES should be the method of choice for molecular diagnosis. In this setting, WES offers a powerful, cost-effective and non-invasive tool for a precise, unequivocal, etiology-based diagnosis. Most importantly, the genetic molecular diagnosis enabled us to provide personalized therapeutic management as well as to alert the clinician to possible life-threatening events, such as malignant hyperthermia and AKI, which can be caused by mutation of some of the rhabdomyolysis genes.
References
Scalco RS, Gardiner AR, Pitceathly RD, Zanoteli E, Becker J, Holton JL, Houlden H, Jungbluth H, Quinlivan R (2015) Rhabdomyolysis: a genetic perspective. Orphanet J Rare Dis 10:51
Vivante A, Kleppa MJ, Schulz J, Kohl S, Sharma A, Chen J, Shril S, Hwang DY, Weiss AC, Kaminski MM, Shukrun R, Kemper MJ, Lehnhardt A, Beetz R, Sanna-Cherchi S, Verbitsky M, Gharavi AG, Stuart HM, Feather SA, Goodship JA, Goodship TH, Woolf AS, Westra SJ, Doody DP, Bauer SB, Lee RS, Adam RM, Lu W, Reutter HM, Kehinde EO, Mancini EJ, Lifton RP, Tasic V, Lienkamp SS, Juppner H, Kispert A, Hildebrandt F (2015) Mutations in Tbx18 cause dominant urinary tract malformations via transcriptional dysregulation of ureter development. Am J Hum Genet 97:291–301
Vivante A, Hwang DY, Kohl S, Chen J, Shril S, Schulz J, Van Der Ven A, Daouk G, Soliman NA, Kumar AS, Senguttuvan P, Kehinde EO, Tasic V, Hildebrandt F (2017) Exome sequencing discerns syndromes in patients from consanguineous families with congenital anomalies of the kidneys and urinary tract. J Am Soc Nephrol 28:69–75
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup (2009) The sequence alignment/map format and Samtools. Bioinformatics 25:2078–2079
Van Der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, Banks E, Garimella KV, Altshuler D, Gabriel S, Depristo MA (2013) From FASTQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinformatics 43:11.10.11–11.10.33
Seelow D, Schuelke M, Hildebrandt F, Nurnberg P (2009) Homozygositymapper—an interactive approach to Homozygosity mapping. Nucleic Acids Res 37:W593–W599
Macarthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Gr A, Adams DR, Altman RB, Antonarakis SE, Ashley EA, Barrett JC, Biesecker LG, Conrad DF, Cooper GM, Cox NJ, Daly MJ, Gerstein MB, Goldstein DB, Hirschhorn JN, Leal SM, Pennacchio LA, Stamatoyannopoulos JA, Sunyaev SR, Valle D, Voight BF, Winckler W, Gunter C (2014) Guidelines for investigating causality of sequence variants in human disease. Nature 508:469–476
Hildebrandt F, Heeringa SF, Ruschendorf F, Attanasio M, Nurnberg G, Becker C, Seelow D, Huebner N, Chernin G, Vlangos CN, Zhou W, O'toole JF, Hoskins BE, Mt W, Hinkes BG, Chaib H, Ashraf S, Schoeb DS, Ovunc B, Allen SJ, Vega-Warner V, Wise E, Harville HM, Rh L, Washburn J, Macdonald J, Nurnberg P, Otto EA (2009) A systematic approach to mapping recessive disease genes in individuals from Outbred populations. PLoS Genet 5:E1000353
Raben N, Sherman J, Miller F, Mena H, Plotz P (1993) A 5′ splice junction mutation leading to exon deletion in an Ashkenazic Jewish family with Phosphofructokinase deficiency (Tarui disease). J Biol Chem 268:4963–4967
Taroni F, Verderio E, Dworzak F, Willems PJ, Cavadini P, Didonato S (1993) Identification of a common mutation in the carnitine palmitoyltransferase ii gene in familial recurrent myoglobinuria patients. Nat Genet 4:314–320
Ptacek LJ, George AL Jr, Barchi RL, Griggs RC, Riggs JE, Robertson M, Leppert MF (1992) Mutations in an S4 segment of the adult skeletal muscle sodium channel cause paramyotonia congenita. Neuron 8:891–897
Toydemir RM, Rutherford A, Whitby FG, Jorde LB, Carey JC, Bamshad MJ (2006) Mutations in embryonic myosin heavy chain (Myh3) cause Freeman–Sheldon syndrome and Sheldon–Hall syndrome. Nat Genet 38:561–565
Baric I, Fumic K, Glenn B, Cuk M, Schulze A, Finkelstein JD, James SJ, Mejaski-Bosnjak V, Pazanin L, Pogribny IP, Rados M, Sarnavka V, Scukanec-Spoljar M, Allen RH, Stabler S, Uzelac L, Vugrek O, Wagner C, Zeisel S, Mudd S (2004) S-Adenosylhomocysteine hydrolase deficiency in a human: a genetic disorder of methionine metabolism. Proc Natl Acad Sci USA 101:4234–4239
Wu S, Ibarra MC, Malicdan MC, Murayama K, Ichihara Y, Kikuchi H, Nonaka I, Noguchi S, Hayashi YK, Nishino I (2006) Central core disease is due to Ryr1 mutations in more than 90% of patients. Brain 129:1470–1480
Klingler W, Heiderich S, Girard T, Gravino E, Heffron JJ, Johannsen S, Jurkat-Rott K, Ruffert H, Schuster F, Snoeck M, Sorrentino V, Tegazzin V, Lehmann-Horn F (2014) Functional and genetic characterization of clinical malignant hyperthermia crises: a multi-centre study. Orphanet J Rare Dis 9:8
Tonin P, Bruno C, Cassandrini D, Savio C, Tavazzi E, Tomelleri G, Piccolo G (2009) Unusual presentation of Phosphoglycerate mutase deficiency due to two different mutations in Pgam-M gene. Neuromuscul Disord 19:776–778
Lee E, Chahin N (2013) A patient with mutation in the Scn4a P.M1592v presenting with fixed weakness, rhabdomyolysis, and episodic worsening of weakness. Muscle Nerve 48:306–307
Martin MA, Rubio JC, Del Hoyo P, Garcia A, Bustos F, Campos Y, Cabello A, Culebras JM, Arenas J (2000) Identification of novel mutations in Spanish patients with musclecCarnitine palmitoyltransferase II deficiency. Hum Mutat 15:579–580
Kaneoka H, Uesugi N, Moriguchi A, Hirose S, Takayanagi M, Yamaguchi S, Shigematsu Y, Yasuno T, Sasatomi Y, Saito T (2005) Carnitine palmitoyltransferase II deficiency due to a novel gene variant in a patient with rhabdomyolysis and ARF. Am J Kidney Dis 45:596–602
Wieser T, Deschauer M, Olek K, Hermann T, Zierz S (2003) Carnitine palmitoyltransferase ii deficiency: molecular and biochemical analysis of 32 patients. Neurology 60:1351–1353
Musumeci O, Bruno C, Mongini T, Rodolico C, Aguennouz M, Barca E, Amati A, Cassandrini D, Serlenga L, Vita G, Toscano A (2012) Clinical features and new molecular findings in muscle phosphofructokinase deficiency (GSD type VII). Neuromuscul Disord 22:325–330
Toscano A, Musumeci O (2007) Tarui disease and distal glycogenoses: clinical and genetic update. Acta Myol 26:105–107
Auranen M, Palmio J, Ylikallio E, Huovinen S, Paetau A, Sandell S, Haapasalo H, Viitaniemi K, Piirila P, Tyynismaa H, Udd B (2015) PFKM gene defect and glycogen storage disease GSD VII with misleading enzyme histochemistry. Neurol Genet 1:E7
Hadjigeorgiou GM, Kawashima N, Bruno C, Andreu AL, Sue CM, Rigden DJ, Kawashima A, Shanske S, Dimauro S (1999) Manifesting heterozygotes in a Japanese family with a novel mutation in the muscle-specific phosphoglycerate mutase (PGAM-M) gene. Neuromuscul Disord 9:399–402
Joshi PR, Knape M, Zierz S, Deschauer M (2009) Phosphoglycerate mutase deficiency: case report of a manifesting heterozygote with a novel E154k mutation and very late onset. Acta Neuropathol 117:723–725
Riazi S, Kraeva N, Sm M, Dowling J, Ho C, Ma P, Parness J, Dirksen R, Rosenberg H (2014) Malignant hyperthermia and the clinical significance of type-1 ryanodine receptor gene (Ryr1) variants: proceedings of the 2013 MHAUS scientific conference. Can J Anaesth 61:1040–1049
Kimlicka L, Lau K, Cc T, Van Petegem F (2013) Disease mutations in the ryanodine receptor N-terminal region couple to a mobile Intersubunit Interface. Nat Commun 4:1506
Gurjar V, Parushetti A, Gurjar M (2013) Freeman-Sheldon syndrome presenting withmicrostomia: a case report and literature review. J Maxillofac Oral Surg 12:395–399
Stender S, Chakrabarti RS, Xing C, Gotway G, Cohen JC, Hobbs HH (2015) Adult-onset liver disease and hepatocellular carcinoma in S-adenosylhomocysteine hydrolase deficiency. Mol Genet Metab 116:269–274
Buist NR, Glenn B, Vugrek O, Wagner C, Stabler S, Allen RH, Pogribny I, Schulze A, Zeisel S, Baric I, Mudd SH (2006) S-adenosylhomocysteine hydrolase deficiency in a 26-year-old man. J Inherit Metab Dis 29:538–545
Strauss KA, Ferreira C, Bottiglieri T, Zhao X, Arning E, Zhang S, Zeisel SH, Ml E, Presnick N, Puffenberger EG, Vugrek O, Kovacevic L, Wagner C, Mazariegos GV, Mudd SH, Soltys K (2015) Liver transplantation for treatment of severe S-adenosylhomocysteine hydrolase deficiency. Mol Genet Metab 116:44–52
Scalco RS, Pitceathly RDS, Gardiner A, Woodward C, Polke JM, Sweeney MG, Olpin SE, Kirk R, Murphy E, Hilton-Jones D, Jungbluth H, Houlden H, Hanna MG, Quinlivan R (2014) G.P.22: Utilising next-generation sequencing to determine the genetic basis of recurrent rhabdomyolysis. Neuromuscul Disord 24:801
Cabrera M, Ghaoui R, Mourdant D, Lamont PJ, Clarke N, Laing NG (2014) T.P.33: clinical and genetic characterization of patients with repeated rhabdomyolysis. Neuromuscul Disord 24:874
Scalco RS, Gardiner AR, Pitceathly RD, Hilton-Jones D, Schapira AH, Turner C, Parton M, Desikan M, Barresi R, Marsh J, Manzur AY, Childs AM, Feng L, Murphy E, Lamont PJ, Ravenscroft G, Wallefeld W, Davis MR, Laing NG, Holton JL, Fialho D, Bushby K, Hanna MG, Phadke R, Jungbluth H, Houlden H, Quinlivan R (2016) Cav3 mutations causing exercise intolerance, myalgia and rhabdomyolysis: expanding the phenotypic spectrum of caveolinopathies. Neuromuscul Disord 26:504–510
Scalco RS, Voermans NC, Piercy RJ, Jungbluth H, Quinlivan R (2016) Dantrolene as a possible prophylactic treatment for Ryr1-related rhabdomyolysis. Eur J Neurol 23:E56–E57
Acknowledgments
We are grateful for the families who contributed to this study. We thank the patients and their families for participating in this study. This research was supported by grants from the National Institutes of Health to RPL and FH (DK088767), from the March of Dimes to FH and from the Yale Center for Mendelian Genomics to RPL (U54HG006504). AV is a recipient of the Fulbright postdoctoral scholar award for 2013 and is also supported by grants from the Manton Center Fellowship Program, Boston Children’s Hospital, Boston, Massachusetts, USA and the Mallinckrodt Research Fellowship Award. ATvdV is supported by a Postdoctoral Research Fellowship, German Research Foundation [VE916/1-1]. FH is the William E. Harmon Professor of Pediatrics at Harvard Medical School.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Disclosures
F.H. is a co-founder of Goldfinch-Bio and receives royalties from Claritas.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Rights and permissions
About this article
Cite this article
Vivante, A., Ityel, H., Pode-Shakked, B. et al. Exome sequencing in Jewish and Arab patients with rhabdomyolysis reveals single-gene etiology in 43% of cases. Pediatr Nephrol 32, 2273–2282 (2017). https://doi.org/10.1007/s00467-017-3755-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3755-8