Abstract
Objective
To study the outcome of extremely low birth weight (ELBW) infants with a history of acute kidney injury (AKI).
Method
In a retrospective, case control study, medical records of all ELBW infants admitted to the neonatal intensive care unit (NICU) between Jan 2002 and Dec 2011 were reviewed. Medical records were reviewed for infants’ demographics, blood pressure (BP) at NICU discharge and at ≥3 years, and estimated glomerular filtration rate (eGFR) at ≥2 years.
Results
During the study period, 222 patients met the inclusion criteria, of whom 10% (23 out of 222) had AKI stage 2 and 3, 39% (87 out of 222) had AKI stage 1, and the rest did not have AKI. At NICU discharge, there was a difference in diastolic BP (DBP) among infants who had AKI stages 2 and 3, those who had stage 1, and those who did not have AKI (53 ± 12 vs 46 ± 9 vs 46 ± 11 mmHg respectively; p = 0.007), and 11% (23 out of 209) had hypertension (HTN). Although there was a significant correlation between the rise in SCr and DBP at NICU discharge in infants with AKI (R = 0.304; p = 0.004), there was no difference in HTN between infants with and those without AKI. At ≥2 years of age, 4% (5 out of 120) across all groups had an eGFR < 90 ml/min/1.73m2 or chronic kidney disease (CKD). At ≥3 years of age, 5% (11 out of 222) had HTN.
Conclusion
At NICU discharge, infants with AKI stages 2 and 3 have a higher DBP than infants with stage 1 AKI and those who did not have AKI. However, there is no difference in the rate of HTN between the two groups. At ≥2 years ELBW infants are at risk for CKD independently of whether or not they develop neonatal AKI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advances in NICU management of extremely preterm and extremely low birth weight (ELBW) infants have led to a significant improvement in the survival of this critically ill population. However, this improvement in survival comes at the cost of short-term and long-term morbidities [1, 2]. It has been previously shown that extreme preterm birth is associated with an elevated systolic blood pressure (SBP) and diastolic blood pressure (DBP) at 2–3 years of age [3]. Adults who were born moderately preterm have increased blood pressure and insulin resistance at 30 years of age [4].
Acute kidney injury (AKI) is common in critically ill pediatric and adult patients. In pediatric patients, its occurrence has been particularly observed following cardiac bypass or in patients treated with extracorporeal membrane oxygenation (ECMO) and it is associated with significant mortality [5, 6]. Pediatric and adult data have shown that AKI has an independent impact upon mortality and morbidity after controlling for co-morbidities, complications, and severity of illness [7, 8].
The incidence of AKI in the NICU has not been well established owing to a lack of unified criteria for diagnosing AKI. More recent data have shown that AKI is prevalent, ranging between 8.4% and 39.8% in premature infants, and it is associated with significant morbidity and mortality [9–17]. We have previously shown that 12.5% of all ELBW infants develop AKI during their NICU stay, and that anuria is associated with an increased mortality [18]. Several studies in newborn infants and a few studies in ELBW infants have identified risk factors that are associated with AKI [10, 11, 13, 18].
It has been previously reported that children with AKI are at an increased risk for developing chronic kidney disease (CKD) and warrant long-term follow-up [19, 20]. However, long-term effects of AKI in ELBW on future growth, kidney function, and blood pressure (BP) have not been well elucidated. Also, little is known about the short-term and long-term effects of AKI on the developing kidneys during the neonatal period. Therefore, in this study, we sought to investigate the short-term and long-term effects of neonatal AKI on growth, renal function (including the development of CKD), and BP in ELBW infants.
Methods
In a retrospective, case–control study, medical records of all ELBW infants admitted to the NICU at Metro Health Medical Center (MHMC) between January 2002 and December 2011 were reviewed. Our inclusion criteria consisted of all ELBW infants (birth weight < 1,000 g) who were born at MHMC between Jan 2002 and Dec 2011 and who had a follow up at 3 years of age and older at MHMC. Our exclusion criteria consisted of patients who died before discharge from the NICU, who were deceased at the time of follow up of the study, who were lost to follow-up ≤ 3 years of age, or who did not have any documented BP or contemporary height measurements (within 1 year of the last documented BP).
Medical records were reviewed for infants’ demographics, including weight, height, and head circumference (HC) measurements at birth and at discharge from the NICU, gender, ethnicity, gestational age (GA) at birth, mode of delivery, Apgar scores, and Score for Neonatal Acute Physiology (SNAP).
Anthropometric measures, including GA-specific percentiles for weight, length, and HC at birth, and at discharge from the NICU, were collected. Blood urea nitrogen (BUN) and serum creatinine values (SCr) during the NICU stay, and the latest available values, were reviewed. First available BUN and SCr values after 72 h of life were recorded as baseline. Subsequent values were compared with the lowest previous SCr to identify infants with AKI. The latest available BUN and SCr values that were available at the time of the study, and contemporary height measurements, were used to calculate the estimated glomerular filtration rate (eGFR) for each patient.
Medical records were also reviewed for a drop in urine output during infants’ NICU stay after the first 24 h of life to identify a decrease in urine output to less than 0.5 mg/kg/h. Patients with AKI were identified and staged using modified Neonatal Kidney Disease: Improving Global Outcomes (KDIGO) criteria, as suggested by the Neonatal Kidney Collaborative (NKC) group [21].
The latest documented SBP and DBP around the time of discharge from the NICU were reviewed to identify patients with hypertension. At the time of discharge from the NICU, hypertension (HTN) was defined as systolic BP and/or diastolic BP, equal or superior to the 95th percentile for corrected gestation age on three or more occasions [22]. The latest available BP readings obtained at ≥ 3 years of age were collected from the medical records. At our institution, BP measurements are obtained by automated oscillometric method during well child visits at ≥ 3 years of age. HTN was defined according to the 4th Report on the Diagnosis, Evaluation, and Treatment of high BP in Children and Adolescents. Hypertension was defined as systolic BP and/or diastolic BP that is superior or equal to the 95th percentile for gender, age, and height on three or more occasions, and BP remaining elevated at the last available visit [23].
The study was approved by the hospital’s Institutional Review Board at Metro Health Medical Center.
Statistical analysis
Data were expressed as means ± standard deviations, medians with interquartile ranges, and as percentages. Chi-squared, and Fisher’s exact tests were used for categorical variables as appropriate. Mann–Whitney U test or t test was used for analysis of parametric and nonparametric continuous variables respectively. Analysis of variance was used for comparison of multiple continuous data variables, and Bonferroni correction for post hoc analysis. A Pearson correlation analysis was conducted to compare the rate of rise in SCr during the NICU stay and DBP at the time of discharge from the NICU. A p value < 0.05 was considered statistically significant.
Results
During the study period, 398 ELBW infants who survived the neonatal period were identified, and only 56% (222 out of 398) met our inclusion criteria, of whom 49% (110 out of 222) had AKI according to the modified neonatal KDIGO staging criteria. Among all infants, 51% (112 out of 222) had no AKI, 39% (87 out of 222) had AKI stage 1, and 10% (23 out of 222) had AKI stage 2 or 3. Of note, 82 patients died in the NICU during the study period, and 83% (68 out of 82) had AKI. Among infants with AKI who died in the NICU, 42.6% (29 out of 68) had stage 1, 17.6% (12 out of 68) had stage 2, and 39.7% (27 out of 68) had stage 3 AKI, according to the KDIGO definition.
As 44% (176 out of 398) of our patients were lost to follow-up, we compared the demographics of infants who were followed up with those of infants who were lost to follow-up. There were no significant differences between the two groups, except that more Caucasians were lost to follow-up (Table 1).
To define the demographics of infants with different stages of AKI, we compared infants with stage 2 and 3 with infants with stage 1 and those with no AKI. There were significant differences among the three groups. Infants with severe AKI had a lower GA, smaller birth weight (BW), smaller length and smaller HC at birth than their controls. However, there were no differences in BW, length or HC percentiles at birth among the three groups (Table 2).
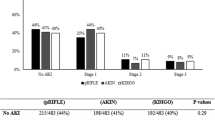
At the time of discharge from the NICU, infants with severe AKI were noted to have a longer NICU stay, and to be older and heavier than infants with stage 1 and infants without AKI. Also, infants with severe AKI had a smaller HC at the time of discharge. However, when all anthropometric measures were adjusted for age, infants with severe AKI had lower weight and length percentiles at the time of discharge from the NICU and a statistically nonsignificant difference in HC percentiles compared with their counterparts (Table 3). There were also statistically significant differences among the three groups regarding DBP and mean arterial BP (MAP). Infants with severe AKI had the highest DBP among the three groups (Table 3). Infants with stages 2 and 3 AKI had a higher DBP than infants with stage 1 AKI and infants without AKI (Fig. 1). However, at the time of discharge from the NICU, there was no difference in the percentage of patients with DBP > 95th percentile for GA between infants with and those without a history of AKI (6% [6 out of 104] vs 3% [3 out of 105] respectively; p = 0.33).
Blood pressure at the time of discharge from the neonatal intensive care unit (NICU) in extremely low birth weight infants (ELBW) with different stages of acute kidney injury (AKI) and those without AKI. SBP systolic blood pressure, DBP diastolic blood pressure. *p value < 0.01 between infants with AKI stage 2 and 3 and infants with AKI stage 1; **p value <0.01 between infants with AKI stage 2 and 3 and those with no AKI (analysis of variance with Bonferroni correction)
At the latest outpatient follow-up, there were no differences in growth parameters or prevalence of hypertension or pre-hypertension among the three groups (Table 4). However, infants with severe AKI were older at the time of their follow-up examination than the group of infants who did not have AKI (Table 4).
There were no differences in serum creatinine at 72 h of life, at discharge from the NICU, or at follow-up among the three groups. However, there were statistically significant differences observed in serum BUN at 72 h of life, at discharge from the NICU, and at follow-up among the three groups (Table 5). There was also a significant difference in age among the groups at the time of follow-up. Infants with AKI were older at the time of follow-up than infants who did not have AKI (Table 5).
To determine if there was any correlation between the rise in serum creatinine during the NICU stay and DBP a correlation analysis was conducted. There was significant correlation between an increase in SCr and DBP at the time of discharge from the NICU (Pearson R = 0.304, p = 0.004; Fig. 2). However, this correlation did not persist by the time of follow-up at ≥3 years of age.
At ≥2 years of age, 4% of infants (5 out of 120) who had an available serum creatinine for analysis had an eGFR < 90 ml/min/1.73 m2 or CKD. However, the prevalence of CKD was not different between infants with and those without AKI (4.2% [3 out of 71] vs 4.1% [2 out of 49] respectively; p = 1.00). Among patients with a history of neonatal AKI and who developed CKD, 1 patient had a history of AKI stage III, and 2 patients had a history of AKI stage I.
Discussion
We have shown that AKI is common during the neonatal period in ELBW infants. Infants with AKI are younger and smaller at birth, and have a longer stay in the NICU than their controls. Among survivors, ELBW infants with AKI have growth restriction at the time of discharge from the NICU and have a higher DBP than infants without AKI. We have also shown a correlation between the degree of rise in SCr during the AKI episode and DBP at the time of discharge from the NICU. However, at follow-up at ≥3 years of age there were no differences in growth restriction or prevalence of high blood pressure between infants with AKI and those without AKI. Overall, 4% of all ELBW infants who had a follow-up SCr had evidence of CKD at ≥2 years of age.
In our study group, we found an AKI prevalence of 49%, which is higher than previous reports. We have previously reported a prevalence of 12.5% among ELBW infants [18]. The differences seen are most likely related to our entry criteria, and definition. In our current study, we used the modified neonatal KDIGO criteria for the definition of AKI. We had previously defined AKI as oliguria of <1 ml/kg/h of urine output that developed 24 h after birth and persisted for at least 24 h and/or a rise of SCr level >1.5 mg/dl 72 h after birth in the presence of normal maternal creatinine levels [18]. When we used the definition based on serum creatinine > 1.5 mg/dl, the occurrence of AKI in our entire cohort (including all patients, those who died in the NICU [82 patients] and those who survived [398 patients]) was 19% (91 out of 483). Also, when we used the definition based on serum creatinine > 1.5 mg/dl, the occurrence of AKI was 35% (29 out of 82) in patients who died in the NICU. In our current study, we are only reporting the rate of AKI amongst the 222 patients who survived and had a follow-up at our institution; hence, the difference in AKI rates. The SCr cut-off level is used for the definition of AKI as SCr level > 1.5 mg/dl is predictive of poor outcome in asphyxiated infants with AKI [24], and a urine output-based definition cannot be used alone as non-oliguric AKI is common in infants [25, 26].
In infants weighing <750 g, Arcinue et al. reported an AKI prevalence of 26% [10]. In their study, AKI was defined according to the Acute Kidney Injury Network (AKIN) criteria, whereas in our study AKI was defined according to the KDIGO criteria. The higher prevalence in our population could be explained by the fact that KDIGO criteria take into account a SCr rise of ≥0.3 mg/dl or ≥50% rise in baseline in SCr levels over 7 days, whereas AKIN only includes similar rises over 48 h, and therefore using the KDIGO criteria can be more inclusive [27, 28].
Previous studies have shown that ELBW infants continue to suffer from growth failure after discharge from the NICU, but a significant number of those infants experience catch-up growth. Dusick et al. have shown that 16% of all ELBW infants are small for gestational age (SGA) at birth, and 89% have growth failure at 36 weeks’ corrected gestation. However, only 40% continue to fail to thrive, or have weight, length, and HC less than the 10th percentile at 18–22 months’ corrected age [29]. Others have also shown similar results in premature and ELBW infants [30, 31]. In our study, we found that infants with AKI were smaller and younger at birth, but did not have any differences in BW, HC or length percentiles when compared with infants without AKI. At the time of their NICU discharge, infants with AKI had lower weight and length percentiles than their counterparts. However, at 3 years of age and older, there was a resolution of the percentile differences in weight and length between infants with and those without a history of AKI. Our findings suggest that AKI during the neonatal period might have an impact on the neonatal growth, which improves over time.
Bonamy et al. have previously shown a rise in SBP and DBP at 2–3 years of age in former extremely premature infants [3]. We found that infants with stage 2 and 3 AKI had higher DBP at the time of discharge from the NICU than infants with stage 1 and infants without AKI. This difference was noted to persist at the most recent follow-up; however, it did not reach statistical significance, probably because our study was too small and underpowered to show a persistent difference. The small differences in DBP between infants with AKI and their controls may not have any significant clinical relevance at the time of discharge from the NICU. However, it remains to be seen if small differences in BP have any future significant clinical relevance; future observational longitudinal and powered studies are needed to answer such a question.
We also noted a correlation between the degree of rise in SCr during the episode of AKI and DBP at the time of discharge from the NICU. This correlation implies an association between the severity of AKI and DBP. However, this correlation did not persist at the time of the latest follow-up. This lack of correlation between the rise in SCr in the newborn period and DBP at ≥3 years of age could have been affected by other factors such as growth and weight gain over time of those ELBW infants. We have previously shown a correlation between weight gain and high blood pressure at ≥3 years of age in EBLW infants [32].
In our study, infants with AKI had higher BUN values at 72 h of life, at the time of discharge from the NICU and at the latest follow-up. However, the statistically significant differences did not correspond to clinically significant differences. To date, GFR has been the most recognized measure of kidney function, but it is low in preterm neonates compared with term neonates. In both preterm and term neonates, it rapidly increases during the first few months of life, reaching adult GFR by the age of 2 years [33, 34]. In our patients, we found that 4% (5 out of 120) of our infants who had an available SCr at follow-up had early CKD based on their eGFR. This prevalence is probably an underestimate of the true prevalence of CKD in those infants, as only 120 patients had follow-ups. Khalsa et al. have recently shown that decreased eGFR occurs with a higher prevalence in adolescents born with a LBW and VLBW [35].
Although the nature of correlation (causal vs correlational) between AKI and downstream adverse outcomes remains a topic of debate, ELBW alone has important implications and it is associated with increased morbidity during the NICU stay. Previous studies have shown that nephrogenesis continues until 34 weeks’ gestation. Most ELBW infants are born before 34 weeks’ gestation. Therefore, their nephron mass at birth is significantly lower, putting them at an even higher risk for injury from the nephrotoxic insults commonly encountered by many NICU patients. This predisposes them not only to developing AKI during hospitalization, but also the microscopic changes that happen from these acute insults have been known to cause long-term renal sequelae. In one study, 59% of children have at least one sign of CKD 3–5 years after the initial insult [20]. In our study, the rate of CKD was lower, probably secondary to the loss of follow-up of a significant number of our patients. Only a quarter of our patients had serum creatinine levels available at ≥2 years of age.
Brenner and Chertow have suggested a direct relationship between BW and nephron number, an inverse relationship between BW and later-life hypertension, and an inverse relationship between nephron number and blood pressure [36]. Data from previously reported animal studies has shown that AKI can induce renal fibrosis, and can affect other vital organs in a deleterious fashion. Thus, despite the fact that clinically overt AKI is typically reversible, there may be subclinical renal and extra-renal damage that persists even after the acute insult is over [37–39]. Although we did not find hypertension as a prominent clinical feature in patients with a history of AKI at follow-up, we did find a statistically significant difference in DBP at the time of discharge from the NICU in infants with severe AKI compared with patients with stage 1 or no AKI. Studies have shown that ELBW and AKI have important implications for the development of cardiovascular risks in later life [1, 2]. In our study, 5% of all infants developed HTN upon follow-up, but the prevalence was not different between patients who did or did not have a history of neonatal AKI. Perhaps longer follow-up of these patients into adulthood may unmask such a difference, if one exists.
Our study has limitations. This is a single center retrospective study. Data were extracted from paper and electronic medical records and therefore some information may have been missing. A significant percentage (44%) of our patients were lost to outpatient follow-up after discharge form the NICU. To determine if our study population is representative of the entire cohort, we compared the demographics and birth characteristics of infants who were followed up with those who were lost to follow-up. There were no differences observed between the two groups, except that more Caucasians were lost to follow-up. Therefore, the difference in ethnicity between the two groups could have influenced our findings, as the prevalence of high blood pressure is different in different ethnic groups. Also, as our study is retrospective in nature and it was conducted over a period of several years, a change in management over time could have influenced our results. Another limitation of our study is that it is underpowered to show a significant relationship between neonatal AKI stages 2 and 3 and CKD later in life, and a relationship between neonatal AKI and long-term changes in blood pressure.
In conclusion, we have shown that AKI is common in ELBW infants, and that infants with AKI are younger and smaller at birth, and have a longer NICU stay than their counterparts. Among survivors, ELBW infants with AKI have growth restriction at the time of discharge from the NICU and a higher DBP than those who did not have AKI. Also, we found a correlation between the degree of rise of SCr during the AKI episode and DBP at discharge. However, this correlation does not persist at follow-up. Overall, 4% of all ELBW infants who had a follow-up SCr had evidence of CKD at ≥2 years of age, regardless of a previous diagnosis of AKI.
Our study underscores the long-term outcome of extreme prematurity and the late consequences of early neonatal kidney insults. To date, ELBW infants with or without a history of AKI have not received routine follow-up nephrology care. This population is at an increased risk for ongoing residual kidney injury and death after AKI; therefore, periodic evaluation after the initial insult is necessary. This study may serve as the basis for future prospective studies to establish a standardized approach for closer surveillance and follow-up of this vulnerable population.
References
Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, Buzas JS (2012) Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 129:1019–1026
Carmody BJ, Charlton JR (2013) Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics 131:1168–1179
Bonamy AK, Källén K, Norman M (2012) High blood pressure in 2.5-year-old children born extremely preterm. Pediatrics 129:e1199–e1204
Dalziel SR, Parag V, Rodgers A, Harding JE (2007) Cardiovascular risk factors at age 30 following pre-term birth. Int J Epidemiol 36:907–915
Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB (2011) Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg 46:630–635
Zwiers AJ, de Wildt SN, Hop WC, Dorresteijn EM, Gischler SJ, Tibboel D, Cransberg K (2013) Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14-year cohort study. Crit Care 17:R151
Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA (2006) Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int 70:1120–1126
Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M (2011) Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care 15:R146. doi:10.1186/cc10269
Bolat F, Comert S, Bolat G, Kucuk O, Can E, Bulbul A, Uslu HS, Nuhoglu A (2013) Acute kidney injury in a single neonatal intensive care unit in Turkey. World J Pediatr 9:323–329
Arcinue R, Kantak A, Elkhwad M (2015) Acute kidney injury in ELBW infants (< 750 grams) and its associated risk factors. J Neonatal Perinatal Med 8:349–357
Weintraub AS, Connors J, Carey A, Blanco V, Green RS (2016) The spectrum of onset of acute kidney injury in premature infants less than 30 weeks gestation. J Perinatol 36:474–480
Carmody JB, Swanson JR, Rhone ET, Charlton JR (2014) Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 9:2036–2043
Stojanović V, Barišić N, Milanović B, Doronjski A (2014) Acute kidney injury in preterm infants admitted to a neonatal intensive care unit. Pediatr Nephrol 29:2213–2220
Choker G, Gouyon J (2004) Diagnosis of acute renal failure in very preterm infants. Biol Neonate 86:212–216
Drukker A, Guignard JP (2002) Renal aspects of the term and preterm infant: a selective update. Curr Opin Pediatr 14:175–182
Bonamy AK, Martin H, Jorneskog G, Norman M (2007) Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med 262:635–642
Chaturvedi S, Ng KH, Mammen C (2017) The path to chronic kidney disease following acute kidney injury: a neonatal perspective. Pediatr Nephrol 32:227–241
Viswanathan S, Manyam B, Azhibekov T, Mhanna MJ (2012) Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr Nephrol 27:303–311
Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG (2012) Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59:523–530
Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL (2006) 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69:184–189
Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL (2015) Neonatal acute kidney injury. Pediatrics 136:e463–e473
Dionne JM, Abitbol CL, Flynn JT (2012) Hypertension in infancy: diagnosis, management and outcome. Pediatr Nephrol 27:17–32
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 114:555–576
Aggarwal A, Kumar P, Chowdhary G, Majumdar S, Narang A (2005) Evaluation of renal functions in asphyxiated newborns. J Trop Pediatr 51:295–299
Jetton JG, Askenazi DJ (2012) Update on acute kidney injury in the neonate. Curr Opin Pediatr 24:191–196
Karlowicz MG, Adelman RD (1995) Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol 9:718–722
Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X (2014) A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 18:R144
Lex DJ, Tóth R, Cserép Z, Alexander SI, Breuer T, Sápi E (2014) A comparison of the systems for the identification of postoperative acute kidney injury in pediatric cardiac patients. Ann Thorac Surg 97:202–210
Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA (2003) Growth failure in the preterm infant: can we catch up? Semin Perinatol 27:302–310
Cooke RJ (2010) Catch-up growth: implications for the preterm and term infant. Eur J Clin Nutr 64:S8–S10
Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M (2006) Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res 60:751–758
Mhanna MJ, Iqbal AM, Kaelber DC (2015) Weight gain and hypertension at three years of age and older in extremely low birth weight infants. J Neonatal Perinatal Med 8:363–369
Abitbol CL, Seeherunvong W, Galarza MG, Katsoufis C, Francoeur D, Defreitas M, Edwards-Richards A, Master Sankar Raj V, Chandar J, Duara S, Yasin S, Zilleruelo G (2014) Neonatal kidney size and function in preterm infants: what is a true estimate of glomerular filtration rate? J Pediatr 164:1026–1031
Brion LP, Fleischman AR, McCarton C, Schwartz GJ (1986) A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr 109:698–707
Khalsa DD, Beydoun HA, Carmody JB (2016) Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr Nephrol. doi:10.1007/s00467-016-3384-7
Brenner B, Chertow G (1994) Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23:171–175
Basile DP (2004) Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13:1–7
Basile DP, Donohoe D, Roethe K, Osborn JL (2001) Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281:F887–F899
Kelly KJ (2006) Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14:1549–1558
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Maqsood, S., Fung, N., Chowdhary, V. et al. Outcome of extremely low birth weight infants with a history of neonatal acute kidney injury. Pediatr Nephrol 32, 1035–1043 (2017). https://doi.org/10.1007/s00467-017-3582-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3582-y