Abstract
Background
Mortality among critically ill children requiring continuous renal replacement therapy (CRRT) is high. Several factors have been identified as outcome predictors. Many studies have specifically reported a positive association between the fluid overload at CRRT initiation and the mortality of critically ill pediatric patients.
Methods
This study is a retrospective single-center analysis including all patients admitted to the pediatric intensive care unit (PICU) of our hospital who received CRRT between 2000 and 2012. One hundred thirty-one patients were identified and subsequently classified according to primary disease. Survival rates, severity of illness and fluid balance differed among subgroups. The primary outcome was patient survival to PICU discharge.
Results
Overall survival to PICU discharge was 45.8 %. Based on multiple regression analysis, mortality was independently associated with onco-hematological disease [odds ratio (OR) 11.7, 95 % confidence interval (CI) 1.3–104.7; p = 0.028], severe multiple organ dysfunction syndrome (MODS) (OR 5.1, 95 % CI 1.7–15; p = 0.003) and hypotension (OR 11.6, 95 % CI 1.4–93.2; p = 0.021). In the subgroup analysis, a fluid overload (FO) of more than 10 % (FO>10 %) at the beginning of CRRT seems to be a negative predictor of mortality (OR 10.9, 95 % CI 0.78–152.62; p = 0.07) only in children with milder disease (renal patients). Due to lack of statistical power, the independent effect of fluid overload on mortality could not be analyzed in all subgroups of patients.
Conclusions
In children treated with CRRT the underlying diagnosis and severity of illness are independent risk factors for mortality. The degree of FO is a negative predictor only in patients with milder disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The epidemiology of pediatric acute kidney injury (AKI) has changed over the past decades, and primary renal diseases are currently no longer the most common causes of AKI [1–3]. The improvement in critical care of children with congenital heart disease and solid organ and bone marrow transplantation have induced a profound change in the etiology of pediatric AKI. Nowadays, cardiac surgery, acute tubular necrosis, sepsis, and use of nephrotoxic drugs represent the most frequent causes of AKI in developed countries [1]. However, the incidence and outcome of pediatric AKI is highly dependent on the characteristics of the patient population.
Moreover, in contrast to adult patients with AKI, children develop multiple organ dysfunction syndrome (MODS) early in the pediatric intensive care unit (PICU) course, with the highest number of organ failures occurring within 72 h from PICU admission (87 % of patients) [4]. In the past decades, the availability of new, child-tailored life support devices has increased the likelihood of more severe critically ill children with AKI receiving renal replacement therapy (RRT) [5].
In this clinical setting, the role of continuous renal replacement therapy (CRRT) in critically ill pediatric patients is expanding. Since the late 1990s, CCRT has become the modality of choice in many PICU in the USA [5], owing to positive effects on hemodynamic stability in allowing predictable fluid and solute removal at slow rates. In critically ill children, AKI is associated with increased length of stay in the PICU and greater mortality [6, 7]. Mortality among children requiring CRRT remains high, with survival rates ranging from 38 to 58 % and varying according to cohort characteristics [8–23]. Several factors have been identified as outcome predictors, including late CRRT initiation [20], hemodynamic instability [13, 18, 21, 22], vasopressor number and dosage dependency [18, 21], underlying diseases [9, 14], low body weight [9, 14, 19], young age [14], need for mechanical ventilation [15], presence of MODS [18], high central venous pressure (CVP) [12] and fluid overload (FO) [8, 10–12, 16, 17, 19].
Many studies have specifically reported a strong association between the magnitude of FO at CRRT initiation and the mortality of critically ill children [8, 10–12, 16, 17, 19]. In 2001, data from a single-center study suggested—for the first time—that a 10–20 % FO at the time of CRRT initiation was associated with increased mortality, independently from the severity of the underlying illness and other clinical factors [8]. Data from the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) Registry on 344 patients treated with CRRT confirmed that a high FO percentage is associated with worsened outcomes. The most recent retrospective ppCRRT analysis revealed an adjusted mortality odds ratio (OR) for FO of 1.03 [95 % confidence interval (CI) 1.01–1.05], which is a 3 % increase in mortality risk with each 1 % increase in FO. However, despite the large body of observational data supporting this association, a number of studies have not been able to confirm these findings [15, 24].
In our hospital (Bambino Gesù Children’s Hospital, Rome, Italy), CRRT is provided to a large number of children and infants requiring critical care, including patients with complex congenital heart defect and liver disease and a large number of onco-hematological patients.
The aim of the study reported here is to describe the clinical characteristics and outcomes of a single-center population of critically ill children requiring CRRT and to identify risks factors for mortality. We have focused particularly on evaluating the association between FO and mortality.
Methods
Patient population and data collection
This was a retrospective single-center study that included all patients receiving CRRT who had been admitted to the PICU of Bambino Gesù Children’s Hospital—IRCCS between 1 January 2000 and 31 December 2012. We retrieved clinical data before PICU admission (pre-PICU), before CRRT initiation (PICU data), at the time of CRRT initiation (CRRT data) and after discharge from PICU (post-PICU data). Data were collected for each CRRT session (CRRT circuit data). A maximum 3-year follow-up after hospital discharge was reviewed.
-
Pre-PICU data comprised patient general information (age, sex, weight, height), renal function {serum creatinine, estimated glomerular filtration rate (eGFR) calculated using the Schwartz equation [25], 24 h before- PICU admission urine output (UO)}, patient primary diagnosis and causes leading to PICU admission.
-
PICU data specific to the PICU included assessment of illness gravity {Pediatric Index of Mortality 2 (PIM2) score [26]}, need for mechanical ventilation for >48 h (MV > 48 h), presence of sepsis and MODS), renal function (serum creatinine and azotemia, eGFR, 24 h post-PICU admission UO), hemodynamic state, total number of inotropic agents and need for diuretic therapy.
-
CRRT data obtained at the time of CRRT initiation included the time interval from PICU admission to CRRT initiation, weight and fluid balance {fluid input and output from PICU admission to CRRT initiation, presence of FO of more than 10 % (FO>10 %)}, renal and hemodynamic state, presence of MODS (number of organs involved) and the need for vasoactive drugs.
-
CRRT circuit data included the indications to initiate CRRT (development of FO, prevention of FO, electrolyte imbalance, metabolic decompensation, sepsis, acute respiratory distress syndrome), reasons for CRRT discontinuation (death, clinical improvement, development of chronic kidney disease) and CRRT parameters (CRRT modality, vascular access, blood, dialysate, replacement fluid flow rates, anticoagulation method, circuit duration). CRRT modality was categorized based on the use of purely convective therapy with replacement fluids [continuous hemofiltration (CVVH)], use of dialysate only [continuous hemodialysis (CVVHD)], or using both dialysate and replacement fluids [continuous hemodiafiltration (CVVHDF)].
AKI was determined by the pediatric-modified RIFLE (p-RIFLE) score [7]. Sepsis and MODS were defined according to the criteria of the International Consensus Conference on Pediatric Sepsis; accordingly, we considered a patient to have MODS if at least two organs were involved [27]. The percentage of FO (%FO) was determined using the PICU admission weight in kilograms as the baseline weight of comparison. We defined %FO using the method described by Goldstein et al [8]: [(fluid in) - (fluid out)/(intensive care unit admission weight) × 100], and considered fluid in (or out) as the amount of fluid from PICU admission to CRRT initiation.
The primary outcome was patient survival to PICU discharge. We also considered hospital discharge survival and renal survival.
Statistical analysis
All tests were performed with SPSS, version 15.0 (IBM Corp., Armonk, NY).The Shapiro–Wilk test was used to test for normality of data. Normally distributed continuous variables were reported as the mean ± standard deviation and compared using the Student t test; non-normally distributed variables were reported as the median with the interquartile range and analyzed using the Mann–Whitney test. Categorical variables were analyzed using the chi-square test. For variables with <5 units for each single subgroup or <30 units for the entire group, the Fisher exact test was used; for subgroups of >5 units or groups with >30 units the chi-square test was utilized. The Kaplan–Meier analysis and Cox regression analysis were performed for survival analyses. Multivariate logistic regression was used to identify predictors of mortality. Variables associated with mortality by univariate analysis with a p value threshold of 0.10 were included as covariates in the multiple regression models. Variables with a p value of >0.2 were eliminated with a stepwise, backward selection approach. The final multivariable-adjusted model included only variables that remained significant at the p < 0.1 level. p values of <0.05 were considered to be significant.
Results
Demographic data
Data from 134 patients were analyzed. Three patients had incomplete records and were excluded from the analysis, leaving 131 patients for review.
Table 1 shows the general and clinical characteristics of our pediatric patient cohort. Patient age ranged from 0 to 17.9 years, of whom 6 % were younger than 1 month at the time of CRRT and 33 % were younger than 1 year. At PICU admission nearly half of the children (47 %) presented MODS. At CRRT initiation children presented a worsening of MODS (30 % had three organ failures). At CRRT initiation, mean fluid balance was 1.81 l/m2 with a mean FO of 7.3 %. At initiation of RRT, 40.5 % patients had FO>10 %.
The most frequent indication for RRT was FO and electrolyte imbalance (88 patients, 67 %). Of the 131 patients, 11 had sepsis as principal indication to begin CRRT therapy. Treatment was performed in three patients who required extracorporeal life support (ECLS). The most commonly used modality was CVVH (71,8 %). The majority of circuits were anticoagulated with heparin (62 %); 36 % received no anticoagulation. Citrate anticoagulation was used only in one case. The femoral vein was the most commonly used vascular access (58.8 %). Median blood flow rate was 4.0 ml/min/kg of body weight (Table 1).
Patients were classified according to their primary disease (Table 2). Onco-hematological and renal patients formed the two largest subgroups, accounting for more than one-half of the patients. The subgroup “other”, accounted for 13 % of the patients and included malformation syndromes, immunological and neurological defects and trauma (6, 4, 4 and 3 patients, respectively). Only one patient had drug intoxication (boric acid).
Registry data were also evaluated according to the time of performance by dividing the observation into two periods (2000–2005 and 2006–2012). The children entered into the earlier period of the registry were older, and only 18.5 % presented MODS at PICU admission (Table 3). In the second period CRRT was initiated earlier (about 1 day after PICU admission), and no differences in CRRT modalities were reported. Both fluid balance and FO% increased in patients entered into the registry in the second period compared to those entered in the first period (Table 3).
Outcome data
Of the 131 patients that received CRRT, 60 (45.8 %) were alive at discharge from the PICU and 55 (42 %) were alive at discharge from the hospital. The Kaplan–Meier survival analysis included all 131 patients. Almost one-half of the patients died within the first 10 days after PICU admission. Survival rates at PICU discharge were not statistically different from those at hospital discharge or at the 12- and 24-month follow-ups, and we therefore used patient survival at PICU discharge as the primary outcome.
Table 2 shows the survival rates for each primary diagnosis. The underlying diseases had a significant impact on the overall mortality (p = 0.003, chi-square test). Survival was best in children with renal disease (85 %) and worst in the stem cell transplantation/oncologic group (20 %).
The severity of illness was also different among subgroups. Children with the worse survival rate (onco-hematological and sepsis) had higher rates of MODS (p = 0.004), a greater need for vasoactive drugs and a higher frequency of FO >10 % at CRRT initiation (p =0.001). Renal patients and children with metabolic disorders had lower PIM2 scores at PICU admission and lower FO compared to the other subgroups.
In the logistic Cox regression analysis, significant risk factors for mortality included age (older children had higher risk of death), sepsis and heart failure as well as admission diagnosis, primary disease (highest in onco-hematological patients; lowest in renal disease patients), clinical severity at PICU admission and at CRRT initiation (PIM2, presence of MODS and number of organ failure, sepsis, CVP, MV > 48 h), hemodynamic state (presence of hypotension and number of vasoactive drugs), AKI, the use of diuretic therapy at CRRT initiation, delay in CRRT initiation and FO (Table 4).
However, in multiple regression model, FO lost its significance when corrected for underlying diseases and illness severity, using the PIM2 score and the presence of MODS at CRRT initiation. According to the multiple regression analysis, mortality was independently associated with onco-hematological disease (OR 11.7, 95 % CI 1.3–104.7; p = 0.028), severe MODS (at least 3 organs involved) (OR 5.09, 95 % CI 1.7–15; p =0.003) and hypotension (OR 11.6, 95 % CI 1.4–93.2; p = 0.021) at CRRT initiation (Table 5).
The renal subgroup showed better survival rates (85 %) and lower illness severity that those patients with other underlying diseases (Tables 1, 2). Compared to all other patients, renal patients had a longer hospitalization stay, but a shorter stay in the PICU (44 and 10 days, respectively), a lower median PIM2 (3.9; p = 0.02) and lower rates of respiratory failure (p = 0.02) and sepsis during the PICU stay (p = 0.01). At CRRT initiation one-third of renal patients had MODS, with a lower mean number of organs involved (p = 0.010), and only 29 % received vasoactive drug infusions (p = 0.005). The median fluid balance was 0.66 l/m2 with a median FO of 2.6 % body weight (p = 0.000).
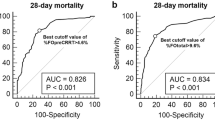
The Cox regression analysis showed that in both subgroups, renal and non-renal, the presence of MODS with more than three organs involvement (renal: OR 10.5, 95 % CI 1–10; p = 0.04; non-renal: OR 5.9, 95 % CI 2–16; p = 0.001) and the presence of hypotension (renal: OR 9, 95 % CI 0.9–89; p = 0.06; non-renal: OR 2.8, 95 % CI 1–7.9; p = 0.04) at CRRT initiation were associated with an increased risk of death. Only in the renal group did a FO of >10 % tend to be positively associated with lower survival rates, although the differences did not reach statistical significance (OR 9, 95 % CI 0.9–98; p = 0.06). Multiple regression analysis confirmed these results: FO lost significance when corrected for illness severity, expressed as presence of at least three organ failures, in both subgroups (renal p = 0.075; non-renal p = 0.875). However, in the renal group it retained a strong association with mortality, with an OR of 10.9 (95 % CI 0.78–152.62) (Table 6). This was also confirmed by cross-table analysis of subgroups of patients (Fig. 1).
Discussion
This retrospective analysis of 131 critically ill children of a single center shows that CRRT is a procedure that can be used to support a very wide range of critically ill children who differ in age, weight, underlying disease and illness severity. CRRT can be safely performed in very small children, as reported in previous studies [9, 14, 19], as well as in those requiring ECLS [24]. The main methodological differences between the data reported here and data from other pediatric studies are our predominant use of convective modalities (71.8 %) and of heparin when anticoagulation was needed [12–14, 18, 19]. These differences are likely to be related to local attitudes, as well as to the availability of citrate anticoagulant systems, which are widely used in the USA but not yet in Europe.
Survival to PICU discharge in our study was 45.8 %, similar to values observed in other pediatric studies [8, 15, 16, 19]. It is probable that onco-hematologic patients, accounting for a 30 % of this cohort, may have strongly affected the global survival and illness severity profile of our study population. In addition, it is of note that any comparison of survival rate between studies is hampered by the many different ways that have been used to categorize patients. In some studies onco-hematological patients are considered as two separate groups, whereas in our study we included them in the same subgroup [10, 14, 16, 23].
Consistent with previous data, we observed that the underlying disease [9, 14], hypotension [13, 18, 21, 22] and MODS [18] at time of CRRT start were independent risk factors for mortality in critically ill children requiring CRRT. However, in our multivariate model, a FO>10 % was not associated with mortality when the entire study population was analyzed. In contrast, our subgroup analysis showed that in renal patients, who were characterized by better survival rates and a lower severity of illness, the presence of FO>10 % at the beginning of CRRT was highly correlated with mortality.
Early and appropriate goal-directed fluid therapy is essential in acute resuscitation of critically ill children with acute hypovolemic and septic shock states [28–30]. However, fluid management in this setting is complex, and an increasing amount of data supports the concept that excessive fluid resuscitation is detrimental [8, 10–12, 16, 17, 19, 31]. It is, however, particularly difficult to differentiate whether FO reflects primarily the severity of the underlying disease or whether is an active player that aggravates the chain of events leading to death. No study to date has clearly demonstrated this causality. Few studies have reported data on FO in selected subgroups of pediatric patients with different underlying diseases and severity of illness. In a subgroup analysis of the ppCRRT Registry, Flores and colleagues [15] analyzed 51 children who underwent stem cell transplantation and required CRRT; these authors found no association between FO and increased mortality in this patient group.
A few pediatric studies have reported survival rates according to the primary diagnosis [14, 19]. Altogether, these are similar to those presented in our study. Specifically, these studies found that patients with renal diseases and metabolic defects had a better outcome, while patients who underwent stem cell transplantation had the worst survival. However, the magnitude of FO and severity of the disease are poorly reported in these selected subgroups of patients. Our study, although limited by its retrospective nature, in part fills this gap.
Our data show that in patients with limited disease severity, as assessed by the severity of MODS, FO represents a significant co-morbidity that is highly associated with outcome. Renal patients are an example of this finding. Conversely, in patients with very severe disease, the ultimate outcome was independent of the magnitude of fluid retention at initiation of CRRT, suggesting that the severity of disease of these patients was the primary negative determinant of outcome and that fluid management policies have little impact on the overall survival. This is in accordance with data reported by Flores et al. [15].
Due to lack of statistical power, the independent effect of FO on mortality could not be analyzed in all subgroups of our patients.
Data from this registry have also been analyzed to evaluate changing attitudes and practice patterns in our hospital over a period of 12 years. Technical improvements have led to an increasing use of CRRT in our PICU, and a growing number of more serious critically ill children have been successfully treated. Data analysis has revealed a reduction of the interval between PICU admission and CRRT initiation, indicating an increased tendency to an earlier initiation of the RRT. In our hospital, CRRT is normally prescribed and handled by the Dialysis Unit in cooperation with the ICU team. This multidisciplinary approach has progressively improved over the years, likely leading to more rapid decision-making and the earlier start of CRRT. Conversely, the registry did not show a decrease of FO at CRRT initiation. A higher percentage of children have presented a FO>10 % at CRRT initiation during last 7 years (2006–2012). Again, this is the expression of more serious critically ill children being treated in recent years. These are decompensated patients with a large amount of fluids gained prior to PICU admission and nephrological evaluation.
In conclusion, our data confirm that the mortality among children requiring CRRT is high. The underlying diagnosis and severity of illness, expressed as severity of MODS and hemodynamic instability, are the principal factors associated with mortality. The degree of FO is a negative predictor only in patients with milder disease, whereas in children with more serious illness FO reflects the severity of the disease.
References
Hui-Stickle S, Brewer ED, Goldstein SL (2005) Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kid Dis 45:96–101
Askenazi D (2011) Evaluation and management of critically ill children with acute kidney injury. Curr Opin Pediatr 23(2):201–207
Williams DM, Sreedhar SS, Mickell JJ, Chan JC (2002) Acute kidney failure: a pediatric experience over 20 years. Arch Pediatr Adolesc Med 156:893–900
Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA (1994) Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med 22(6):1025–1031
Warady BA, Bunchman T (2000) Dialysis therapy for children with acute renal failure: survey results. Pediatr Nephrol 15(1–2):11–13
Schneider J, Khemani R, Grushkin C, Bart R (2010) Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 38:933–939
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Goldstein SL, Currier H, Cd G, Cosio CC, Brewer ED, Sachdeva R (2001) Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107:1309–1312
Symons JM, Brophy PD, Gregory MJ, McAfee N, Somers MJ, Bunchman TE, Goldstein SL (2003) Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis 41:984–989
Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA (2004) Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med 32:1771–1776
Gillespie RS, Seidel K, Symons JM (2004) Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol 19:1394–1399
Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, Bunchman TE, Baker C, Mottes T, McAfee N, Barnett J, Morrison G, Rogers K, Fortenberry JD (2005) Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement. Kidney Int 67:653–658
Fernandez C, Lopez-Herce J, Flores JC, Galaviz D, Ruperez M, Brandstrup KB, Bustiniza A (2005) Prognosis in critically ill children requiring continuous renal replacement therapy. Pediatr Nephrol 20:1473–1477
Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL (2007) Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2(4):732–738
Flores FX, Brophy PD, Symons JM, Fortenberry JD, Chua AN, Alesander SR, Mahan JD, Bunchman TE, Blowey D, Somers MJ, Baum M, Hackbarth R, Chand D, Benfield M, Goldstein SL (2008) Continuous renal replacement therapy (CRRT) after stem cell transplantation. A report from the prospective pediatric CRRT Registry Group. Pediatr Nephrol 23(4):625–630
Hayes LW, Oster RA, Tofil NM, Tolwani AJ (2009) Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24(3):394–400
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortnberry JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55(2):316–325
Santiago MJ, Lopez-Herce J, Urbano J, Solana MJ, del Castillo J, Ballestero Y, Botran M, Bellon JM (2010) Clinical course and mortality risk factors in critically ill children requiring continuous renal replacement therapy. Intensive Care Med 36(5):843–849
Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, Blowey D, Bunchman TE, Brophy PD, Symons J, Chua A, Flores F, Somers MJ (2013) Continuous Renal Replacement Therapy for Children ≤10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr 162(3):587–592
DiCarlo JV, Alexander SR, Agarwal R, Schiffman JD (2003) Continuous veno-venous hemofiltration may improve survival from acute respiratory syndrome after bone marrow transplantation or chemotherapy. J Pediatr Hematol Oncol 25(10):801–805
Santiago MJ, López-Herce J, Urbano J, Solana MJ, del Castillo J, Sánchez A, Bellón JM (2013) Continuous renal replacement therapy in children after cardiac surgery. J Thorac Cardiovasc Surg 146(2):448–454
Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxyold NY, Brophy PD (2001) Pediatric acute renal failure: out come by modality and disease. Pediatr Nephrol 16(12):1067–1071
Selewski DT, Cornell TT, Lombel RM, Blatt NB, Han YY, Mottes T, Kommareddi M, Kershaw DB, Shanley TP, Heung M (2011) Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med 37(7):1166–1673
Boschee ED, Cave DA, Garros D, Lequier L, Granoski DA, Guerra GG, Ryerson LM (2014) Indications and outcomes in children receiving renal replacement therapy in pediatric intensive care. J Crit Care 29(1):37–42
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34(3):571–590
Slater A, Shann F, Pearson G, Paediatric Index of Mortality (PIM) Study Group (2003) PIM2: a revised version of the Pediatric Index of Mortality. Intensive Care Med 29(2):278–285
Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis (2005) International pediatric sepsis consensus conference: definition for sepsis and organ dysfunction inpediatrics. Pediatr Crit Care Med 6(1):2–8
Davison D, Basu RK, Goldstein SL, Chawla LS (2014) Fluid management in adults and children: core curriculum 2014. Am J Kidney Dis 63(4):700–712
Prowle JR, Chua HR, Bagshaw SM, Bellomo R (2012) Clinical review: volume of fluid resuscitation and the incidence of acute kidney injury—a systematic review. Crit Care 16(4):230
Akech S, Ledermann H, Maitland K (2010) Choice of fluids for resuscitation in children with severe infection and shock: systematic review. BMJ 341:c4416
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA (2011) Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 39(2):259–265
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Galasso, L., Emma, F., Picca, S. et al. Continuous renal replacement therapy in children: fluid overload does not always predict mortality. Pediatr Nephrol 31, 651–659 (2016). https://doi.org/10.1007/s00467-015-3248-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3248-6