Abstract
Background
The aim of this study was to analyze the long-term efficacy and safety of angiotensin-converting enzyme inhibitor (ACEi) and ACEi + angiotensin receptor blocker (ARB) treatments in a cohort of children with Alport syndrome (AS).
Methods
This was a respective review of 79 Chinese children with AS who received ACEi alone or combined ACEi + ARB therapy.
Results
The mean age of the pediatric patients with AS at onset of treatment was 8.6 ± 4.1 (range 1.5–16.3) years. The mean duration of follow-up was 2.5 ± 1.8 (range 0.5–7.8) years. For analysis, we separated the children into three groups according to proteinuria level before treatment, namely, <25, 25–50, and ≥50 mg/kg/day, respectively; after 1 year of treatment the proteinuria had decreased from 11.0 to 9.7 mg/kg/day, from 34.6 to 15.2 mg/kg/day, and from 73.0 to 50.0 mg/kg/day in each group, respectively. Proteinuria decreased significantly during the first 2 years of treatment and was stable from the third to fifth years of treatment. There was no statistically significant difference in the antiproteinuric effect of the ACEi and ACEi + ARB treatments in patients with severe or less severe mutations after 1 year of therapy. Five children stopped the ACEi + ARB treatment due to a decline in creatinine clearance.
Conclusion
Our findings demonstrate that early and long-term ACEi and ARB treatments in children with AS is efficient and well tolerated. The antiproteinuric effect of ACEi and ARB is of equal value in children with severe and less severe mutations in the COL4An gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alport syndrome (AS) is a hereditary renal disease characterized by persistent hematuria, proteinuria, and progressive renal failure [1]. Approximately 85 % of cases follow the X-linked pattern of inheritance and are caused by mutations in the COL4A5 gene [2]. The other 15 % of cases are caused by mutations in the COL4A3/COL4A4 genes, and the majority of these cases follow an autosomal recessive pattern of inheritance [3]. Males with X-linked AS (XLAS) and males and females with autosomal recessive AS (ARAS) almost always progress to end-stage renal disease (ESRD) during their second or third decade of life [4–6]. Thus, slowing the progression of AS is a meaningful treatment strategy before gene therapy can be applied.
Clinical practice recommendations for the treatment of children with AS, published in 2013 [7], recommend angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II type I receptor blockers (ARB) as the first-line and second-line agents, respectively, for treatment of these children. In 2011, a retrospective observational study [8] in Europe reported that use of ACEi delayed onset of dialysis and improved life expectancy. In addition, therapy initiated earlier in younger patients significantly delayed dialysis by 13 years compared to later therapy or no therapy in older siblings. A subsequent study [9] in the UK reported that losartan and enalapril had comparable efficacies for the reduction of proteinuria in children with AS. Moreover, there have been some reports of the efficacy and safety of ACEi and ARB in children with AS [10–12]. However, most AS patients presenting with ESRD are older than 20–30 years, and therefore whether the degree of proteinuria can be used to evaluate the efficacy of treatment in children with AS remains unclear.
To understand the efficacy and safety of ACEi and ARB treatments, we study analyzed the long-term efficacy and safety of ACEi and ARB treatments in a Chinese cohort of children with AS.
Patients and methods
Inclusion and exclusion criteria
Patients were selected for enrolment in this study from the registry database of hereditary kidney diseases in children in China. The clinical data and laboratory data compiled in the database were collected during patient visits to our hospital. The registry database was updated for each patient every 6 months. The inclusion criteria were (1) patients with a definitive diagnosis of AS proven by at least two methods, including kidney biopsy, skin biopsy, and/or mutation analysis; (2) age at onset of treatment ≤18 years; (3) treatment with ACEi and/or ARB; (4) follow-up of >6 months. The exclusion criteria were (1) uncertain diagnosis of AS or other hereditary kidney diseases and (2) follow-up of <6 months.
Clinical and laboratory data
This study was retrospective. Primary data, including age, gender, gene mutations, age at onset of treatment and at follow-up, degree of proteinuria (mg/kg/day), serum creatinine and creatinine clearance, and side effects of treatment, such as hyperkalemia, cough, hypotension and others, were collected. Deaths of any cause were recorded. Creatinine clearance (Ccr) was calculated using the standard formula U × V/P (ml/min), where (U is urine creatinine level, V is 24-h urine volume, and P is the serum creatinine level), and then corrected according to body surface area. The initial treatment was always with ACEi; if the proteinuria did not decrease after 6 months of treatment, ARB was added to the treatment regimen. Proteinuria levels at 1, 2, 3, 4, and 5 years after the initiation of treatment were compared to the proteinuria level before treatment. Gene mutations were divided into severe mutations (nonsense, deletion, insertion, and splicing mutations) and less severe mutations (missense mutations) [7].
Statistical analysis
Data are presented as mean ± standard deviation (SD) or as the median and range. The nonparametric paired Wilcoxon test was used to assess the differences in proteinuria from before treatment to each follow-up time point. A p value of <0.05 was considered to be statistically significant (SPSS software; IBM Corp., Armonk, NY).
Results
Patient characteristics
A total of 293 pediatric patients from 272 families were diagnosed with AS through to March 2014 in the genetics outpatient unit of Peking University First Hospital. Of these, 126 patients received ACEi treatment with or without ARB, and 79 of these were followed for >6 months and enrolled in this study. Among the 79 children enrolled in the study, 73 had XLAS (67 males, 6 females) and six had ARAS. Thirty-seven children were treated with ACEi, and 42 children were treated with ACEi + ARB. The average age at onset of treatment and at last follow-up was 8.6 ± 4.1 (range 1.5–16.3) and 11.1 ± 4.6 (mean 2.9–23.0) years, respectively. The mean duration of follow-up was 2.5 ± 1.8 (0.5–7.8) years.
Proteinuria in male patients with XLAS and male and female patients with ARAS
In this study, 67 male children with XLAS and six female and male children with ARAS were combined to form a single group of 73 children based on the similarity in their disease courses. The median age of these 73 children at onset of treatment was 7.9 (range 1.5–16.8) years. Of these 73 children, 70, 47, 37, 18, and 18 children had follow-up data for the time points of 1, 2, 3, 4, and 5 years after the initiation of treatment, respectively. Proteinuria data before and after treatment initiation are shown in Table 1.
Of the 73 children, 32 were treated with ACEi, and the other 41 were treated with ACEi +ARB. In the ACEi treatment group, the median age at onset of treatment was 7.0 (range 1.5–14.9) years, the median proteinuria level before treatment was 19.0 (6.7 mg–122.1) mg/kg/day, and the proteinuria level decreased to 9.2 (3.8–79.3) mg/kg/day after 1 year of treatment. In the ACEi + ARB treatment group, the median age at onset of treatment was 9.1 (3.8–16.8) years, median proteinuria level before treatment was 42.9 (range 5.9–93.1) mg/kg/day, and the proteinuria level decreased to 30.2 (range 4.2–86.2) mg/kg/day after 1 year of treatment.
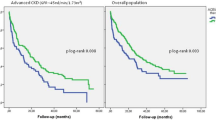
These 73 children were separated into three groups according to their proteinuria level before treatment: <25 mg/kg/day (group 1, n = 35); 25–50 mg/kg/day (group 2, n = 15); ≥50 mg/kg/day (group 3, n = 23). The changes in proteinuria in these three groups from before treatment initiation to 1 year after the initiation of treatment are shown in Table 2. Proteinuria was stable in group 1 and decreased significantly in groups 2 and 3, although it was still pronounced in group 3 after 1 year of treatment. Proteinuria at each time point after treatment in the three groups of children is shown in Fig. 1.
Follow-up of proteinuria at various time points after treatment in the three groups of children. a Group 1, proteinuria level prior to initiation of treatment with <25 mg/kg/day, b group 2, proteinuria level before start of treatment with 25–50 mg/kg/day, c group 3, proteinuria level before start of treatment with ≥50 mg/kg/d
At the beginning of treatment, six children (8 %) were <3 years old, 18 (25 %) were 3–6 years old, and 49 (67 %) were 6–18 years old. The proteinuria level before initiation of treatment in each of these three age groups was 37.1 ± 16.6 (<3 years old), 18.1 ± 14.6(3–6 years old), and 44.0 ± 35.2 mg/kg/day (6–18 years old). After 1 year of treatment, proteinuria had decreased in six (100 %), eight (57 %), and 34 (71 %) of the children in these three groups (<3 years old, 3–6 years old, and 6–18 years old at beginning of treatment, respectively).
Proteinuria in the heterozygous XLAS carriers
Six of the children with AS in the study were female heterozygous XLAS carriers. Only one of these was treated with ACEi + ARB; the other five patients were treated with ACEi alone. The age at onset of treatment among these six patients ranged from 7.1 to 16.3 years. The proteinuria level before treatment ranged from 11.6 to 21.5 mg/kg/day; after 2 years of treatment, the proteinuria level had decreased to <11.0 mg/kg/day in all six patients.
Proteinuria and gene mutations
Pathogenic mutations in the COL4An gene were detected in 64 children (64/71, 90.1 %). To examine the correlation between gene mutation status and the antiproteinuric effect of ACEi and ARB in children with ARAS and male patients with XLAS, we analyzed 57 children. Twenty-eight children were found to have less severe mutations (missense mutations) in the COL4An gene, and 29 children had severe mutations (deletion, nonsense and splicing mutations). In the group of children with less severe mutations, the average age at onset of treatment was 7.6 years; at 1 year after treatment-initiation, the urinary protein level had decreased or remained the same in 50 % of children, and the median urinary protein level had decreased from 40.8 to 28.2 mg/kg/day. In the group of children with severe mutations, the average age at onset of treatment was 8.6 years old; at 1 year after treatment initiation, the urinary protein level had decreased or remained the same in 45 % of children, and the median urinary protein had decreased from 41.0 to 25.9 mg/kg/day. None of these values were significantly different between the two groups (p > 0.05).
Renal function
In our study cohort, 72 of the 79 (91 %) children had normal Ccr during follow-up, whereas Ccr declined in the remaining seven (9 %) children during follow-up (Ccr <80 ml/min × 1.73 m2) (Table 3). All patients who experienced a decline in Ccr were males with XLAS and treated with ACEi + ARB. Two children presented with lower than normal Ccr at the beginning of treatment (patients no. 5 and 27). For the children with lower than normal and normal Ccr, the age at onset of therapy was 10.8 ± 3.1 and 8.4 ± 4.1 years, respectively (p < 0.05), and the proteinuria level at the start of therapy was 67.2 and 23.0 mg/kg/day, respectively (p < 0.05). The median age at initiation of a decline in Ccr was 13.0 (range 9.5–18.0) years. Five patients with lower than normal Ccr stopped treatment during the follow-up period. One patient died at 16 years of age from a cerebrovascular event due to a lack of renal replacement therapy; he had received ACEi + ARB treatment since the age of 9.7 years, and the treatment was stopped at 13.8 years due to a decline in Ccr.
Safety
One male patient with XLAS developed hyperkalemia after 3 years of ACEi + ARB therapy, which resolved after the medicine was stopped. Five children stopped ACEi + ARB treatment due to a decline in Ccr. Other recorded adverse effects included dry cough in three children receiving ACEi treatment and symptomatic hypotension in two children receiving ACEi + ARB treatment.
Discussion
The results of this study demonstrate that the long-term use of ACEi and ARB efficiently decreased proteinuria in our study population of children with AS and that this therapy was well tolerated. Of the 79 children enrolled in the study, 73 were males with XLAS or female and male children with ARAS. The other six children were female heterozygous XLAS carriers. The average age at the initiation of treatment in this study was 8.6 years. Of the 73 children with XLAS or ARAS, 70, 47, 37, 18 and 18 children had follow-up data for the time points of 1, 2, 3, 4, and 5 years after the initiation of treatment, respectively. This is the largest retrospective study of ACEi and ARB therapy in a cohort of children with AS in China.
We found that proteinuria decreased significantly in the first 2 years of ACEi and ARB treatment in these children but that unfortunately it gradually returned to its level before the onset of treatment or even increased beyond that level after 3 years of treatment. This trend is similar to that reported by Gross et al. [8] who observed that ESRD was delayed by therapy with ACEi in a time-dependent manner, with earlier treatment correlating with better efficacy. However, data on the efficacy and safety of the early use of ACEi and ARB in children with AS remain scarce.
In 2002, Adler et al. [13] reported that routine ACEi treatment may not be warranted in children with early AS, based on observations of 11 children with early AS (mean urine albumin:creatinine ratio 1.2 mg/mg) who were treated with enalapril for 14 days. In 2004, a study of ten children (median age 10.25 years) with AS who had been treated with enalapril for 5 years was reported [14]. The results showed that enalapril reduced urinary protein excretion and preserved glomerular filtration in AS patients; one of the patients in this study developed chronic kidney disease within 5 years of treatment. Recently, a report from the UK demonstrated that losartan significantly lowered proteinuria and was well tolerated after 12 weeks of treatment in 15 children with AS (median age 12 years) [12]. The average age at initiation of the ACEi and ARB treatments in the 79 children enrolled in our study was 8.6 years, which is younger than that reported in previous studies. After 1 year of treatment, proteinuria had decreased in all six children aged <3 years at treatment initiation and in 8/18 children who were 3–6 years old at the start of treatment. All children who aged <6 years at the start of the treatment had normal Ccr throughout the course of treatment. In addition, after 1 year of treatment, proteinuria had decreased significantly in children with proteinuria of 25–50 mg/kg/day before treatment initiation, and proteinuria was maintained at a low level in children with proteinuria of <25 mg/kg/day before treatment initiation. In children with proteinuria of ≥50 mg/kg/day before the start of treatment, proteinuria decreased significantly from 73.0 to 50.0 mg/kg/day, but the proteinuria level obtained during treatment remained high. These results demonstrate that the early application of ACEi and ARB therapy in children with AS reduced proteinuria and was well tolerated. Thus, our data support of the early use of ACEi and ARB in children with AS.
In the children enrolled in our study, ACEi was the first therapy to be used; if the proteinuria did not decrease after 6 months of treatment, ARB was added to the treatment regimen. For the children treated with ACEi alone and those with ACEi + ARB, the median age at onset of treatment was 7.0 and 9.1 years, respectively, and the proteinuria level before treatment was 19.0 and 42.9 mg/kg/day, respectively. After 1 year of treatment, the proteinuria levels in both groups of children had decreased. Due to the differences between the ACEi alone and the ACEi + ARB treatment groups, we did not compare them in terms of their effect on proteinuria outcome. It is worth noting that there were no severe side effects recorded in children who underwent ACEi treatment. In contrast, of the children who received ACEi + ARB treatment, seven presented with declined Ccr and one developed hyperkalemia. Based on this result, we propose that Ccr should be monitored during the follow-up period in children receiving ACEi or ACEi + ARB treatment, especially those receiving ACEi + ARB treatment.
Seven children in our study presented with reduced Ccr (Ccr <80 ml/min × 1.73 m2) during the follow-up. Compared to the children with normal Ccr, the mean age at initiation of treatment of these 7 children was older and the proteinuria more severe. Therefore, we recommend the early use of ACEi and ARB in children with AS, as well as regular follow-up by a nephrologist. For the children who presented with declined Ccr after therapy with ACEi or ACEi + ARB, terminating the drug therapy did not result in the Ccr returning to the normal level. This suggests that the observed decline in Ccr in children with AS in our study may not have been related to the choice of treatment. However, ACEi and ARB should be used with great caution in children presenting with declined Ccr. Side effects of ACEi and ARB were observed in six (7.6 %) children in this study, including dry cough, symptomatic hypotension, and hyperkalemia. In general, ACEi and ARB therapies were well tolerated.
In conclusion, early and long-term treatment with ACEi and ARB in children with AS is efficient and well tolerated. Nevertheless, we suggest monitoring Ccr during follow-up for children with AS being treated with ACEi and ARB. In the future, we will continue to follow the children included in this study to confirm that a reduction of proteinuria helps delay ESRD in AS patients. Moreover, future studies are required to identify new biomarkers of disease progression and treatment efficiency for children with AS.
References
Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K (1990) Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248:1224–1227
Nagel M, Nagorka S, Gross O (2005) Novel COL4A5, COL4A4, and COL4A3 mutations in Alport syndrome. Hum Mutat 26:60
Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schröder CH, Smeets HJ (1994) Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8:77–81
Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Verellen C, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC (2000) X-linked Alport syndrome: natural history in 195 families and genotype- phenotype correlations in males. J Am Soc Nephrol 11:649–657
Wang F, Zhao D, Ding J, Zhang H, Zhang Y, Yu L, Xiao H, Yao Y, Zhong X, Wang S (2012) Skin biopsy is a practical approach for the clinical diagnosis and molecular genetic analysis of x-linked Alport’s syndrome. J Mol Diagn 14:586–593
Zhang Y, Wang F, Ding J, Zhang H, Zhao D, Yu L, Xiao H, Yao Y, Zhong X, Wang S (2012) Genotype-phenotype correlations in 17 Chinese patients with autosomal recessive Alport syndrome. Am J Med Genet A 158A:2188–2193
Kashtan CE, Ding J, Gregory M, Gross O, Heidet L, Knebelmann B, Rheault M, Licht C, Alport Syndrome Research Collaborative (2013) Clinical practice recommendations for the treatment of Alport syndrome: a statement of the Alport Syndrome Research Collaborative. Pediatr Nephrol 28:5–11
Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Study Group Members of the Gesellschaft für Pädiatrische Nephrologie, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M (2012) Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81:494–501
Webb NJ, Shahinfar S, Wells TG, Massaad R, Gleim GW, McCrary Sisk C, Lam C (2013) Losartan and enalapril are comparable in reducing proteinuria in children with Alport syndrome. Pediatr Nephrol 28:737–743
Li JG, Ding J, Wang F, Zhang HW (2009) Drugs controlling proteinuria of patients with Alport syndrome. World J Pediatr 5:308–311
Proesmans W, Knockaert H, Trouet D (2000) Enalapril in paediatric patients with Alport syndrome: 2 years’ experience. Eur J Pediatr 159:430–433
Webb NJ, Lam C, Shahinfar S, Strehlau J, Wells TG, Gleim GW, Le Bailly De Tilleghem C (2011) Efficacy and safety of losartan in children with Alport syndrome—results from a subgroup analysis of a prospective, randomized, placebo- or amlodipine-controlled trial. Nephrol Dial Transplant 26:2521–2526
Adler L, Mathew R, Futterweit S, Frank R, Gauthier BG, Kashtan CE, Trachtman H (2002) Angiotensin converting enzyme inhibitor therapy in children with Alport syndrome: effect on urinary albumin, TGF-beta, and nitrite excretion. BMC Nephrol 3:2
Proesmans W, Van Dyck M (2004) Enalapril in children with Alport syndrome. Pediatr Nephrol 19:271–275
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical disclosure
This study was approved by the Ethical Committee of Peking University First Hospital and parental/patient consent was not required due to the retrospective nature of the study.
Financial statement
This study was supported by grants from the National Nature Science Foundation (30801252 81070545, and 81400685), the Beijing Nature Science Foundation (7102148), and the National “Twelfth Five-Year” Science and Technology Support Project (2012BAI03B02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, F., Ding, J. et al. Long-term treatment by ACE inhibitors and angiotensin receptor blockers in children with Alport syndrome. Pediatr Nephrol 31, 67–72 (2016). https://doi.org/10.1007/s00467-015-3184-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3184-5