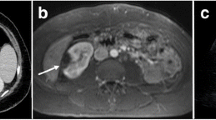
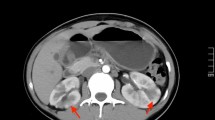
Abstract
Background-Aim
Acute focal bacterial nephritis (AFBN), renal abscess and pyonephrosis are uncommon and not fully addressed forms of urinary tract infection (UTI) which may be underdiagnosed without the appropriate imaging studies. Here, we review the characteristics and outcome of these renal entities in children managed at a single medial centre.
Patients and Methods
The medical files of all children hospitalized for episodes of AFBN, renal abscess and pyonephrosis during a 10-year period (2003–2012) were reviewed.
Results
Among the 602 children hospitalized for UTI, 21 presented with AFBN, one with abscess and three with pyonephrosis. All 25 children (13 girls), ranging in age from 0.06 to13.4 years, were admitted with fever and an impaired clinical condition, and 18 had urological abnormalities. More than one lesion, often of different types, were identified in 11 episodes. Urine cultures from 13 episodes grew non-Escherichia coli pathogens and those from two episodes were negative. Antibiotics were administered for 14–60 days, and emergency surgery was required in three cases. During follow-up, 13 patients underwent corrective surgery. Permanent renal lesions were identified in 16 patients.
Conclusions
AFBN, renal abscess and pyonephrosis should be suspected in children with severe presentation and urological history. Appropriate imaging is crucial for management planning. Prognosis is often guarded despite appropriate treatment. Based on the results of this study we propose a management algorithm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI), a common clinical problem in infants and children, usually runs an uncomplicated course but has the potential of severe manifestations and long-term sequelae [1]. The severity of UTI depends on diverse factors, including urinary tract malformations, pathogen–host interactions and promptness of management [2, 3]. Febrile UTI usually takes the form of pyelonephritis. The more serious forms, including acute focal bacterial nephritis (AFBN), renal abscess and pyonephrosis, are uncommon and may be clinically indistinguishable from pyelonephritis, or may mimic abdominal inflammatory processes or even tumours [4–6]. Timely diagnosis is important, as longer antibiotic treatment and occasionally surgery are required [5–7].
Progress in imaging has greatly facilitated the diagnosis of these entities. However, ultrasound (US) has a low sensitivity for renal lesions and, consequently, more sensitive modalities, such as computed tomography (CT) or scintigraphy, are often required for an accurate diagnosis [8–10]. Magnetic resonance urography (MRU) has recently emerged as a technique which enables noninvasive evaluation of many abnormalities of the urinary tract, providing combined structural and functional information; such attributes have led to claims that it is the most powerful diagnostic tool currently available [11].
These improvements in imaging techniques have resulted in an increasing number of AFBN cases being recognised, suggesting that AFBN is not as unusual as previously considered [12–21]. AFBN is a localised, bacterial, inflammatory renal mass without liquefaction which affects one or more renal lobules; it is regarded as the midpoint between pyelonephritis and renal abscess [8]. Renal abscess is better defined as a focal, walled-off purulent parenchymal cavity, but it remains a diagnostic challenge due to diverse origins of infection and different pathogenetic mechanisms among patients, such as complicating UTI arising from haematogenous spread, or staphylococcal carbuncle in rare cases of proximity to an infected area [5, 10]. Information on pyonephrosis remains more scanty and is based mainly on case reports [6, 22].
As policies for UTI management have recently called for a radical reduction in the use of imaging for clinical purposes [23] and/or the limitation of imaging mainly to US [24], the forms of UTI described above may be underdiagnosed. In this study we report our clinical experience with children with AFBN, renal abscess and pyonephrosis in a referral centre over a 10-year period, focusing on the distinct clinical, laboratory and imaging characteristics. Based on our results, we propose a management algorithm.
Patients and methods
Patients
We reviewed the medical records of all children hospitalized with UTI in the Department of Pediatrics, Heraklion University Hospital during the 10-year period (January 2003 to December 2012), looking for severe, complicated forms of infection. Heraklion University Hospital is the referral centre for the island of Crete, with a coverage population of 105,000 children aged <15 years. All medical records of children with AFBN, renal abscess and pyonephrosis were retrieved, and the clinical, laboratory, imaging and follow-up information were recorded. All children had a complete workup with urine and blood culture before treatment was initiated and also underwent US investigation of kidneys and bladder within 3 days of admission. Both investigations are in accordance with our Department’s policy for inpatient UTI management, which includes an acute phase US for every child sick enough to require hospitalization for a UTI. The decision for further imaging during hospitalization was based on US findings of focal hyperechogenic or hypoechogenic renal lesions, unilateral or bilateral nephromegaly or inconclusive abnormal findings and/or complicated clinical course, i.e. no improvement after 72 h on antibiotics. MRU was preferred over CT when available to avoid exposure to radiation.
Definitions
Abnormal urinalysis was defined as positive leucocyte esterase and/or nitrites or a leucocyte count of >10 per high power field. Positive urine cultures were defined as any bacterial growth in specimens obtained by suprapubic aspiration, isolation of ≥104 colony-forming units (CFU)/ml of a single organism in specimens obtained by transurethral catheterization or the isolation of >105 CFU/ml of a single organism in mid-stream specimens. The diagnosis of AFBN, renal abscess and pyonephrosis was based on US and/or CT/MRU findings [7, 16, 18, 19].
Follow-up
All but two cases involved children with complicated UTI who underwent voiding cystourethrography (VCUG) and dimercaptosuccinic acid (DMSA) scan on average 4 weeks and 6 months, respectively, after the acute episode. Repeat US was performed on demand for each case during hospitalization and in all patients before discharge and at 1 and 3 months thereafter. All children were followed-up in the hospital’s paediatric nephrology outpatient clinic.
Statistics
Children with and without underlying conditions were compared for a number of variables, including age, microbiology, clinical presentation and course and laboratory indexes, using the chi-square test for categorical variables and either the t test or the Mann–Whitney test for numerical variables, with 0.05 as the cut-off for statistical significance.
Results
Frequency
From a total of 683 episodes of UTI in 602 children, 23 episodes of AFBN (3.4 %) were identified in 21 children; one child presented with 3 distinct episodes (Patient 9; Table 1). Isolated renal abscess was observed in a single child (patient 22) and was of ascending origin, while in 2 children (patients 20 and 21) AFBN progressed to abscess. Pyonephrosis was observed in three children and coexisted with AFBN in an additional three children (patients 1, 3 and 12). Thus, AFBN coexisted with either pyonephrosis or abscess in five of the 21 children (23.8 %) diagnosed with AFBN. No other forms of complicated UTI, such as carbuncles or xanthogranulomatous pyelonephritis, were identified.
Clinical presentation
The children included in the study ranged in age from 0.06 to 13.4 (median 4.9) years. All presented with an impaired condition and high temperature (≥39° C in 20/27 episodes) of 1 h to 12 days (average 56 h) duration prior to admission. In 11 episodes, the diagnosis at admission was not UTI, including appendicitis (patients 3, 9a and 17), gastroenteritis (patients 2 and 9c), renal tumour (patients 5 and 20), meningitis (patients 10 and 13) and renal stone (patient 21). A urological history was recalled in 15 children (Table 1), of whom 12 were on antibiotic prophylaxis. With the exception of two (patients 12 and 25) with chronic renal failure, chronic diseases were not reported.
Laboratory findings
Laboratory findings on admission included leucocytosis (range 5,200–31,360, median 17,300 white blood cells/mm3), elevated erythrocyte sedimentation rate (range 13–140, median 68 mm/h) and C-reactive protein (range 2.21–41.2, median 15.4 mg/dL). A reversible increase of creatinine was observed in 15 episodes, as well as in the two children with chronic renal failure. Abnormal urinalysis was found in 16 episodes, and urine cultures grew Eschericia coli in 12 episodes, Pseudomonas aeruginosa in eight episodes, Klebsiella pneumoniae in three episodes and Proteus mirabilis and P. stuartii in one episode each; the cultures were sterile in the two recurrent episodes of patient 9. Blood cultures were positive in one child with AFBN (patient 13) and in all three cases of pyonephrosis (patients 23, 24 and 25); all of these grew P. aeruginosa, the same isolates as identified in these patients’ urine cultures. Three of the youngest patients (patients 10, 13 and 23) had cerebrospinal fluid pleocytosis (32, 23, and 15 white cells/mm3, respectively).
Diagnosis
Renal US identified the type of lesion in 17/27 episodes, revealed non-specific findings of nephromegaly and/or increased parenchymal echogenicity in seven episodes, was inconclusive in one episode and was falsely negative in two episodes. In 20 and five episodes the diagnosis was confirmed by MRU and CT, respectively, both of which were performed within the first week of admission. The decision for MRU/CT was based on fever persistence while under treatment (14 episodes), suspicion of complicated anatomy (5 episodes) and inconclusive US imaging (6 episodes); US imaging was sufficiently diagnostic in the remaining two episodes.
Treatment
All children received intravenous antibiotics for 6–52 (median 13) days and completed their course with oral medication for a total of 14–60 (median 21) days. The switch to oral treatment was guided by defervescence, improvement of inflammatory indexes and repeat US findings. Fever subsided after a median time of 48 h (range 12 h to 5 days) from treatment onset. Fever subsided in two children (patients 22 and 25) following surgical drainage on the fifth and third day after hospitalization, respectively, and in one patient (patient 24) after spontaneous drainage from an ectopic orifice on the fourth day after hospitalization. Patient 25 was severely septic and hypotensive and remained in the Intensive Care Unit for 5 days. A male neonate (patient 23) underwent nephrectomy of the affected kidney due to persistent bacteraemia, despite appropriate antibiotic treatment for 1 month. A 4.5-year-old girl (patient 9), with left side grade III vesicoureteral reflux (VUR), was readmitted with new AFBN episodes within 6 months of the initial one, despite complete resolution of the initial lesion and administration of prophylaxis.
Further imaging
Voiding cystourethrography was performed on 23 patients and revealed VUR in 14 patients (VUR grades III–V in 12 patients, bilateral in 7 patients; Table 1), with seven being newly diagnosed. Two adolescents were not investigated (patients 6 and 7), but one of these had tested negative for VCUG in infancy. All children had a DMSA scan 6 months after admission; parenchymal defects were identified in 23 of these children, and these defects persisted on the subsequent DMSA scans of 16 of them.
Children with underlying urological conditions (n = 18) differed from those with normal urinary tracts (n = 7) in age of presentation (median ages 3.1 and 6.7 years, respectively; p = 0.04) and microbiology (non-E.coli pathogens 0/7 and 11/18, respectively; p = 0.009). No other differences were identified.
Outcome
Children were followed-up for 0.9–10 (median 3.5) years. Surgical VUR correction was required for eight children with high-grade VUR and recurrent infection. Corrective surgery was required for an additional two children with duplication and obstructive upper moiety (semi-nephrectomy on affected side): a boy with hypospadias and urethral diverticulum (urethral diverticulotomy and reconstruction) and a boy with acute ureterostomies (sequential ureteral re-implantation). The remaining 13 children with AFBN were medically managed—five for mild VUR, three for unspecified bladder dysfunction, one for hypercalciuria and four for recurrent mild UTI. One of the latter children, who had a renal graft and an augmented bladder, had a favourable course with self-catheterization.
Discussion
Acute focal bacterial nephritis, renal abscess and pyonephrosis, once considered to be unusual manifestations in children, are being increasingly reported due to improvements in the sensitivity of imaging techniques that enables them to be visualised [17]. The exact frequency of these three clinical entities is not known, and our literature search failed to identify studies that included the whole spectrum of severe UTI. In our paediatric patient population, AFBN, renal abscess and pyonephrosis were identified in nearly 4 % of children hospitalized for UTI, which is in accordance to the rates of AFBN reported from the USA, Germany and Israel [17–19], whereas studies from Taiwan have reported AFBN rates of 8–10 % [21, 25]. Most of the reported episodes occurred in children beyond infancy with a previous urological history. Initial presentation often mimicked other febrile conditions and resulted in diagnostic delays [5, 16, 18, 19, 22].
Multiple lesions and the co-existence of different types of lesions were found in nearly half of our patients. Cheng et al. found that abscess was accompanied by either AFBN or pyelonephritis in all of the paediatric patients included in their study [10] and suggested that this co-existence argues against the concept that AFBN is the midpoint between pyelonephritis and abscess [26]. We rather believe that the renal infectious process takes the form of a continuum from pyelonephritis to abscess of ascending origin and xanthogranulomatous pyelonephritis, with the different forms of the same process possibly associated with urinary tract anatomy, renal vascular bed, bacterial properties or host response to infection. Pyonephrosis is also included in the same inflammatory spectrum in obstructive or dilated collecting systems, as it was noted in both of our patients with obstructive malformations and in those children with dilated VUR and AFBN. These latter cases, representing infected hydronephrosis rather than pure pyonephrosis, can be considered as a stage of the infectious process in the presence of severe dilatation and probably intrarenal reflux, as was the case in two of our patients.
The bacterial spectrum of AFBN and of renal abscess of ascending origin does not differ from that of other forms of UTI [6, 7, 10, 18–20, 23, 27]. In accordance with the findings reported in a study from Germany that included children with acute focal bacterial nephritis [19], we also found that non-E. coli pathogens prevailed in our patients, probably due to the inclusion of pyonephrosis cases, which were all due to P. aeruginosa, and to high rates of urinary malformations and prophylaxis [3, 28]. In our study, urine cultures were sterile in two AFBN episodes, despite abnormal urinalysis, as has already been reported but not adequately explained in earlier studies [9, 16, 18, 19]. Haematogenous spread of infection could be a potential mechanism in these cases.
Acute phase renal US can provide diagnostic help, but less extensive lesions may be missed [8]. In our paediatric patient population, 17 cases had suggestive US presentation, including all of the pyonephrosis cases, and seven additional cases had non-specific findings. CT is considered to be the most sensitive and specific diagnostic method for imaging parenchymal and suppurative renal lesions [4, 5, 10, 18]. To our knowledge, MRU is not yet routinely used for the diagnosis of AFBN, abscess and pyonephrosis in medical institutions but in our experience it provides accurate information on the type of renal lesions and underlying urological abnormalities that can guide early or late surgery decisions.
The administration of intravenous antimicrobials until 2–3 days of defervescence and a total treatment duration of at least 2 weeks are generally recommended in AFBN [4, 7, 16–18]. Our experience with AFBN suggests at least 1 week of intravenous treatment and a total antibiotic course of 3 weeks. Abscesses often require drainage, but medical management alone might be sufficient, as was the case for two of our patients [5, 10]. Antibiotic treatment for 4 weeks is required in any case [5, 10]. Nephrectomy is exceptional as a treatment for pyonephrosis; however drainage is unavoidable in most cases [22]. All of our children with pyonephrosis and the one patient with renal/perirenal abscess required corrective surgery after the acute phase. High rates of renal scarring despite antibiotic treatment have been reported in children with AFBN, and this was also the case in 16/25 of our patients, in whom persistent parenchymal defects corresponding with the acute site of infection were identified [21]. Because most of our patients had pre-existing urological abnormalities, the possibility of congenital lesions cannot be completely excluded. During the follow-up period eight children were referred for surgical correction of VUR and remained UTI-free after surgery. All except the two children with chronic renal failure maintained normal renal function and blood pressure.
Although we acknowledge the limitations of our retrospective, single-center study, we believe that our findings are representative of the single referral unit of Crete with both medical and surgical facilities for children and allow for the proposal of a management algorithm for severe UTI forms (Fig. 1). According to current suggestions for UTI management, guidance for children of older ages is very limited [23], and although ultrasound is favoured as a first-line, non-invasive imaging modality [24, 29], concerns of non-specific and probably misleading findings have been raised regarding its use during acute phase [24]. Our policy of acute phase US of severely affected children, irrespective of age and treatment response, facilitated the diagnosis of severe manifestations and reduced the possibility of missed cases of AFBN with a more benign course which, however, cannot be completely eliminated. However, it should be stressed that not only the clinical presentation but also the patient’s history and the diagnostic questions which needed to be answered were important in guiding the decision for acute imaging, which cannot be proposed unconditionally.
Conclusions
In conclusion, AFBN, renal abscess and pyonephrosis may co-exist and should be suspected in children—especially in older children—with severe clinical presentation and urological abnormalities. Acute phase US is essential for the diagnosis of these entities, but further imaging is usually required. It is important that future studies address the true incidence and the optimal management of AFBN, abscess and pyonephrosis among children with febrile UTI.
References
Wald E (2004) Urinary tract infections in infants and children: a comprehensive overview. Curr Opin Pediatr 16:65–68
Jahnukainen T, Chen M, Celsi G (2005) Mechanisms of renal damage owing to infection. Pediatr Nephrol 20:1043–1053
Bitsori M, Maraki S, Raissaki M, Bakantaki A, Galanakis E (2005) Community-acquired enterococcal urinary tract infections. Pediatr Nephrol 20:1583–1586
Cheng CH, Tsau YK, Lin TY (2006) Effective duration of antimicrobial therapy for the treatment of acute lobar nephronia. Pediatrics 117:e84–e89
Angel C, Shu T, Green J, Orihuela E, Rodriguez G, Hendrick E (2003) Renal and peri-renal abscess in children: proposed physio-pathologic mechanisms and treatment algorithm. Pediatr Surg Int 19:35–39
Sharma S, Mohta A, Sharma P (2004) Neonatal pyonephrosis—a case report. Int Urol Nephrol 36:313–315
Rathore NH, Barton LL, Luisiri A (1991) Acute lobar nephronia: a review. Pediatrics 87:728–734
Cheng CH, Tsau YK, Hsu SY, Lee TL (2004) Effective ultrasonographic predictor for the diagnosis of acute lobar nephronia. Pediatr Infect Dis J 23:11–14
Lavocat MP, Granjon D, Allard D, Gay C, Freycon MT, Dubois F (1997) Imaging of pyelonephritis. Pediatr Radiol 27:159–165
Cheng CH, Tsai MH, Su LH, Wang CR, Lo WC, Tsau YK, Lin GJ, Huang YC, Chiu CH, Lin TY (2008) Renal abscess in children: a 10-year clinical and radiologic experience in a tertiary medical center. Pediatr Infect Dis J 27:1025–1027
Cerwinka WH, Grattan-Smith D, Kirsch AJ (2008) Magnetic resonance urography in pediatric urology. J Pediatr Urol 4:74–83
Rosenfield AT, Glickman MG, Taylor KJ, Crade M, Hodson J (1979) Acute focal bacterial nephritis (acute lobar nephronia). Radiology 132:553–561
Lawson GR, White FE, Alexander FW (1985) Acute focal bacterial nephritis. Arch Dis Child 60:475–477
Shimizu M, Katayama K, Kato E, Miyayama S, Sugata T, Ohta K (2005) Evolution of acute focal bacterial nephritis into a renal abscess. Pediatr Nephrol 20:93–95
Greenfield SP, Montgomery P (1987) Computerized tomography and acute pyelonephritis in children, a clinical correlation. Urology 29:137–140
Kline MW, Kaplan SL, Baker CJ (1988) Acute focal bacterial nephritis: diverse clinical presentations in pediatric patients. Pediatr Infect Dis J 7:346–349
Uehling DT, Hahnfeld LE, Scanlan KA (2000) Urinary tract abnormalities in children with focal bacterial nephritis. BJU Int 85:885–888
Klar A, Hurvitz H, Berkun Y, Nadjari M, Blinder G, Israeli T, Halamish A, Katz A, Shazberg G, Branski D (1996) Focal bacterial nephritis (lobar nephronia) in children. J Pediatr 128:850–853
Seidel T, Kuwertz-Bröking E, Kaczmarek S, Kirschstein M, Frosch M, Bulla M, Harms E (2007) Acute focal bacterial nephritis in 25 children. Pediatr Nephrol 22:1897–1901
Yang CC, Shao PL, Lu CY, Tsau YK, Tsai IJ, Lee PI, Chang LY, Huang LM (2010) Comparison of acute lobar nephronia and uncomplicated urinary tract infection in children. J Microbiol Immunol Infect 43:207–214
Cheng CH, Tsau YK, Chang CJ, Chang YC, Kuo CY, Tsai IJ, Hsu YH, Lin TY (2010) Acute lobar nephronia is associated with a high incidence of renal scarring in childhood urinary tract infections. Pediatr Infect Dis J 29:624–628
Patel R, Nwokoma N, Ninan GK (2013) Primary neonatal MRSA pyonephrosis. Int Urol Nephrol 45:939–942
National Institute for Health and Clinical Excellence (2007) Urinary tract infection in children: diagnosis, treatment and long-term management. Available at: http://www.nice.org.uk/nicemedia/pdf/CG54fullguideline.pdf
Roberts KB, Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management (2011) Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128:595–610
Cheng CH, Tsau YK, Chen SY, Lin TY (2011) Clinical courses of children with acute lobar nephronia correlated with computed tomographic patterns. Pediatr Infect Dis J 28:300–303
Cheng CH, Tsau YK, Lin TY (2010) Is acute lobar nephronia the midpoint in the spectrum of upper urinary tract infections between acute pyelonephritis and renal abscess? J Pediatr 156:82–86
Stojanovic MM, Micic SR, Milovanovic DR, Jancovic SM (2009) Risk factors for treatment failure in renal suppurative infections. Int Urol Nephrol 41:319–325
Bitsori M, Maraki S, Koukouraki S, Galanakis E (2012) Pseudomonas aeruginosa urinary tract infection in children: risk factors and outcomes. J Urol 187:260–264
Ammenti A, Cataldi L, Chimenz R, Fanos V, La Manna A, Marra G, Materassi M, Pecile P, Pennesi M, Pisanello L, Sica F, Toffolo A, Montini G (2012) Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow-up. Acta Paediatr 101:451–457
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bitsori, M., Raissaki, M., Maraki, S. et al. Acute focal bacterial nephritis, pyonephrosis and renal abscess in children. Pediatr Nephrol 30, 1987–1993 (2015). https://doi.org/10.1007/s00467-015-3141-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3141-3