Abstract
Background
To evaluate the long-term efficacy of single-balloon enteroscopy endoscopic retrograde cholangiography (SBE-ERC) for the treatment of biliary obstruction and to analyze the factors affecting the recurrence of benign bilioenteric anastomotic stricture after SBE-ERC treatment.
Methods
The clinical data of patients with biliary diseases treated with SBE-ERC after choledochojejunostomy in our hospital from January 2015 to December 2021 were analyzed retrospectively for the success rates of diagnosis and treatment and the incidence of complications. Patients who were diagnosed with benign bilioenteric anastomotic stricture were followed up. The independent factors affecting recurrence were obtained by univariate and multivariate analyses using the Kaplan‒Meier method and Cox proportional hazard regression model.
Results
A total of 289 SBE-ERCs were performed in 165 patients. The overall success rate was 83.0% (240/289). The incidence of postoperative complications was 5.2% (15/289). The 108 successfully treated patients diagnosed with benign bilioenteric anastomotic stricture were followed up. Twenty-six percent (29/108) of patients had recurrent stricture after SBE-ERC. The biliary patency rates at 1 year, 2 years and 5 years after SBE-ERC were 90.1%, 69.3%, and 53.9%, respectively. Single-factor analysis revealed the absence of intrahepatic biliary gas imaging during endoscopy (\({\chi }^{2}\)=5.366, P = 0.021), a diameter of balloon dilatation during the last endoscopic treatment less than 0.8 cm (\({\chi }^{2}\)=4.552, P = 0.033), and the presence of a thread in the anastomosis (\({\chi }^{2}\)=8.921, P = 0.003) as risk factors for recurrence. A non-indwelling biliary plastic stent (\({\chi }^{2}\)=14.868, P < 0.001) and undergoing only one ERCP treatment (\({\chi }^{2}\)=13.313, P = 0.001) were risk factors for the recurrence of benign stricture after SBE-ERC resection. Multivariate analysis revealed that the absence of a stent (HR = 0.15, 95% CI 0.06–0.40, P = 0.001), absence of intrahepatic biliary gas imaging during endoscopy (HR = 0.39, 95% CI 0.17–0.91, P = 0.03) and the presence of a thread in the anastomosis (HR = 3.69, 95% CI 1.59–8.57, P = 0.002) were independent risk factors for stricture recurrence.
Conclusions
Treating biliary disease after choledochojejunostomy with SBE-ERC is safe and effective, with a good immediate technical success rate and an acceptable incidence of complications. SBE-ERC has long-term efficacy in the treatment of benign bilioenteric anastomotic stricture. The absence of intrahepatic biliary gas imaging during endoscopy, non-indwelling biliary stents and the existence of anastomotic threads are independent risk factors for the recurrence of benign bilioenteric anastomotic stricture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Choledochojejunostomy is widely used in digestive tract reconstruction after bile duct amputation, such as in radical resection of hepatobiliary and pancreatic malignant tumors, congenital biliary disease diversion and repair of biliary tract injury [1, 2]. While the long-term survival rate of patients after surgery has improved, the incidence of long-term complications after choledochojejunostomy has also increased. These biliary complications include bilioenteric stricture, cholangitis and cholangiolithiasis [3]. The recurrence rate of hepatolithiasis after choledochojejunostomy is 27.3%, and the incidence of choledochojejunostomy stricture is 8.2% [4]. Many patients suffer repeated recurrences and surgical treatments. How to properly address biliary complications after choledochojejunostomy is an urgent problem for surgeons.
In the past, secondary reconstruction via choledochojejunostomy or percutaneous transhepatic biliary drainage (PTCD) was often done for choledochojejunostomy stricture. While the reconstruction of choledojejunal anastomosis can completely solve the problem of anastomotic stricture, the abdominal adhesion caused by the primary operation creates a great challenge for the second surgeon, and restenosis of the reconstructed choledojejunal anastomosis is still possible. PTCD is generally used to relieve biliary obstruction quickly and plays a role in drainage before surgery. However, for patients whose bile duct dilatation is not obvious, the difficulty of puncture is much higher. In addition, bleeding and other complications are more likely [5]. The surgeon cannot intuitively observe the stricture of the choledojejunal anastomosis, so it plays a limited role in making follow-up treatment plans. For patients with multiple stenoses, the treatment cycle of PTCD is long, and long-term catheterization is highly inconvenient [6]. Therefore, there is an urgent need for a minimally invasive, effective and repeatable operation for treating choledochojejunostomy stricture.
Endoscopic retrograde cholangiopancreatography (ERCP), an advanced minimally invasive endoscopic technique, is an important method for the diagnosis and treatment of biliary diseases, as it has a better curative effect and long-term prognosis than PTCD [5]. Thanks to recent advances in endoscopic technology, many types of instrument-assisted enteroscopy have been developed. These include double-balloon enteroscopy (DBE), single-balloon enteroscopy (SBE) and spiral enteroscopy (spiral enteroscopy,SE) [7, 8]. Endoscopic retrograde cholangiography (ERC) is increasingly used to treat such patients. At present, there are a wide range of studies on the application of enteroscopy in gastrointestinal Roux-en-Y anastomosis after gastrointestinal ERC [9]. Because this technique is difficult and challenging, few centers have applied it, and there is no consensus on the optimal treatment technology and strategy. Further, its safety data and long-term efficacy still need more study. On this background, we analyzed the cases of SBE-ERC-treated patients after choledochojejunostomy in our center. To analyze the safety and efficacy of surgery, the patency rate of patients with benign bilioenteric anastomotic stricture was evaluated over long-term follow-up, and the risk factors for stricture recurrence were analyzed to determine the appropriate treatment for such patients.
Methods
Patients
We retrospectively studied patients with biliary tract diseases after choledochojejunostomy treated with SBE-ERC from January 2015 to December 2021 at Xinhua Hospital Affiliated with the Medical School of Shanghai Jiaotong University. The indications for ERCP were the following: (1) history of digestive tract reconstruction with choledochoenterostomy; (2) cholangitis, such as fever, abdominal pain or jaundice; and (3) preoperative CT or MRCP that revealed bile duct stones or obstructions. General patient information, including sex, age, surgical history, and laboratory and radiological examination results, was collected. This study met the relevant ethical standards and was approved by the Ethics Committee of Xinhua Hospital Affiliated with the Medical School of Shanghai Jiaotong University.
Major devices and endoscopic procedures
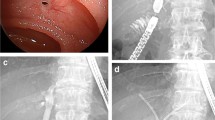
The enteroscopy and therapeutic equipment included an Olympus CV-260 central unit, an SIF-260 small-balloon enteroscope (working length 200 cm, outer diameter 9.2 mm, biopsy channel 2.8 mm) and an ST-SB 1 single-use overtube (working length 132 cm, outer diameter 13.2 mm, internal diameter 11 mm). A cotton sphincterotome (COOK, 320 cm length, United States), Glo-Tip ERCP catheter (COOK, 320 cm, United States), Tracer Metro Direct guidewire (COOK, 600 cm length, United States), OASIS stent introducer (COOK, 320 cm length, United States), 5 Fr pushing catheter (COOK, 320 length, United States), Quantum TTC biliary balloon dilator (COOK, 320 cm length, United States), Tri-EX triple lumen balloon extractor (Cook, 275 cm length, United States), and other conventional ERCP devices were used. The patient was placed in the horizontal position under general anesthesia via airway intubation. Endoscopic carbon dioxide (CO2) insufflation was used in all procedures. The enteroscopy process included repeated inflation and overtube, deflation and drag movements. Abdominal compression was applied manually if in-depth insertion was technically difficult. During the operation, the X-ray image was frequently monitored to track and guide the progress of the enteroscopy. After the bilioenteric anastomosis was reached, the endoscopists performed the biliary duct cannulation, cholangiography, anastomosis dilation, stone extraction or biliary stent insertion, and subsequent ERCP operations (Fig. 1). If the anastomotic stricture was severe, a needle knife was used to cut the scar tissue (Video1). For patients with choledocholithiasis, bile duct stones were removed with a basket after anastomotic dilatation according to the size of the stones. If the stones were larger, they were removed with mechanical lithotripsy or laser lithotripsy. For patients with a blocked stent or anastomotic thread, all foreign bodies were removed (Fig. 2 and Video2). According to the patients’ condition, if ERC failed, we tried endoscopic treatment again within 1 week or tried PTCD or surgical choledocholithotomy plus choledochojejunostomy reconstruction.
Intraoperative picture of a patient with bilienteric anastomosis stricture undergoing SBE-ERC. A endoscope through the intestinal anastomosis; B endoscope reaching the strictured biliary-intestinal anastomosis; C and D insertion of a catheter for contrast and intrahepatic bile duct visualization shows anastomosis stricture and calculus of intrahepatic duct; E balloon dilation of the anastomosis, waistline indicates high stricture recurrence risk; F appearance of bilioenteric anastomosis after balloon dilation; G placement of plastic stents; H status after stents placement complete; I endoscopic image of multiple stents through bilioenteric anastomosis
Postoperative observation indicators and treatment
All patients were monitored for vital signs under fasting conditions and were given IV fluid. Abdominal signs were closely observed. Routine blood examination, liver function tests and follow-up examinations were conducted the next day. According to the results, drugs such as liver protection, acid inhibition, antiemesis, hemostasis, and antibiotics were given for further treatment. Food intake time was determined by the general condition of the patients and the laboratory results. Under normal conditions, patients could drink and eat 24 h after surgery.
Definitions of outcomes and adverse events
The criteria for successful endoscopy were the single-balloon enteroscope successfully entering the input loop and finding the choledojejunal anastomosis.
The standard for successful radiography was that the guidewire catheter entered the anastomosis, the bile duct was intubated, and cholangiography was performed after the injection of contrast agent. The combination of successful endoscopy and successful radiography was defined as diagnostic success. The standard of successful treatment was the completion of corresponding intervention treatments, such as balloon dilatation, lithotripsy, stone removal, stent removal, and biliary stent placement. Because all patients in this group underwent choledochojejunostomy, intubation and therapeutic procedures were carried out through choledochojejunostomy, not involving the pancreas, so there was no possibility of PEP. Other definitions of cholangitis, bleeding, perforation and so on were the same as those used for conventional ERCP [10].
Follow-up and definition of recurrence of bilioenteric anastomotic stricture
Patients who were diagnosed with benign bilioenteric anastomotic stricture who achieved successful treatment were followed up. Routine blood tests, liver function tests, abdominal CT and MRCP were done regularly. The presence of symptoms such as abdominal pain, fever, jaundice and cholangiectasis on imaging indicated recurrence of the bilioenteric anastomotic stricture. The patient was admitted to the hospital for enteroscopy-assisted examination to confirm the status of the bilioenteric anastomosis.
Statistical analysis
The analyses were performed using SPSS version 23.0. The results are expressed as median (interquartile range). The Kaplan‒Meier method was used to evaluate cumulative biliary patency after SBE-ERC treatment, and the differences in cumulative biliary patency between groups were compared using the log-rank test. Variables with P < 0.2 in the Kaplan‒Meier analysis were included in the Cox proportional-hazards regression model for multifactor analysis, yielding the independent factors affecting the recurrence of benign bilioenteric anastomotic stricture. A P value < 0.05 was considered statistically significant.
Results
Patient characteristics
The general information of patients who underwent SBE-ERC after cholangiojejunostomy was as follows (Table 1). A total of 165 patients met the inclusion criteria and so were included in this study. Among them were 82 males (49.7%) and 83 females (50.3%). The median age was 57 (4–83) years. The original surgical methods included 90 cases of choledochojejunostomy Roux-en-Y anastomosis, and other surgical methods in 3 patients (partial gastrectomy + choledochotomy + bilioenteric anastomosis in 2 patients and Whipple + choledochojejunostomy Roux-en-Y anastomosis in 1 patient).
ERCP treatment details and success rate
A total of 120 of the 165 patients were treated successfully by SBE-ERC on the first attempt. A total of 289 SBE-ERC operations were performed. The time since the original surgery to the first SBE-ERC ranged from 2 months to 30 years, with a median of 4 years. The main symptoms before ERC included abdominal pain in 130 patients, fever in 124 patients, and elevated total bilirubin in 83 patients. Among the 289 ERC operations, the success rates of endoscopy and radiography were 87.2% and 97.6%, respectively, so the technical success rate of diagnosis was 85.1% (246/289). Eighteen patients underwent only diagnostic ERC without therapeutic treatment, and the success rate of treatment was 97.4% (222/228). The overall success rate was 83.0% (240/289). As for the diagnoses made from SBE-ERC, 173 (74.6%) were bile duct stones, 171 (70.1%) were anastomotic stenosis, 8 (3.0%) were intrahepatic bile duct stenosis, 35 (8.0%) were anastomotic foreign bodies, and 6 (2.1%) were tumor recurrence. The median hospital stay after ERCP was 3 (1–180) days. The treatment details of the 289 operations are shown in Table 2.
Causes of surgical failure and subsequent treatment
The failure rates of endoscopy and cholangiography were 12.8% (37/289) and 2.4% (6/252), respectively. Therefore, the overall diagnosis and treatment failure rates were 14.9% (43/289) and 2.6% (6/228), respectively. The most common reason for failed endoscopy was too large an angle of the input loop for Roux-en-Y anastomosis (13 cases) and an excessively twisted bowel lumen (22 cases). There were four cases of failed anastomosis identification. Of the six cholangiography failures, five were caused by needle-like stenosis of the anastomosis leading to unsuccessful bile duct insertion, and one case was caused by erosion of the anastomosis. Among the cases of treatment failure, one was because the stones filled the bile duct and could not be removed, three were due to anastomotic stenosis through which the incision knife or basket could not pass. In another case, we failed to remove the stone because the ductuli hepaticus communis above the anastomosis was extremely twisted. The last case was because the intrahepatic bile duct stone was located too high and could not be reached. Among the 45 patients who failed the first ERC treatment, 28 (62.2%) patients underwent conservative medical treatment and were discharged, 7 (15.6%) patients were successfully treated with another SBE-ERC attempt after conservative treatment, 8 (17.8%) patients were successfully treated with PTCD, and 2 patients (4.4%) underwent surgery to reconstruct the choledojejunal anastomosis.
Complications and management
As shown in Table 2, the incidence of postoperative complications was 5.2% (15/289). There were 12 cases of postoperative cholangitis, with an incidence of 4.2%. The patient had fever, chills and increased white blood cells after operation, which improved after the use of antibiotics and hepatic protective drugs. Gastrointestinal perforation occurred in 2 patients, for an incidence of 0.7%. One patient had a small intestinal perforation due to a twisted input loop near the transverse colonic mesenteric poke and was converted to laparotomy. In one patient, the endoscope failed to enter due to severe adhesion of the small intestine at the input loop, and the patient experienced aggravated abdominal pain after the operation. Exploratory laparotomy was performed 3 days after SBE-ERC. The small intestine laceration of the input loop was found and sutured. Another patient had sudden anesthesia-related cardiac and respiratory arrest during the operation, which was successfully rescued by CPR and ICU support treatment.
Long-term efficacy of SBE-ERCP treatment for benign bilioenteric anastomotic stricture
A total of 108 patients with benign choledojejunal anastomotic stricture successfully diagnosed and treated by SBE-ERC were followed up. The median follow-up time was 38.6 (1.6–73.5) months, and 29 patients (26.9.0%) developed stricture recurrence at a median of 16.2 (1–56.3) months after SBE-ERC treatment. Among them, 16 patients were treated with SBE-ERC again, 10 patients were treated conservatively, 2 patients underwent laparotomy to remove stones, and 1 patient underwent PTCD at another hospital. Among the 16 patients who underwent repeat SBE-ERC, 7 had a secondary recurrence at a median time of 13.7 months, 4 of whom were treated with SBE-ERC a third time, one who was treated with PTCD, one who underwent surgery, and one who experienced imaging recurrence but no obvious symptoms and who received no further treatment (Fig. 3). The biliary patency rates of 108 patients at 1 year, 2 years and 5 years after SBE-ERC treatment were 90.1% (95% CI 83.8–96.4), 69.3% (95% CI 58.7–79.9), and 53.9% (95% CI 37.8–70.0), respectively. The curve of the cumulative biliary patency rate after SBE-ERC treatment was drawn by the Kaplan‒Meier method (Fig. 4).
Risk factors for bilioenteric anastomotic stricture recurrence in patients treated with SBE-ERC
The Kaplan‒Meier method was used to analyze risk factors for bilioenteric anastomotic stricture recurrence. The absence of intrahepatic biliary gas imaging during endoscopy (Fig. 5) (\({\chi }^{2}\) = 5.366, P = 0.021), a diameter of balloon dilatation during the last endoscopic treatment less than 0.8 cm (\({\chi }^{2}\) = 4.552, P = 0.033),the presence of a thread in the anastomosis (\({\chi }^{2}\) = 8.921, P = 0.003), without biliary stent placement (\({\chi }^{2}\) = 14.868, P < 0.001) and receiving only one ERCP treatment (\({\chi }^{2}\) = 13.313, P = 0.001) were risk factors for the recurrence of benign stricture after SBE-ERC (Table 3). Factors with P < 0.2 were input into the Cox proportional-hazards regression model for multivariate analysis, and the results showed that without biliary stent placement (HR = 0.15, 95% CI 0.06–0.40, P = 0.001), absence of intrahepatic biliary gas imaging (HR = 0.39, 95% CI 0.17–0.91, P = 0.03) and the presence of threads in the anastomosis (HR = 3.69, 95% CI 1.59–8.57, P = 0.002) were independent risk factors for stricture recurrence.
Discussion
With the development of endoscopic technology, balloon-assisted enteroscopic ERCP has become a first-line intervention for the treatment of bile duct diseases after choledochojejunostomy, and its advantages of minimally invasiveness and repeatability make it far superior to traditional surgery. Due to the changes in the anatomical structure of the digestive tract and biliary system, there are some difficulties and challenges in endoscopy, cannulation and treatment, which require the operator to have extensive experience and great skill. The overall success rate of treatment in this study was 83.0%, which was slightly higher than that in other reports [11,12,13]. The difficulty of enteroscopy lies in how to pass through the growing and distorted intestines. For such patients, our center often treats them in a cooperative way involving general surgeons and endoscopic physicians. Surgeons have a deeper understanding of digestive tract structure and can guide the enteroscopy path. Endoscopic physicians have better endoscopic skills, and the combination of both can improve the success rate of endoscopy to a certain extent. When reaching the bilioenteric anastomosis, identifying the input loop is most important. The opening of the input loop can be judged by observing changes in the intestinal mucosa, which is referred to as the folding break sign. During intestinal anastomosis, the input loop is not the same as that of the intestine passed by the previous endoscope, so the mucosal texture is interrupted at the input loop and extends to the output loop [14]. The opening can also be judged by filling the intestinal cavity with carbon dioxide gas or contrast agent, which has a higher success rate than relying solely on endoscopic imaging (69.2% vs. 92.3%, P = 0.02) [15]. In this study, by observing the changes in the endoscopic mucosa, the diameter of the intestinal cavity and the angle between the input loop and the previously passed intestinal canal, we determined the correct path more accurately. The interrupted mucosa, the smaller diameter of the intestine and the steep entry angle are often suggested to open the input loop [16]. How to insert the endoscope to pass through the twisted intestinal loop is also a major difficulty. After reconstruction, some intestines are in a relatively fixed position, and the large angle of the intestinal loop can cause continuous bending of the enteroscope and failure to advance further. Under these circumstances, patient and meticulous manipulation is essential. Surgical techniques such as "push-pull-hook-spin" have been used in endoscopic insertion, which improved the success rate of treatment [16, 17].
In this study, the incidence of complications was 5.2%, which was comparable to that in other reports [11,12,13]. The most common complication was cholangitis. Poor bile duct drainage, including bile duct stone residue and unsolved bilioenteric anastomotic stricture, is considered the main risk factor [18, 19]. For patients with complete obstruction and suspected poor drainage, the use of prophylactic antibiotics before and after operation is the prevention method recommended by the current guidelines [18, 20]. For patients with severe stricture and stone obstruction, the choice between an indwelling biliary stent and nasobiliary drainage tube can reduce the occurrence of postoperative cholangitis. In this group, there were 2 cases of gastrointestinal perforation, both of which occurred in the input loop during endoscopy. The main reason was the small intestinal adhesion and stenosis of the input loop. Both cases were repaired successfully by surgery. If it is impossible to judge the route of the endoscope when the intestinal cavity is narrow and distorted, the contrast agent should be injected to adjust the direction under the guidance of X-ray, and the action should be slow and gentle to avoid putting the lens in blindly.
In this study period, our center first tried to remove stones and foreign bodies that caused obstructions and then treated patients with balloon dilatation alone or combined with plastic stent implantation according to the degree of stenosis of the anastomosis. At present, there is no consensus on the standard scheme for the treatment of benign obstructions after choledochojejunostomy. In patients with benign stricture of the choledojejunal anastomosis, the recurrence rate of biliary obstruction after ERCP has been higher than that after simple choledocholithiasis, which often requires multiple endoscopic treatments. The long-term effect of endoscopic treatment needs to be further studied. Here, during the median follow-up of 38.6 (1.6–73.5) months in 108 patients with benign bilioenteric anastomotic stricture, 29 (26.9%) patients experienced stricture recurrence. The biliary patency rates after SBE-ERC treatment at 1 year, 2 years and 5 years after treatment were 90.1%, 69.3% and 53.9%, respectively. Mizukawa et al. [21] reported that the cumulative anastomotic patency rates at 1, 2 and 3 years after endoscopic balloon dilatation in the treatment of stricture after choledochoenterostomy were 73%, 55% and 49%, respectively. Cantwell et al. [22] reported that the 5 years patency rate after treatment with a 10–12-mm balloon and a 10–12-Fr stent was 53% [23]. Our study showed that SBE-ERC is a good long-term treatment for benign biliary obstruction after choledochojejunostomy.
Balloon dilatation is most commonly used to treat bilioenteric anastomotic stricture. The short-term remission rate of patients with stricture is 74%. During long-term follow-up, the recurrence rate of stricture is 50–71% [21, 24]. This may be because the cause of anastomotic stenosis is local ischemia and fibrous scar tissue hyperplasia, and only intermittent balloon dilation cannot achieve the purpose of continuously expanding the anastomosis. In this study, our balloon diameter standard was to make the waistline of the anastomosis disappear and avoid rip the mucosa around it. When the dilation diameter of the last treatment was less than 0.8 cm, the probability of stricture recurrence increased, which may indicate thicker scar tissue around the anastomosis. Another important finding of this study is that intrahepatic biliary gas imaging during endoscopy is an independent protective factor against stricture recurrence. In the process of reaching the anastomosis, if gas flows into the bile duct, it often indicates that the biliary anastomosis is not incompletely closed or needle-like strictured, and there is still some elasticity in the tissue around the anastomosis. This and the final balloon dilatation diameter can also reflect the degree of anastomotic obstruction and tissue characteristics, thus indirectly predicting the probability of recurrence of the anastomotic stricture after treatment.
In this study, indwelling biliary stent during treatment was a protective factor against the recurrence of anastomotic stricture. The selection of stents to achieve long-term stable dilatation has more advantages in improving the long-term biliary patency rate. Tomoda et al. [25] reported that the 1-and 3-year biliary patency rates of the balloon dilatation combined with plastic stent group and simple balloon dilatation group were 62.5% vs. 89.4% and 46.6% vs. 84.7%, respectively (P = 0.015), and simple balloon dilatation was identified as an independent risk factor for the recurrence of choledojejunal anastomosis stenosis. In addition to plastic stents, fully coated metal stents are effective in the treatment of bilioenteric anastomotic stricture, with stenosis resolution rates of 85% and 100%, respectively, but the probability of stent displacement within 3 months is as high as 10% [25]. The higher cost of metal stents also limits their preferability to a certain extent [26]. Patients with indwelling biliary stents are likely to undergo multiple endoscopic treatments; therefore, in this study, patients who underwent only one SBE-ERC treatment had a higher probability of postoperative stenosis recurrence. On the other hand, it confirm the effectiveness of plastic stents in reducing the recurrence rate of stenosis.
In a study of patients with a previous history of surgery, Song et al. [27] reported that residual threading leads to the deposition of bile mud on the wall of the bile duct, leading to the formation of stones. Another study reported [28] that the removal of the thread head can significantly reduce the recurrence rate of stones. Our study further confirmed that the existence of an anastomotic site thread was an independent risk factor for the recurrence of bilioenteric anastomotic stricture after SBE-ERC treatment. We believe that in addition to the deposition of bile mud, the existence of a thread head leads to anastomotic blockage and changes in biliary fluid dynamics, resulting in cholestasis and then stone recurrence, but its specific mechanism remains to be determined. Either way, this finding suggests that in clinical practice, removing the thread thoroughly is helpful for preventing the recurrence of stones and stenosis. The absorbable sutures used in biliary surgery, such as PDS sutures, are also recommended to reduce the residual sutures at the anastomotic site and prevent long-term complications.
This study has some limitations. First, this was a retrospective study, which inevitably had a certain degree of bias in the process of data collection. Second, this was a single-center study, and the overall number of patients after choledochojejunostomy was small. We did not compare uncontrolled variables between patients receiving different treatment methods. Larger, multicenter, prospective studies are needed to analyze the efficacy of SBE-assisted balloon dilation or stent placement. In summary, SBE-ERC is a safe and effective treatment for patients with biliary tract diseases after cholangioenterostomy, with a good immediate technical success rate and a lower incidence of adverse events. For patients with risk factors for stricture recurrence, more attention should be paid to thorough dilatation of the anastomosis and removal of the thread and stones. Bile stents should also be placed to enhance the long-term effect of endoscopic therapy and reduce the recurrence rate.
References
Corff M, Berger S, Gershon-Cohen J (1952) Choledochostomy with cholangiography; a review of 50 cases. Surg Gynecol Obstet 94:394–400
Tocchi A, Mazzoni G, Liotta G, Lepre L, Cassini D, Miccini M (2001) Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1,000 patients. Ann Surg 234:210–214
Lau WY, Lai EC, Lau SH (2010) Management of bile duct injury after laparoscopic cholecystectomy: a review. ANZ J Surg 80:75–81
Li SQ, Liang LJ, Peng BG, Hua YP, Lv MD, Fu SJ, Chen D (2012) Outcomes of liver resection for intrahepatic stones: a comparative study of unilateral versus bilateral disease. Ann Surg 255:946–953
Hammad H, Brauer BC, Smolkin M, Ryu R, Obuch J, Shah RJ (2019) Treating biliary-enteric anastomotic strictures with enteroscopy-ERCP requires fewer procedures than percutaneous transhepatic biliary drains. Dig Dis Sci 64:2638–2644
Yang DH, Lee SK, Moon SH, Park DH, Lee SS, Seo DW, Kim MH (2009) Percutaneous transhepatic cholangioscopic intervention in the management of complete membranous occlusion of bilioenteric anastomosis: report of two cases. Gut Liver 3:352–355
Tomoda T, Kato H, Ueki T, Ogawa T, Hirao K, Akimoto Y, Matsumoto K, Horiguchi S, Tsutsumi K, Okada H (2022) Efficacy of double-balloon enteroscopy-assisted endoscopic balloon dilatation combined with stent deployment for hepaticojejunostomy anastomotic stricture. Dig Endosc 34:604–611
Kurzynske FC, Romagnuolo J, Brock AS (2015) Success of single-balloon enteroscopy in patients with surgically altered anatomy. Gastrointest Endosc 82:319–324
Haruta H, Yamamoto H, Mizuta K, Kita Y, Uno T, Egami S, Hishikawa S, Sugano K, Kawarasaki H (2005) A case of successful enteroscopic balloon dilation for late anastomotic stricture of choledochojejunostomy after living donor liver transplantation. Liver Transpl 11:1608–1610
Tryliskyy Y, Bryce GJ (2018) Post-ERCP pancreatitis: pathophysiology, early identification and risk stratification. Adv Clin Exp Med 27:149–154
Inamdar S, Slattery E, Sejpal DV, Miller LS, Pleskow DK, Berzin TM, Trindade AJ (2015) Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc 82:9–19
Yane K, Katanuma A, Maguchi H, Takahashi K, Kin T, Ikarashi S, Sano I, Yamazaki H, Kitagawa K, Yokoyama K, Koga H, Nagai K, Nojima M (2017) Short-type single-balloon enteroscope-assisted ERCP in postsurgical altered anatomy: potential factors affecting procedural failure. Endoscopy 49:69–74
Tanisaka Y, Ryozawa S, Mizuide M, Araki R, Fujita A, Ogawa T, Tashima T, Noguchi T, Suzuki M, Katsuda H (2021) Status of single-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy: systematic review and meta-analysis on biliary interventions. Dig Endosc 33:1034–1044
Kanno Y, Ohira T, Kozakai F, Miyamoto K, Kusunose H, Sakai T, Yonamine K, Okano H, Koshita S, Ogawa T, Shimizu T, Ito K (2022) Accurate endoscopic identification of the afferent limb at the Y anastomosis using the fold disruption sign after gastric resection with Roux-en-Y reconstruction. Dig Endosc 34:238–243
Niwa Y, Nakamura M, Kawashima H, Yamamura T, Maeda K, Sawada T, Mizutani Y, Ishikawa E, Ishikawa T, Kakushima N, Furukawa K, Ohno E, Honda T, Ishigami M, Fujishiro M (2020) Accuracy of carbon dioxide insufflation for endoscopic retrograde cholangiopancreatography using double-balloon endoscopy. World J Gastroenterol 26:6669–6678
Wu WG, Qin LC, Song XL, Zhao MN, Zhang WJ, Gu J, Weng H, Liu YB, Zhang Y, Qu CY, Xu LM, Wang XF (2019) Application of single balloon enteroscopy-assisted therapeutic endoscopic retrograde cholangiopancreatography in patients after bilioenteric Roux-en-Y anastomosis: experience of multi-disciplinary collaboration. World J Gastroenterol 25:5505–5514
Wu WW, Zhang WJ, Gu J, Zhao MN, Weng H, Weng MZ, Zhang Y, Qu CY, Xu LM, Liu YB, Wang XF (2018) Endoscopicretrograde cholangio-pancreatography management of long-term complications after pancreaticoduodenectomy. Zhonghua Wai Ke Za Zhi 56:833–836
Kang X, Zheng L, Zeng W, Yang S, Sun H, Zhang R, Wang X, Wang B, Tao Q, Yao S, Chen J, Pan Y, Guo X (2018) Risk factors for post-ERCP pancreatitis in high-risk patients receiving post-procedure rectal indomethacin. J Gastrointest Surg 22:1903–1910
Thosani N, Zubarik RS, Kochar R, Kothari S, Sardana N, Nguyen T, Banerjee S (2016) Prospective evaluation of bacteremia rates and infectious complications among patients undergoing single-operator choledochoscopy during ERCP. Endoscopy 48:424–431
Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM, ASGE Standards of Practice Committee (2017) Adverse events associated with ERCP. Gastrointest Endosc 85:32–47
Mizukawa S, Tsutsumi K, Kato H, Muro S, Akimoto Y, Uchida D, Matsumoto K, Tomoda T, Horiguchi S, Okada H (2018) Endoscopic balloon dilatation for benign hepaticojejunostomy anastomotic stricture using short double-balloon enteroscopy in patients with a prior Whipple’s procedure: a retrospective study. BMC Gastroenterol 18:14
Bonnel DH, Fingerhut AL (2012) Percutaneous transhepatic balloon dilatation of benign bilioenteric strictures: long-term results in 110 patients. Am J Surg 203:675–683
Cantwell CP, Pena CS, Gervais DA, Hahn PF, Dawson SL, Mueller PR (2008) Thirty years’ experience with balloon dilation of benign postoperative biliary strictures: long-term outcomes. Radiology 249:1050–1057
Kamei H, Imai H, Onishi Y, Ishihara M, Nakamura M, Kawashima H, Ishigami M, Ito A, Ohmiya N, Hirooka Y, Goto H, Ogura Y (2015) Considerable risk of restenosis after endoscopic treatment for hepaticojejunostomy stricture after living-donor liver transplantation. Transplant Proc 47:2493–2498
Tomoda T, Kato H, Miyamoto K, Saragai Y, Mizukawa S, Yabe S, Takata S, Muro S, Uchida D, Matsumoto K, Horiguchi S, Tsutsumi K, Hirao K, Ogawa T, Okada H (2020) Comparison between endoscopic biliary stenting combined with balloon dilation and balloon dilation alone for the treatment of benign hepaticojejunostomy anastomotic stricture. J Gastrointest Surg 24:1352–1358
Shibuya H, Hara K, Mizuno N, Hijioka S, Imaoka H, Tajika M, Tanaka T, Ishihara M, Hirayama Y, Yoshida T, Okuno N, Hieda N, Bhanthumkomol P, Shimizu Y, Senda Y, Natsume S, Niwa Y, Yamao K (2017) Treatment of biliary strictures with fully covered self-expandable metal stents after pancreaticoduodenectomy. Endoscopy 49:75–79
Song ME, Chung MJ, Lee DJ, Oh TG, Park JY, Bang S, Park SW, Song SY, Chung JB (2016) Cholecystectomy for prevention of recurrence after endoscopic clearance of bile duct stones in Korea. Yonsei Med J 57:132–137
Leborgne J, Basque Y, Horeau JH, Sudry P, Le Neel JC (1981) Calcul cholédocien sur fil migrateru. A propos de 3 observations [common bile duct stone formation on migrating ligature threads. A report on three cases (author’s transl)]. J Chir (Paris) 118:343–347
Funding
This study was supported by National Natural Science Foundation of China (82272691), Science Foundation of Shigatse City (RKZ2024ZR-006 (Z)).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Hao Weng、Xue-Feng Wang and Yi-Jun Shu; (II) Administrative support: Jun Gu (III) Provision of study materials or patients: Ming-Zhe Weng, Wen-Jie Zhang, Lei-Ming Xu, Yi Zhang; (IV) Collection and assembly of data: Qing-quan Fan; (V) Data analysis and interpretation: Hao Weng and Qing-quan Fan; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Disclosures
Hao Weng, Qing-quan Fan, Jun Gu, Ming-Zhe Weng, Wen-Jie Zhang, Lei-Ming Xu, Yi Zhang, Yi-Jun Shu and Xue-Feng Wang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 129835 KB)
Supplementary file2 (MP4 117264 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Weng, H., Fan, Qq., Gu, J. et al. Efficacy and long-term outcomes of single-balloon enteroscopy-assisted treatment for biliary obstruction after choledochojejunostomy. Surg Endosc (2024). https://doi.org/10.1007/s00464-024-11096-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00464-024-11096-z